 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Outbreaks of Pseudo-Infection with Cyclospora and Cryptosporidium -- Florida and New York City, 1995Efforts to expand the scope of surveillance and diagnostic testing for emerging infectious diseases (1) also may increase the potential for identifying pseudo-outbreaks (2,3) (i.e., increases in incidence that may result from enhanced surveillance) and outbreaks of pseudo-infection (i.e., clusters of false-positives for infection). This report describes the investigations of outbreaks of pseudo-infection with Cyclospora in Florida and Cryptosporidium in New York City in 1995 after health departments in those jurisdictions had initiated surveillance for these emerging organisms. These investigations emphasize 1) the need for laboratory training in the identification of emerging pathogens and 2) the importance of confirmation by reference laboratories as an early step in the investigation of any apparent outbreak caused by an emerging pathogen. Cyclosporiasis in Florida Cyclosporiasis is caused by infection with Cyclospora cayetanensis, a recently identified coccidian parasite (4) that can cause prolonged, relapsing diarrhea; treatment with trimethoprim-sulfamethoxazole relieves symptoms and accelerates clearance of the parasite (5). Until 1996, most cases of cyclosporiasis in the United States occurred among international travelers (6), and information about modes of transmission of C. cayetanensis was limited. Waterborne transmission had been documented, but direct person-to-person transmission was considered unlikely (4). During the summer of 1995, in response to an outbreak of Cyclospora infection among Florida residents with no history of international travel (7), the state health department initiated surveillance for the organism. All state laboratories began routine testing with a modified acid-fast stain for C. cayetanensis in stool specimens submitted for parasitologic examination (8). On July 25, 1995, the Florida Department of Health (FDH) designated cyclosporiasis a reportable disease. On August 11, a 3-year-old boy at a children's shelter had onset of diarrhea and abdominal pain; Giardia cysts were identified in a stool specimen obtained from the child. Because of previous giardiasis outbreaks at the shelter, county public health officials recommended testing the 13 shelter residents who were preschool classmates or roommates of the index patient. State branch laboratory A reported that stool specimens from six children tested positive for Giardia, and six tested positive for Cyclospora. The high proportion of specimens positive for Cyclospora prompted testing of 81 persons, including all children residing at the shelter and the shelter's staff and volunteers. Overall, branch laboratory A identified Cyclospora oocysts in specimens from 31 (86%) of 36 staff, 16 (64%) of 25 children, and nine (45%) of 20 volunteers. In response to this apparent outbreak, the residence was closed to new admissions, the children's outside activities were restricted, and trimethoprim-sulfamethoxazole was prescribed for all 25 children. On September 17, FDH was notified about the apparent outbreak and joined the investigation. The local community hospital, which had begun testing for Cyclospora in 1995, was contacted for information about laboratory-identified infections in the community during July-September. Questionnaires were administered to shelter staff, volunteers, the older children, and the infants' caretakers, and medical records for the children were reviewed. A case was defined as onset of nausea, vomiting, or diarrhea in a resident, employee, or volunteer at the shelter during August- September. Branch laboratory A sent portions of stool specimens from 23 shelter residents and staff to the reference laboratories at CDC and the University of Arizona for Cyclospora testing. Questionnaires were completed for 79 of the 81 children and adults. Symptoms among the 56 persons whose stool specimens were positive for Cyclospora at branch laboratory A included abdominal pain (30%), nausea (26%), fatigue (22%), diarrhea (three or more loose or watery stools in 24 hours) with median duration of 2 days (20%), vomiting (19%), anorexia (15%), fever (10%), and weight loss (8%). The 23 persons with negative stool specimens had symptoms and onset dates similar to those of persons with positive specimens (Figure_1). In addition, the likelihood of being asymptomatic was similar among persons who were test-positive (35%) and test-negative (46%) (p=0.4). Potential risk factors (e.g., consumption of food or water at the shelter or participation in field trips) were not associated with the likelihood of being ill or testing positive. During the time of the apparent outbreak at the shelter, the local community hospital examined 357 stool specimens for ova and parasites and identified Cyclospora oocysts in specimens from two patients; neither person was associated with the shelter. Of the 23 stool specimens submitted by branch laboratory A to the two reference laboratories, branch laboratory A reported that 17 (74%) specimens were positive for Cyclospora; in comparison, the reference laboratories at both CDC and the University of Arizona reported that all the specimens were negative for Cyclospora. The state central laboratory and the University of Arizona laboratory reviewed slides from branch laboratory A and identified pollen grains and other artifacts similar to Cyclospora oocysts in size and staining characteristics but lacking the appropriate internal morphology. CDC examined stool specimens obtained from 19 other persons at the shelter; rare Cyclospora oocysts were identified in specimens from two children: one child who had been asymptomatic, and one who had vomited. Based on these findings, FDH asked all laboratories in the state that had reported detecting Cyclospora oocysts in specimens from symptomatic patients in 1995 to forward their positive slides to the state central laboratory or CDC for confirmation. Branch laboratory A submitted slides from 130 patients not associated with the shelter; of these, 38 (29%) were confirmed as Cyclospora, and 92 (71%) were considered to have been false positives. In response to the investigation, FDH revised the case definition for cyclosporiasis to include confirmation of Cyclospora infection by a reference laboratory, and the state central laboratory initiated a proficiency training program at all state laboratories to teach laboratorians how to identify Cyclospora and Cryptosporidium spp. In 1996, in a subsequent outbreak of cyclosporiasis (9), Florida laboratories initially identified 188 specimens from patients as positive for Cyclospora; 32 (17%) were not confirmed by the state central laboratory. Cryptosporidiosis in New York City To improve disease reporting and identify exposures associated with infection, New York City designated cryptosporidiosis a reportable disease in January 1994, and the New York City Department of Health (NYCDOH) initiated active surveillance in November 1994. Each of the clinical laboratories are routinely contacted (usually monthly) for reports of new cases, and each case is investigated by telephone interview and/or chart review. Of the 289 cases of cryptosporidiosis reported in New York City during 1994, most (72%) occurred among men and among persons aged 20-44 years (63%). Laboratory B, a commercial laboratory in New York City, examines approximately 400 stool specimens per month for ova and parasites. Although these examinations do not routinely include testing for Cryptosporidium parvum, requests for this test increased fourfold from 1993 (143 {3%} of 4344) to 1995 (587 {11%} of 5333). Before April 1995, laboratory B used a modified acid-fast technique to test for Cryptosporidium oocysts. From January 1994 through March 1995, laboratory B reported four cases of cryptosporidiosis. In April 1995, after switching to an enzyme-linked immunosorbent assay (ELISA) method to test for Cryptosporidium antigen (ProSpecT Cryptosporidium Microplate Assay 21/96, Alexon Incorporated, Sunnyvale, California *), laboratory B began reporting an increased number of positive tests: 24 in April and a mean of 52 per month from May through September, for a total of 281 in 6 months. Demographic characteristics of these 281 patients differed from those of patients reported to have been positive by other New York City laboratories; specifically, patients who were test-positive by laboratory B were more likely to be aged greater than or equal to 60 years (36% versus 5%, pless than 0.01) and female (59% versus 25%, pless than 0.01). Because of these findings, in August 1995 the NYCDOH initiated a validation study at laboratory B. Stool specimens submitted to laboratory B for Cryptosporidium testing were split and sent for parallel testing either to the New York City Bureau of Laboratories, which performed ELISA and acid-fast testing, or to the New York State Wadsworth Center, David Axelrod Institute for Public Health, which performed ELISA, direct immunofluorescence testing (MERIFLUOR Cryptosporidium/Giardia Direct Immunofluorescent Detection Procedure, Meridian Diagnostics Incorporated, Cincinnati, Ohio), and modified acid-fast testing. ELISA testing was performed with the same kit used by laboratory B. Of 84 split specimens, laboratory B reported 57 (68%) positive test results, and the two reference laboratories each reported one positive result. Based on these findings, all 280 unconfirmed positive ELISA results for Cryptosporidium identified at laboratory B from April through September were considered to have been false positives. Physicians for these patients were notified that previously reported positive results may have been the result of laboratory error. Reported by: CR Sterling, PhD, YR Ortega, MS, Dept of Veterinary Science, Univ of Arizona, Tucson. EC Hartwig, Jr, ScD, MB Pawlowicz, MS, MT Cook, Bur of Laboratories, RS Hopkins, MD, State Epidemiologist, Florida Dept of Health. JR Miller, MD, M Layton, MD, Bur of Communicable Disease, A Ebrahimzadeh, PhD, Bur of Laboratories, New York City Dept of Health; J Ennis, J Keithly, PhD, Wadsworth Center, David Axelrod Institute for Public Health, New York State Dept of Health. Div of Parasitic Diseases, National Center for Infectious Diseases; Div of Applied Public Health Training (proposed), Epidemiology Program Office, CDC. Editorial NoteEditorial Note: Pseudo-outbreaks associated with increased surveillance for disease and outbreaks of pseudo-infection resulting from false-positive laboratory results may occur with increasing frequency because of changes in surveillance and rapid developments in the technologies and tests available to identify organisms -- particularly new and emerging pathogens. Cyclosporiasis and cryptosporidiosis are emerging infectious diseases in the United States, and many health departments and laboratories are unfamiliar with the identification and diagnosis of these parasitic infections. The outbreaks described in this report resulted from a combination of laboratory error and enhanced surveillance -- factors identified in previous pseudo-outbreaks (2,10). Consequences of such events include misdirection of resources; unnecessary treatment, anxiety, and disruption of patients' lives; and loss of confidence in laboratories and public health agencies (3). To prevent the occurrence or minimize the impact of pseudo-outbreaks, the first steps in most outbreak investigations should be to confirm the diagnosis and the occurrence of the outbreak. Confirmation of the diagnosis entails validation of the laboratory findings and assessment of the concordance between the clinical features and test results. In the Florida investigation, an outbreak of pseudo-infection was suspected initially because of an inability to document an association between patients with specific clinical manifestations and positive findings for Cyclospora oocysts, while in New York City, the investigation was prompted, in part, by the atypical demographic characteristics of patients with cryptosporidiosis reported by one laboratory. Although such patterns were important in the investigations described in this report, they typically are more reliable for well-characterized pathogens than for emerging pathogens, for which critical epidemiologic and clinical information may be limited. Because local laboratories may lack experience and optimal techniques for identifying emerging pathogens, these organisms may be more likely to be associated with outbreaks of pseudo-infection; therefore, confirmation of the diagnosis by an experienced reference laboratory may be critical in confirming outbreaks associated with these pathogens. The outbreaks of pseudo-infection in Florida and New York City began after laboratory personnel implemented new testing procedures -- in one instance, for a newly-identified pathogen and, in the other, with a different technique. The investigations of these incidents emphasize the potential for the occurrence of such outbreaks when efforts are made to enhance laboratory surveillance. In addition, these incidents indicate the needs for training and proficiency testing in conjunction with the introduction of new laboratory techniques and for reporting laboratories to submit a proportion of their positive and negative specimens for confirmation by a reference laboratory following the initiation of surveillance or testing for new pathogens. References
* Use of trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services. Figure_1 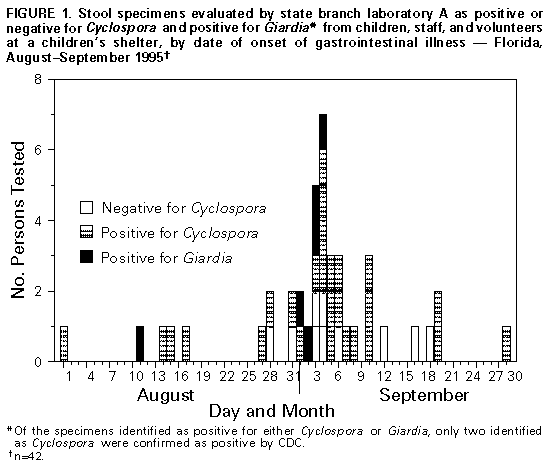 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|