 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommendations of the Immunization Practices Advisory Committee Pneumococcal Polysaccharide VaccineThese recommendations update the last statement by the Immunization Practices Advisory Committee (ACIP) on pneumococcal polysaccharide vaccine (MMWR 1984;33:273-6, 281) and include new information regarding 1) vaccine efficacy, 2) use in persons with human immunodeficiency virus (HIV) infection and in other groups at increased risk of pneumococcal disease, and 3) guidelines for revaccination. INTRODUCTION Disease caused by Streptococcus pneumoniae (pneumococcus) remains an important cause of morbidity and mortality in the United States, particularly in the very young, the elderly, and persons with certain high-risk conditions. Pneumococcal pneumonia accounts for 10%-25% of all pneumonias and an estimated 40,000 deaths annually (1). Although no recent data from the United States exist, in the United Kingdom pneumococcal infections may account for 34% of pneumonias in adults who require hospitalization (2). The best estimates of the incidence of serious pneumococcal disease in the United States are based on surveys and community-based studies of pneumococcal bacteremia. Recent studies suggest annual rates of bacteremia of 15-19/100,000 for all persons, 50/100,000 for persons greater than or equal to 65 years old, and 160/100,000 for children less than or equal to 2 years old (3,4). These rates are 2-3 times those previously documented in the United States. The overall rate for pneumococcal bacteremia in some Native American populations can be six times the rate of the general population (5). The incidence of pneumococcal pneumonia can be 3-5 times that of the detected rates of bacteremia. The estimated incidence of pneumococcal meningitis is 1-2/100,000 persons. Mortality from pneumococcal disease is highest in patients with bacteremia or meningitis, patients with underlying medical conditions, and older persons. In some high-risk patients, mortality has been reported to be greater than 40% for bacteremic disease and 55% for meningitis, despite appropriate antimicrobial therapy. Over 90% of pneumococci remain very sensitive to penicillin. In addition to the very young and persons greater than or equal to 65 years old, patients with certain chronic conditions are at increased risk of developing pneumococcal infection and severe pneumococcal illness. Patients with chronic cardiovascular diseases, chronic pulmonary disease, diabetes mellitus, alcoholism, and cirrhosis are generally immunocompetent but have increased risk. Other patients at greater risk because of decreased responsiveness to polysaccharide antigens or more rapid decline in serum antibody include those with functional or anatomic asplenia (e.g., sickle cell disease or splenectomy), Hodgkin's disease, lymphoma, multiple myeloma, chronic renal failure, nephrotic syndrome, and organ transplantation. In a recent population-based study, all persons 55-64 years old with pneumococcal bacteremia had at least one of these chronic conditions (4). Studies indicate that patients with acquired immunodeficiency syndrome (AIDS) are also at increased risk of pneumococcal disease, with an annual attack rate of pneumococcal pneumonia as high as 17.9/1000 (6-8). This observation is consistent with the B-cell dysfunction noted in patients with AIDS (9,10). Recurrent pneumococcal meningitis may occur in patients with cerebrospinal fluid leakage complicating skull fractures or neurologic procedures. PNEUMOCOCCAL POLYSACCHARIDE VACCINE The current pneumococcal vaccine (Pneumovax (R) 23, Merck Sharp & Dohme, and Pnu-Imune (R) 23, Lederle Laboratories) is composed of purified capsular polysaccharide antigens of 23 types of S. pneumoniae (Danish types 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, 33F). It was licensed in the United States in 1983, replacing a 14-valent vaccine licensed in 1977. Each vaccine dose (0.5 mL) contains 25 ug of each polysaccharide antigen. The 23 capsular types in the vaccine cause 88% of the bacteremic pneumococcal disease in the United States. In addition, studies of the human antibody response indicate that cross-reactivity occurs for several types (e.g., 6A and 6B) that cause an additional 8% of bacteremic disease (11). Most healthy adults, including the elderly, show a twofold or greater rise in type-specific antibody, as measured by radioimmunoassay, within 2-3 weeks of vaccination. Similar antibody responses have been reported in patients with alcoholic cirrhosis and diabetes mellitus requiring insulin. In immunocompromised patients, the response to vaccination may be less. In children less than 2 years old, antibody response to most capsular types is generally poor. In addition, response to some important pediatric pneumococcal types (e.g., 6A and 14) is decreased in children less than 5 years old (12,13). Following vaccination of healthy adults with polyvalent pneumococcal vaccine, antibody levels for most pneumococcal vaccine types remain elevated at least 5 years; in some persons, they fall to prevaccination levels within 10 years (14,15). A more rapid decline in antibody levels may occur in children. In children who have undergone splenectomy following trauma and in those with sickle cell disease, antibody titers for some types can fall to prevaccination levels 3-5 years after vaccination (16,17). Similar rates of decline can occur in children with nephrotic syndrome (18). Patients with AIDS have been shown to have an impaired antibody response to pneumococcal vaccine (10,19). However, asymptomatic HIV-infected men or those with persistent generalized lymphadenopathy respond to the 23-valent pneumococcal vaccine (20). VACCINE EFFICACY In the 1970s, pneumococcal vaccine was shown to reduce significantly the occurrence of pneumonia in young, healthy populations in South Africa and Papua New Guinea, where incidence of pneumonia is high (21,22). It was also demonstrated to protect against systemic pneumococcal infection in hyposplenic patients in the United States (23). Since then, studies have attempted to assess vaccine efficacy in other U.S. populations (24-30; CDC, unpublished data) (Table_1). A prospective, ongoing case-control study in Connecticut has shown an overall protective efficacy of 61% against pneumococcal bacteremia caused by vaccine- and vaccine-related serotypes. The protective efficacy was 60% for patients with alcoholism or chronic pulmonary, cardiac, or renal disease and 64% for patients greater than or equal to 55 years old without other high-risk chronic conditions (25,26). In another multicenter case-control study, vaccine efficacy in immunocompetent persons greater than or equal to 55 years old was 70% (27). A smaller case-control study of veterans failed to show efficacy in preventing pneumococcal bacteremia (28), but determination of the vaccination status was judged to be inadequate and the selection of controls was considered to be potentially biased. Studies based on CDC's pneumococcal surveillance system suggest an efficacy of 60%-64% for vaccine-type strains in patients with bacteremic disease. For all persons greater than or equal to 65 years of age (including persons with chronic heart disease, pulmonary disease, or diabetes mellitus), vaccine efficacy was 44%-61% (29; CDC, unpublished data). In addition, estimates of vaccine efficacy for serologically related types were 29%-66% (29). Limited data suggest that clinical efficacy may decline greater than or equal to 6 years after vaccination (CDC, unpublished data). A randomized, double-blind, placebo-controlled trial among high-risk veterans showed no vaccine efficacy against pneumococcal pneumonia or bronchitis (30); however, case definitions used were judged to have uncertain specificity. In addition, this study had only a 6% ability to detect a vaccine efficacy of 65% for pneumococcal bacteremia (31). In contrast, a French clinical trial found pneumococcal vaccine to be 77% effective in reducing the incidence of pneumonia in nursing home residents (32). Despite conflicting findings, the data continue to support the use of the pneumococcal vaccine for certain well-defined groups at risk. RECOMMENDATIONS FOR VACCINE USE Adults
Children
Special Groups Persons living in special environments or social settings with an identified increased risk of pneumococcal disease or its complications (e.g., certain Native American populations). ADVERSE REACTIONS Approximately 50% of persons given pneumococcal vaccine develop mild side effects, such as erythema and pain at the injection site. Fever, myalgia, and severe local reactions have been reported in less than 1% of those vaccinated. Severe systemic reactions, such as anaphylaxis, rarely have been reported. PRECAUTIONS The safety of pneumococcal vaccine for pregnant women has not been evaluated. Ideally, women at high risk of pneumococcal disease should be vaccinated before pregnancy. TIMING OF VACCINATION When elective splenectomy is being considered, pneumococcal vaccine should be given at least 2 weeks before the operation, if possible. Similarly, for planning cancer chemotherapy or immunosuppressive therapy, as in patients who undergo organ transplantation, the interval between vaccination and initiation of chemotherapy or immunosuppression should also be at least 2 weeks. REVACCINATION In one study, local reactions after revaccination in adults were more severe than after initial vaccination when the interval between vaccinations was 13 months (33) (Table_2 (p. 73)). Reports of revaccination after longer intervals in children and adults, including a large group of elderly persons revaccinated at least 4 years after primary vaccination, suggest a similar incidence of such reactions after primary vaccination and revaccination (unpublished data; 17,34-38). Without more information, persons who received the 14-valent pneumococcal vaccine should not be routinely revaccinated with the 23-valent vaccine, as increased coverage is modest and duration of protection is not well defined. However, revaccination with the 23-valent vaccine should be strongly considered for persons who received the 14-valent vaccine if they are at highest risk of fatal pneumococcal infection (e.g., asplenic patients). Revaccination should also be considered for adults at highest risk who received the 23-valent vaccine greater than or equal to 6 years before and for those shown to have rapid decline in pneumococcal antibody levels (e.g., patients with nephrotic syndrome, renal failure, or transplant recipients). Revaccination after 3-5 years should be considered for children with nephrotic syndrome, asplenia, or sickle cell anemia who would be less than or equal to 10 years old at revaccination. STRATEGIES FOR VACCINE DELIVERY Recommendations for pneumococcal vaccination have been made by the ACIP, the American Academy of Pediatrics, the American College of Physicians, and the American Academy of Family Physicians. Recent analysis indicates that pneumococcal vaccination of elderly persons is cost-effective (39). The vaccine is targeted for approximately 27 million persons aged greater than or equal to 65 years and 21 million persons aged less than 65 years with high-risk conditions (1). Despite Medicare reimbursement for costs of the vaccine and its administration, which began in 1981, annual use of pneumococcal vaccine has not increased above levels observed in earlier years (40) (Figure_1). In 1985, less than 10% of the 48 million persons considered to be at increased risk of serious pneumococcal infection were estimated to have ever received pneumococcal vaccine (1). Opportunities to vaccinate high-risk persons are missed both at time of hospital discharge and during visits to clinicians' offices. Two thirds or more of patients with serious pneumococcal disease had been hospitalized at least once within 5 years before their pneumococcal illness, yet few had received pneumococcal vaccine (40). More effective programs for vaccine delivery are needed, including offering pneumococcal vaccine in hospitals (at the time of discharge), clinicians' offices, nursing homes, and other chronic-care facilities. Many patients who receive pneumococcal vaccine should also be immunized with influenza vaccine (41), which can be given simultaneously at a different site. In contrast to pneumococcal vaccine, influenza vaccine is given annually. VACCINE DEVELOPMENT A more immunogenic pneumococcal vaccine preparation is needed, particularly for children less than 2 years old. The development of a protein-polysaccharide conjugate vaccine for selected capsular types holds promise. References
Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Clinical effectiveness of pneumococcal vaccination in U.S. populations
========================================================================================================
Vaccine
Type efficacy
Location Method No. persons infection (%) 95% C.I.
--------------------------------------------------------------------------------------------------
Connecticut Case-control * 543 cases VT +, VT-related 61 42, 73
(25,26) 543 controls
Philadelphia Case control * 122 cases All serotypes 70 37, 86
(27) 244 controls
Denver Case control * 89 cases All serotypes -21 -221, 55
(28) 89 controls
CDC-1 Epidemiologic * 249 vaccinated VT 64 47, 76
(29) 1638 unvaccinated
CDC-2 Epidemiologic * 240 vaccinated VT 60 45, 70
(unpublished) 1527 unvaccinated
VA cooperative Randomized 1145 vaccinated All serotypes -34 @ -119, 18 @
study (30) controlled trial & 1150 controls VT -19 @ -164, 47 @
--------------------------------------------------------------------------------------------------
* Only patients with isolates from normally sterile body sites were included.
+ Vaccine-type pneumococcal infection.
& Pneumococcal pneumonia and bronchitis were diagnosed primarily by culture of respiratory secretions.
@ Values calculated from the published data.
========================================================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Reactions to revaccination with pneumoccal vaccine
====================================================================================================================
Vaccinees
------------------------ Revaccination
Study Condition Age No. period Reactions
-----------------------------------------------------------------------------------------
Borgono, et al. Normal Adults 7 13 mos Increase in
1978 (33) local reactions
Carlson, et al. Normal 21-62 yrs 23 12-18 mos Increase in
1979 (34) local reactions
Rigau-Perez, et al. Sickle >=3 yrs 28 28-35 mos No increase in
1983 (35) cell disease reactions compared
with primary
vaccination
Lawrence, et al. Normal 2-5 yrs 52 35 mos Increase in
1983 (36) (mean) local reactions
Mufson, et al. Normal 23-40 yrs 12 24-48 mos No increase in
1984 (37) reactions compared
with primary
vaccination
Weintrub, et al. Sickle 10-27 yrs 17 8-9 yrs No "serious"
1984 (17) cell disease local reactions
Kaplan, et al. Sickle 4-23 yrs 86 37-53 mos Four "severe"
1986 (38) cell disease reactions *
-----------------------------------------------------------------------------------------
* Severe reaction was defined as presence of local pain, redness, swelling, and auxiliary temperature >100 F (37.8
C); two patients aged 21 and 23 years had temperatures of 102 F (38.9 C).
====================================================================================================================
Return to top. Figure_1 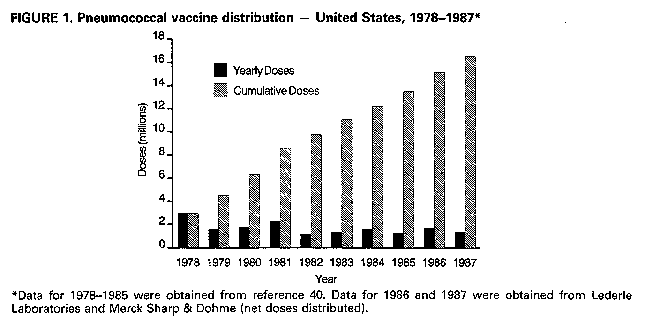 Return to top. Disclaimer All MMWR HTML documents published before January 1993 are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 08/05/98 |
|||||||||
This page last reviewed 5/2/01
|