 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Cardiac Valvulopathy Associated with Exposure to Fenfluramine or Dexfenfluramine: U.S. Department of Health and Human Services Interim Public Health Recommendations, November 1997Fenfluramine and dexfenfluramine are appetite suppressants that were in widespread use in the United States. On July 8, 1997, 24 cases of valvular heart disease in women who had been treated with fenfluramine and phentermine were publicly reported (1). Although valvular lesions were observed on both sides of the heart, a left-sided valve was affected in all cases. The histopathologic features were similar to those observed in carcinoid-induced valvular disease, a serotonin-related syndrome. Based on these data, the Food and Drug Administration (FDA) issued a public health advisory on July 8, followed by letters from FDA to 700,000 U.S. health-care practitioners and institutions requesting information about any additional similar patients (2). Subsequently, reports of fenfluramine- or dexfenfluramine-associated valvulopathy increased. This report summarizes the data used by FDA in its decision to request voluntary withdrawal of these drugs from the market and presents interim public health recommendations for persons exposed to these drugs. As of September 30, FDA had received 144 individual, provider-initiated (i.e., "spontaneous") reports involving fenfluramine or dexfenfluramine, with or without phentermine, in association with valvulopathy (this total included the 24 publicly reported cases {1}). Minimal degrees of regurgitation (i.e., trace or mild mitral regurgitation {MR} or trace aortic regurgitation {AR}) are relatively common in the general population and are not generally considered abnormal. Therefore, in this analysis, a case of fenfluramine- or dexfenfluramine-associated cardiac valvulopathy was defined as documented AR of mild or greater severity and/or MR of moderate or greater severity after exposure to these drugs. Of the 132 spontaneous reports with complete information, 113 (86%) met the case definition. Of these 113 cases, 111 (98%) occurred among women; the median age of case-patients was 44 years (range: 22-68 years). Of these 113 cases, two (2%) used fenfluramine alone; 16 (14%), dexfenfluramine alone; 89 (79%), a combination of fenfluramine and phentermine; and six (5%), a combination of all three drugs. None of the cases used phentermine alone. The median duration of drug use was 9 months (range: 1-39 months). Overall, 87 (77%) of the 113 cases were symptomatic. A total of 27 (24%) case-patients required cardiac valve-replacement surgery; of these, three patients died after surgery. Because symptoms frequently occur relatively late during the course of valvular incompetence, the prevalence of valve lesions was assessed for patients who were exposed to these drugs but who had no obvious history of cardiac disease or cardiac symptoms. In early September, FDA received echocardiographic reports from five independent, unpublished echocardiographic prevalence surveys of patients who had received dexfenfluramine or fenfluramine alone or in combination with phentermine (Table_1). Although the methodology of these surveys differed, the prevalence of valvular disease meeting the case definition was similar in all five survey populations, ranging from 30.0% to 38.3% (overall: 32.8%; 95% confidence interval=27.7%-38.9%) (Figure_1) (Division of Pharmacovigilance and Epidemiology, Center for Drug Evaluation and Research, FDA, personal communication, 1997). Where the echocardio-graphic diagnostic classfication was intermediate, the classification was upgraded to the higher level: for example, the classification of mild to moderate was upgraded to moderate. Downgrading of the diagnostic classification did not substantially alter the prevalence of valvulopathy that met the case definition. The duration of exposure to the drugs was determined for patients based on the time they were treated by the centers providing the prevalence survey data. Preliminary data suggest that the prevalence of valvulopathy may be higher among persons exposed for greater than or equal to 6 months: for persons with less than 3 months' exposure, the prevalence was 22% (five of 23 cases); for persons with 3-5 months' exposure, 22% (five of 23); and for persons with greater than or equal to 6 months' exposure, 35% (83 of 236). However, some patients may have been treated with these drugs before visiting the centers; therefore, these patients may have been exposed for longer durations. Of patients with valvulopathy in these surveys, 86% had AR, and 19% had MR either alone or in combination. An audible cardiac murmur was auscultated in 17% of the patients meeting the case definition. The 32.8% overall prevalence of valvular lesions meeting the case definition in exposed persons is substantially higher than would be expected in the general population (3). Preliminary reports from large population-based studies of adults indicate that the prevalence of valvular regurgitation meeting the FDA case definition is an estimated less than or equal to 5% and may be lower among obese persons than among nonobese persons (4; R. Devereux, New York Hospital-Cornell Medical Center, personal communication, 1997). However, the results of studies specifically designed to estimate the prevalence of regurgitant valvular lesions among obese adults who have lost weight or who have not been exposed to these drugs have not yet been reported. Based on data from the five prevalence surveys, FDA requested the voluntary withdrawal of fenfluramine and dexfenfluramine from the U.S. market; on September 15, the manufacturers and FDA announced the withdrawal of the drugs. Reported by: R Bowen, MD, Naples, Florida. A Glicklich, MD, Milwaukee, Wisconsin. M Khan, MD, Minneapolis, Minnesota. S Rasmussen, Danville, Indiana. T Wadden, PhD, Philadelphia, Pennsylvania. J Bilstad, MD, D Graham, MD, L Green, M Lumpkin, MD, R O'Neill, PhD, S Sobel, MD, Food and Drug Administration. VS Hubbard, MD, S Yanovski, MD, G Sopko, MD, National Institutes of Health. Div of Adult and Community Health, Div of Diabetes Translation, Div of Nutrition and Physical Activity (proposed), and Div of Oral Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Editorial NoteEditorial Note: In 1959, FDA approved the prescription appetite suppressant phentermine (Adipex{Registered}, Fastin{Registered}, and Ionamin{Registered}) for single-drug, short-term ("a few weeks") treatment of obesity. In 1973, fenfluramine (Pondimin{Registered}) also was approved for single-drug, short-term use as a prescription appetite suppressant, and in 1996, FDA approved dexfenfluramine (the dex-isomer of fenfluramine, Redux{Registered}) as a single-drug, prescription appetite suppressant for longer term use in markedly obese persons, noting that safety beyond 1 year of use had not been established in clinical trials. Both fenfluramine and dexfenfluramine appear to act by affecting the metabolism of the neurotransmitter serotonin in the brain. Recently, fenfluramine has been widely used both in combination with phentermine ("fen-phen") and for periods longer than a few weeks. Since 1995, approximately 14 million prescriptions have been written for either fenfluramine or dexfenfluramine; most of the product use was in women and persons aged less than 60 years (5). Based on an assumed median treatment course of 3-12 months and an average prescription length of 1 month, an estimated 1.2-4.7 million persons in the United States have been exposed to these drugs. The findings in this report indicate that a higher than expected prevalence of cardiac valvulopathy may have occurred among persons exposed to fenfluramine or dexfenfluramine. Factors potentially associated with these lesions but not yet determined are 1) the natural history of these lesions, including the relation between the development of the lesions and duration of drug use and whether the lesions generally resolve, progress, or remain unchanged when the drug is discontinued; 2) the clinical importance of mild valvulopathy in asymptomatic persons without audible murmurs; and 3) what, if any, characteristics might predispose a person to develop cardiac valve abnormalities during exposure to these drugs. Based on the preliminary data indicating a higher than expected prevalence of valvulopathy in exposed, asymptomatic persons without murmurs, history, and physical examination alone do not appear to be sufficiently sensitive to detect this valvulopathy in all exposed patients, particularly in those in whom obesity impedes auscultation of murmurs. Patients with acquired, primarily left-sided, valvular heart disease may be at increased risk for development of bacterial endocarditis following certain invasive procedures. FDA is aware of one person whose condition met the case definition and who presented with fever and signs and symptoms of cardiac failure and, on echocardiogram, had both AR, MR, and a large endocarditic vegetation; blood cultures from this patient were positive for streptococci (H. Connolly, Mayo Clinic, personal communication, 1997). Although the degree to which patients with these valvular lesions are at risk for developing endocarditis has not yet been determined, prudent medical management of these patients should include appropriate antimicrobial prophylaxis before certain invasive procedures and should be based on 1997 American Heart Association (AHA) recommendations for preventing bacterial endocarditis (6). The U.S. Department of Health and Human Services (DHHS) is issuing the following interim recommendations for persons previously exposed to fenfluramine or dexfenfluramine. These recommendations have been developed collaboratively by CDC, FDA, and the National Institutes of Health (the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases) in consultation with the American Heart Association, the American College of Cardiology, and the American Dental Association and are based on data associating exposure to these drugs (as single agents or as part of combination therapy) with cardiac valvulopathies. As more definitive data about the natural history of the disease become available, these DHHS interim recommendations may be revised. To determine whether valvulopathy is present in potentially affected persons and to provide such persons with optimal care, DHHS recommends that:
Optimal timing of follow-up echocardiography to determine progression, regression, or stabilization of valvular lesions is currently unknown. DHHS anticipates that within 1 year, sufficient data will become available to make recommendations about the need for continued echocardiographic monitoring. During the interim, because patients with documented valvular disease who are at risk for bacterial endocarditis should be offered antimicrobial prophylaxis after their initial echocardiogram and because no other intervention in asymptomatic patients is indicated, DHHS is not issuing recommendations for follow-up echocardiography. Practitioners should use their best judgment, based on the individual patient's history, clinical presentation, and current valvular or pulmonary hypertension status, to determine the need for additional echocardiographic follow-up. Health-care practitioners should continue to report to FDA those patients with cardiac valvular lesions who have been exposed to fenfluramine, dexfenfluramine, phentermine, or any combination of these products. The specific information requested can be obtained from FDA's World-Wide Web site at http://www.fda.gov/cder (click on "What's Happening" or "Drug Information") or by calling FDA, telephone (301) 827-3172. These reports can be sent directly to FDA through FDA's MedWatch program (either by using the postage-paid MedWatch form or by fax {(800) 332-0178} or can be given over the phone {(800) 332-1088}). References
Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Selected characteristics of five echocardiographic prevalence surveys of persons exposed to fenfluramine or
dexfenfluramine *, by reporting area, 1997
================================================================================================================================================================
Reporting area Sample size % Females Median age (yrs) Median initial weight (lbs) Median dose of drug(s)+ (mg/d) Median duration of exposure (mos)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Florida 115 & 87% 48 190 F,20.0 12
P,30.0
D,15.0
Minnesota
Fenfluramine 47 @ 85% 51 234 F,60.0 30
P,30.0
Dexfenfluramine 20 @ 80% 46 239 D,30.0 9
P,30.0
Wisconsin 50 & 94% 48 239 F,60.0 14
P,37.5
D,30.0
Indiana 31 & 77% 47 234 F,20.0 6
P,37.5
D,15.0
Pennsylvania 21 ** 100% 48 213 F,60.0 24
P,15.0
Total 284 87% 48 219 F,40.0 14
P,30.0
D,30.0 ++
----------------------------------------------------------------------------------------------------------------------------------------------------------------
* Alone or in combination with phentermine.
+ D=dexfenfluramine, F=fenfluramine, and P=phentermine.
& Confidence sample.
@ Random sample.
** Complete study sample (n=19); convenience sample (n=2).
++ A total of 15 persons received dexfenfluramine alone; 21, dexfenfuramine and phentermine; and 45, dexfenluramine (with or
without phentermine) and fenfluramine (with or without phentermine) sequentially.
================================================================================================================================================================
Return to top. Figure_1 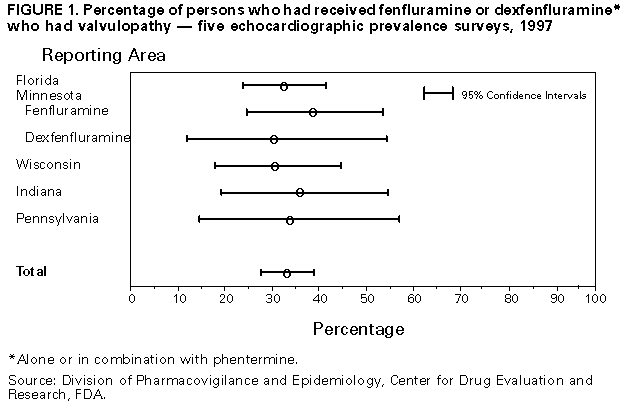 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|