 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Lactic Acidosis Traced to Thiamine Deficiency Related to Nationwide Shortage of Multivitamins for Total Parenteral Nutrition -- United States, 1997Since November 1996, there has been a nationwide shortage of intravenous (IV) multivitamins (MVIs) used in U.S. hospitals and home-health-care agencies for total parenteral nutrition (TPN). Patients receiving TPN without MVI supplementation are at risk for thiamine deficiency and life-threatening complications associated with severe deficiency of thiamine, a coenzyme necessary for oxidation of keto acids (Figure_1). This report describes three patients receiving TPN who had thiamine deficiency-related lactic acidosis in 1997 and presents recommendations for alternatives to parenteral MVI during the shortage. Case 1 On February 3, a 32-year-old man underwent a total coloproctectomy with ileostomy as treatment for fulminant ulcerative colitis. TPN was initiated immediately postoperatively and included 2087 mL per day of amino acids (92 g) and dextrose (382.5 g) with 21 g fat emulsion, electrolytes, and minerals per day; however, no MVIs were added to the solution because the hospital's supply was exhausted. Attempts to introduce clear liquids orally on February 7 and 8 were unsuccessful because of persistent severe anorexia, nausea, and vomiting. On February 10, an upper gastrointestinal barium imaging study revealed delayed transit time, but no mechanical obstruction. During February 10-22, TPN was continued without MVIs. On February 22, the patient was lethargic and weak, and abnormal laboratory findings included severe acidosis (pH 6.87 {normal: 7.35-7.45}; HCO3, 5 mEq/L {normal: 24-28 mEq/L}; pCO2, 28 mm Hg {normal: 35-45 mm Hg}; pO2, 131 mm Hg {normal: 80-100 mm Hg}; and base excess, -13 mEq/L), glucose level of 570 mg/dL (normal: 65-110 mg/dL), and serum lactic acid of 16 mmol/L (normal: 0.8-2.5 mmol/L); serum ketones were negative. Lactic acidosis of unknown etiology was diagnosed, and broad-spectrum antimicrobials were initiated after appropriate cultures were obtained. During the next 8 hours, 600 mEq/L of bicarbonate was administered with only modest elevation of pH (to 7.20) and no change in base excess (-16.2 mEq/L). Because the patient's clinical condition continued to deteriorate, an exploratory laparotomy was performed; however, no focus of infection or bowel necrosis was found. An analysis for serum thiamine measured the lowest detectable level of 0.2 mg/dL (normal: 1.1-1.6 mg/dL), and 400 mg of thiamine was administered intravenously. Two hours later, a blood gas specimen contained a serum pH of 7.50 and an HCO3 of 11.3 mEq/L. Acid/base and clinical status improved; a second dose of 400 mg thiamine was administered intravenously, and pH, pCO2, and HCO3 levels returned to normal. Case 2 On March 10, an 11-year-old girl with chronic idiopathic intestinal pseudo-obstruction syndrome, maintained on home TPN, sought care following a 3-4 day history of abdominal pain, vomiting, and decreased ostomy output. Outpatient treatment with trimethoprim/sulfamethoxazole was initiated for suspected intestinal bacterial overgrowth. Because she did not improve, she was hospitalized on March 14. A history obtained on admission revealed that she had been started on oral MVIs on January 16 because TPN supplemented with MVI was not available. However, on February 27, she had discontinued her oral MVI supplementation without notifying her physician or home-care provider. Physical examination revealed lethargy, pallor, 10% dehydration, orthostatic hypotension, tachycardia, hyperemic gingival mucosa, alterations in papillae on her tongue, and buccal mucosa ulcerations. Laboratory findings included a sodium level of 134 mmol/L (normal: 136-146 mmol/L); potassium, 2.7 mmol/L (normal: 3.5-5.3 mmol/L); blood urea nitrogen, 44 mg/dL (normal: 7-22 mg/dL); glucose, 154 mg/dL (normal: 70-100 mg/dL); and hemoglobin, 12.6 mg/dL (normal: 11.0-14.5 mg/dL). Rehydration therapy was continued with the home TPN formulation; however, she developed encephalopathy, generalized tremors, aphonia (hoarseness), progressive hyperglycemia, and acidosis with an increased anion gap. Vitamin deficiencies were confirmed with low red blood cell (RBC) transketolase activity of 0.17 IU/gm Hbg (normal: 0.75-1.3 IU/gm Hbg) and decreased levels of vitamin B12 (less than 100 pg/mL; normal: 200-1140 pg/mL) and 25-OH cholecalciferol (12.5 ng/mL; normal: 16-74 ng/mL) levels. Her RBC folate level was within normal limits, and lactate levels were not measured. Treatment with parenteral thiamine and other vitamin B supplementation improved her encephalopathy and tremors. Case 3 On January 21, a 19-year-old man began to receive home TPN for treatment of gastrointestinal dysmotility associated with antecedent chronic cholecystitis and complications of abdominal surgeries. The initial TPN formula consisted of 2750 mL per day of amino acids (120 g) and dextrose (600 g) with 250 mL per day of 20% fat emulsion, electrolytes, minerals, vitamins, and trace elements. On March 5, he was admitted to the hospital because of nonbloody diarrhea and fever. Findings on examination included an oral temperature of 102 F (39 C); pulse, 150 beats per minute; systolic blood pressure, 150 mm Hg; respiration rate, 20 per minute; oral thrush; an abnormally smooth tongue with decreased papillae; dry mucous membranes; diminished bowel sounds; left abdominal tenderness and rebound; rectal tenderness (without blood or abnormal mass); and a grade 2/6 systolic ejection murmur. His serum lactate level on admission was 16 mmol/L (normal: 0.93-1.65 mmol/L). The central IV catheter was removed and cultured, and TPN was temporarily discontinued. During the subsequent 5 days, the patient's neurologic status deteriorated markedly, and he became confused and complained of blurred vision, diplopia, and dyspnea. New findings included slurred speech, diminished deep tendon reflexes, ophthalmoplegia, and evidence of cortical blindness despite a normal fundoscopic examination. Magnetic resonance imaging (MRI) scan of the brain was consistent with Wernicke's encephalopathy. As a result of the MRI findings, treatment was initiated with 100 mg per day of thiamine parenterally. The home TPN provider was contacted and reported that the patient did not receive IV MVIs during February 5-March 3 because of a national shortage. Within 24 hours after thiamine supplementation, the ophthalmoplegia and cortical blindness improved substantially. During the next 4-5 days, his mental status improved and his serum lactate level became normal. Reported by: B Silverman, MD, GM Franklin, MD, R Bolin, MD, Memorial Clinic, Olympia, Washington. DD Hensrud, MD, Mayo Clinic, Rochester, Minnesota. WP Zeller, MD, Loyola Medical Center, Maywood, Illinois. American Society for Parenteral and Enteral Nutrition, Silver Spring, Maryland. Hospital Infections Program, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: TPN may be used for short periods to treat severely ill patients and for prolonged periods for patients with chronic or permanent gastrointestinal failure. However, the decision to initiate TPN must be balanced against the potential risks for serious sequelae, including life-threatening lactic acidosis resulting from metabolic causes other than alcoholism (type B lactic acidosis). In 1989, three adults developed fatal severe lactic acidosis associated with acute thiamine deficiency while receiving TPN during a nationwide shortage of IV MVI preparations (2). The three cases in 1997 described in this report also were associated with a nationwide shortage of IV MVIs that began in November 1996. These cases were characterized by low thiamine levels and rapid reversal after a single dose of IV thiamine, as well as by initially refractory lactic acidosis, hyperglycemia, and absence of sepsis. The time for development of severe lactic acidosis in these and other reported episodes (range: 7-34 days) is consistent with the time required to deplete body stores of thiamine in healthy adults deprived of this vitamin. The large glucose load found in most TPN preparations results in additional metabolic needs for thiamine. To reduce the risk for complications related to thiamine deficiency, health-care providers should consider administration of thiamine if multivitamins are unavailable. The current shortage of MVI supplement for TPN solution for adults first occurred in November 1996 after one of the U.S. distributors (Schein Industries, Florham Park, New Jersey *) of this product discontinued production of the supplement. Of the two remaining distributors, Astra USA (Westborough, Massachusetts) markets a supplement (MVI-12 Injection) that is identical to the product that was produced by Schein, and Fujisawa (Deerfield, Illinois) manufactures a preparation (Multi Vitamin Concentrate) that lacks three of the vitamins (folic acid, cyanocobalamin, and biotin) present in Schein or Astra's MVIs. Because Astra's supplier of the product (a contract manufacturer) has had and continues to have production difficulties, Astra has limited supplies of the product, which it is conserving for urgent/emergent situations. Fujisawa reports an increasing demand for their product. The American Society for Parenteral and Enteral Nutrition (ASPEN) has recommended alternative options for parenteral MVI use in adults (see box(Table_B1)). Because of reports of limited availability of IV MVIs for pediatric patients, ASPEN has recommended that supplements intended for neonates be reserved for use in this group (see box). Since the shortage began, the Food and Drug Administration has been working with Astra USA and ASPEN to identify alternative therapies and to identify alternate suppliers of acceptable product. Physicians who prescribe TPN should recognize the potential risks for acute thiamine deficiency and lactic acidosis in patients who are not receiving adequate supplements. Until the manufacture of MVIs for TPN for adults increases, shortages of these products may continue. Patients who are receiving TPN for prolonged periods (greater than 7 days) are at increased risk for lactic acidosis. Complications associated with inadequate MVIs for TPN should be reported to CDC's Hospital Infections Program, National Center for Infectious Diseases, telephone (404) 639-6413. References
* Use of trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services. Table_B1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
Recommendations to Health-Care Providers to Reduce the Risk for
Vitamin Deficiency in Adult and Pediatric Patients Receiving Total
Parenteral Nutrition During Intravenous Multivitamin Shortages *
Recommendations for Adult Patients
1. DO NOT use pediatric parenteral multivitamins (MVIs) for
adults.
2. Use oral vitamin preparations whenever possible.
3. Reserve use of MVI-12 Injection (Astra USA, Westborough,
Massachusetts) ** for patients receiving total parenteral nutrition
(TPN) or those with a medical need for intravenous MVIs.
4. Ration MVI-12 (e.g., reduce the daily dose or give vitamins
three times a week).
5. Use 5 mL of Multi Vitamin Concentrate (MVC) (Fujisawa,
Deerfield, Illinois) three times a week along with intravenous
supplementation of folic acid and monthly intramuscular or
subcutaneous administration of cyanocobalamin (no parenteral
biotin product is commercially available to use for
supplementation). Because of the differences in the vitamin
profiles of MVC and MVI-12, this provides an approximation of
equivalency to MVI-12; health-care providers should remain
vigilant for clinical signs of deficiency.
6. If an MVI preparation is needed and not available, then use
individual vitamin preparations (oral or injectable). Optimally,
patients should receive daily intravenous doses (unless otherwise
clinically indicated) of 3 5 mg thiamine, 0.4 1.0 mg folate, 100
mg ascorbic acid, 5 10 mg pyridoxine, and 40 50 mg niacin. In the
home setting, such patients should receive at a minimum 50 mg
thiamine intravenously three times a week and folate three times
a week. All patients should receive monthly doses of 100 mcg
cyanocobalamin intramuscularly or subcutaneously.
Recommendations for Pediatric Patients ***
1. Reserve use of MVI-Pediatric (Astra USA) for neonates.
2. MVI-Pediatric may deliver insufficient quantities of vitamin A
in very-low birthweight infants. Vitamin A supplementation in
addition to MVI-Pediatric should be decided for each patient
individually.
3. MVI-12 contains propylene glycol and similar quantities of
polysorbate, which can cause adverse consequences:
a. Polysorbate has been associated with hepatotoxicity resulting
from use of intravenous vitamin E.
b. Propylene glycol has been associated with hyperosmolality and
seizures.
c. Concomitant administration of drugs using propylene glycol as
an excipient could increase the risk for toxicity.
Therefore, to avoid propylene glycol toxicity, reserve
MVI-Pediatric for use in low-birthweight infants (less than 3 lb
4 oz {less than 1500 g}) for which greater than2 weeks of TPN is
anticipated.
4. Use adult MVIs for infants weighing less than or equal to 3 lb
4 oz (less than or equal to 1500 g) and children. Supplementation
with additional vitamins is necessary.
* Based on recommendations from the American Society for
Parenteral and Enteral Nutrition (ASPEN).
** Use of trade names and commercial sources is for identification
only and does not imply endorsement by the Public Health Service
or the U.S. Department of Health and Human Services.
*** Because of the complex nature of the recommendations for
pediatric patients receiving TPN, these recommendations are only
a summary. Complete detailed recommendations are available from
ASPEN, telephone (301) 587-6316 or e-mail [email protected].
Return to top. Figure_1 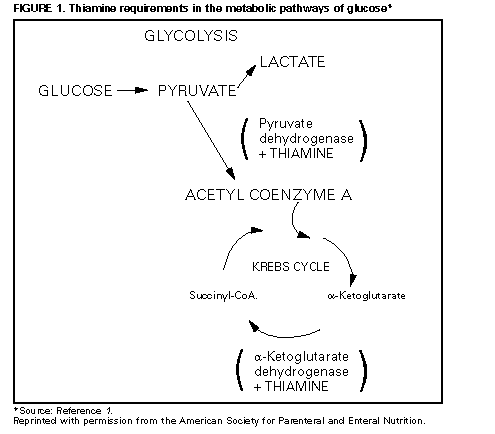 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|