 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication -- Indonesia, 1995In 1988, Indonesia (1991 population: 181 million) adopted the goal of eradicating poliomyelitis by the year 2000. Although routine coverage with three doses of oral poliovirus vaccine (OPV3) has been greater than 90% among 1-year-old children since 1991, cases of polio continue to be reported (Figure_1). To interrupt poliovirus transmission, National Immunization Days (NIDs) * were conducted during September 13-17 and October 18-22, 1995, resulting in vaccination of greater than 22 million children aged less than 5 years (representing approximately 100% of the target population). NIDs are planned for September 10-14 and October 15-19, 1996, and in 1997. This report presents the polio surveillance data for Indonesia for 1995, which indicate that substantial improvements are necessary to meet the objectives of the polio-eradication initiative. Activities are under way to intensify surveillance for acute flaccid paralysis (AFP) and to identify the remaining reservoirs of wild poliovirus transmission. Indonesia, which instituted AFP surveillance in 1995, requires all cases of AFP to be reported immediately to health authorities. A case of suspected polio is defined as AFP, including Guillain-Barre syndrome, in a child aged less than 15 years for which no other cause can be immediately identified, or any patient in whom a clinician suspects polio regardless of age. Stool specimens collected from AFP case-patients were sent to one of three designated laboratories in Bandung, Jakarta, and Surabaya for virus isolation. Poliovirus isolates were sent to the national laboratory in Jakarta to distinguish wild and vaccine-type polioviruses. Genetic sequencing of wild polioviruses was conducted at CDC. The number of reported polio cases has decreased substantially since the start of the national Expanded Program on Immunization (Figure_1). In 1995, a total of 22 cases of AFP were reported, representing an AFP rate of 0.04 per 100,000 children aged less than 15 years; seven (33%) cases were investigated within 48 hours of report, 17 (78%) had stool specimens taken for virus isolation, and two (10%) cases had stool specimens taken within 2 weeks of paralysis onset. Of the 22 cases of suspected polio, 12 have been classified as confirmed polio cases (using the standard WHO case definition **) by virus isolation (five cases -- two in case-patients and three in contacts of case-patients), with residual paralysis at 60 days' follow-up (four), or lost to follow-up (three). Four culture-confirmed cases of polio were associated with type 1 and one with type 3 wild polioviruses with onset of paralysis during March and August 1995. Four of these cases, in Java and Sumatra, occurred in unvaccinated children. To define the molecular epidemiology of the type 1 wild polioviruses isolated from the four culture-confirmed cases in 1995, genetic sequencing was conducted on a 150-nucleotide interval at the VP1/2A junction of the viral genome (1). Because the poliovirus genome mutates at a rate of approximately two nucleotide substitutions per week, it is possible to quantify the genetic relatedness of isolates from different geographic areas and determine chains of transmission. Data summarizing the sequence relatedness among wild poliovirus isolates recently identified as endemic in South and East Asia suggested that the type 1 wild polioviruses isolated from Indonesia in 1995 form a distinct cluster and have been circulating in the country for at least 10 years. The genetic sequences of the isolates from Java and Sumatra, although found at the same time on adjacent islands, differed enough to suggest that the two cases were not related epidemiologically. Reported by: H Wibosono, MD, H Roespandi, MD, Surveillance Unit, Ministry of Health, Indonesia; W Gendro, Polio Laboratory, Indonesia National Institute of Health, Research, and Development, Jakarta; L Soemara, MD, PT Biofarma, S Rusliana, MD, Public Health Laboratory of Surabaya; S Prihatini, MD, Provincial Health Svcs, East Java. United Nations Children's Fund; World Health Organization, Jakarta, Indonesia. Respiratory and Enterovirus Br, National Center for Infectious Diseases; Polio Eradication Activity, National Immunization Program, CDC. Editorial NoteEditorial Note: Indonesia successfully conducted its first NIDs in 1995 and is improving surveillance for polio by requiring AFP reporting. The AFP reporting rate in Indonesia during 1995 (0.04 per 100,000) was substantially lower than the expected rate of one AFP case per 100,000 persons aged less than 15 years (the rate used to define a sensitive AFP surveillance system {653 cases would be expected each year in Indonesia if the rate reached one per 100,000}). To meet the objectives of the polio-eradication initiative, performance indicators other than the AFP rate (i.e., proportion of case investigations conducted within 48 hours of notification and proportion of cases for which two stool specimens were obtained within 2 weeks of paralysis onset) should be at least 80%, which indicates adequate surveillance. Major efforts are under way to strengthen the surveillance system as a guide to further polio-eradication activities in Indonesia; these efforts include establishing active AFP reporting (i.e., reviewing hospital discharge records for AFP in hospitalized patients) linked with timely AFP case investigations. The low level of AFP reporting in Indonesia and the retrospective reviews of hospital records indicate that many persons with AFP or with physician-diagnosed polio are admitted to hospitals but are not reported to public health officials. Although a hospital early-warning system is in place to immediately report high-priority infectious diseases, active surveillance systems probably will be needed to increase sensitivity. These active systems will be valuable especially in densely populated urban areas. For the global polio-eradication initiative, genetic analysis of wild poliovirus isolates has provided critically important information. The isolation of type 1 wild poliovirus in Java and Sumatra indicate that the virus is indigenous to Indonesia and has circulated for many years, and confirmed the independent evolution of at least two poliovirus reservoirs in the country. These findings suggest that wild poliovirus has remained endemic in Indonesia despite routine high coverage with OPV3 in recent years and underscore the need for supplementary vaccination strategies (i.e., NIDs) to interrupt poliovirus transmission. Indonesia, the fourth most populous country in the world, is of critical importance to the global polio-eradication initiative. Improved virologic surveillance already has identified at least two indigenous poliovirus reservoirs. The government of Indonesia, in cooperation with the major partner agencies contributing to the polio-eradication initiative (including WHO, United Nations Children's Fund {UNICEF}, and Rotary International), will need to establish a sensitive AFP surveillance system. Adequate surveillance is necessary to identify the remaining poliovirus reservoirs and target areas for supplemental vaccination activities (i.e., mopping-up vaccination ***) and prepare Indonesia for eventual certification of polio-free status (2). References
* Mass campaigns over a short period (days to weeks) in which two doses of oral poliovirus vaccine are administered to all children in the target age group, regardless of prior vaccination history, with an interval of 4-6 weeks between doses. ** A confirmed case of polio is defined as AFP and at least one of the following: 1) laboratory-confirmed wild poliovirus infection, 2) residual paralysis at 60 days, 3) death, or 4) no follow-up investigation at 60 days. *** House-to-house administration of two doses of oral poliovirus vaccine at an interval of 4-6 weeks to all children aged less than 3 years who reside in areas where risk for wild poliovirus transmission is highest. Figure_1 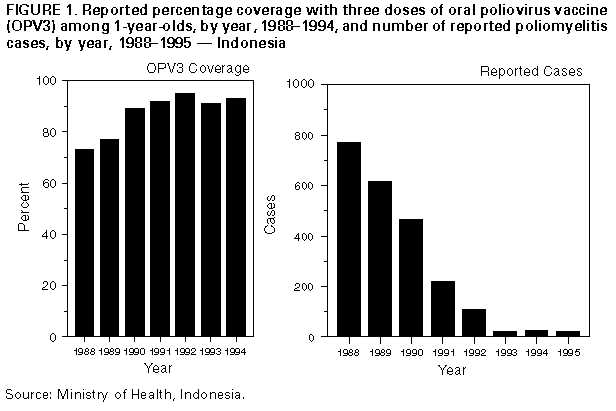 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|