 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Measles Outbreak -- Romania, 1997During December 1, 1996-September 30, 1997, a total of 20,034 cases of measles (incidence: 88.7 per 100,000 population) were reported to the Ministry of Health (MOH) in Romania (Figure_1); 13 cases were fatal. The outbreak began in December 1996, peaked in May 1997, then declined. Cases occurred in the capital (Bucharest) and all 40 other districts (1996 total population: 22.6 million). District-specific attack rates were highest in the northwest and lower in the south, ranging from 10 to 258 cases per 100,000 population. This report describes the investigation of this epidemic by MOH and estimates the efficacy of measles vaccine using the screening method (1); the findings of the investigation suggest that high routine vaccination coverage with a single dose of measles vaccine with an estimated efficacy of 77%-90% was not sufficient to prevent periodic outbreaks of measles. In May 1979, Romania introduced routine measles vaccination with an imported, live attenuated measles vaccine. Since 1981, measles vaccine (Schwarz strain, greater than 1000 median tissue culture infectious doses) produced by Institute Cantacuzino in Bucharest has been used exclusively in Romania. From 1979 through 1994, a single dose of measles vaccine was administered to children aged 9-15 months during mass campaigns conducted in February and September each year (2). In 1994, a second dose of measles vaccine was introduced for children entering school at age 7 years. On October 1, 1995, measles vaccination policy was changed from administration in biannual campaigns to continuous administration to all children attaining age 9 months by means of minicampaigns conducted during the last week of each month. During 1983-1996, reported coverage with one dose of measles vaccine by age 2 years averaged 93%. Since 1994, reported coverage with the second dose in each school-entry cohort has been 95%. Notification of a patient with measles diagnosed by a physician is compulsory by law in Romania. Supplementary information about the vaccination status, disease complications (recorded in mutually exclusive categories), and outcome of individual notified cases is collected by district epidemiologists. Data entry has been completed for all 3969 cases with onset during December 1, 1996-March 31, 1997, and for a systematic sample of every fifth case (n=3002 cases) with onset during April 1-September 30, 1997. Because the mean age and the vaccination status of cases occurring during these two periods were similar, these groups were combined (n=6971) in the analysis. Children aged less than 2 years and 10-18 years accounted for the highest number of cases (Figure_2). Overall, students (kindergarten-12th grade) accounted for 3834 (55%) cases. Of the 762 cases reported among infants, 40% occurred in infants aged 9-11 months; 38%, in those aged 6-8 months; and 22%, in those aged less than 6 months. Overall, 4019 (58%) persons with measles were reported to have received at least one dose of measles vaccine. A history of receipt of vaccine was established for 5% of infants with measles; among ill persons aged 1-4 years, 5-9 years, 10-14 years, 15-19 years, and 20-24 years, history of receipt of vaccine was established for 56%, 80%, 75%, 64%, and 20%, respectively. Based on the screening method, vaccine efficacy was estimated to be 90% among persons aged 1-4 years and 77% among persons aged 10-14 years. Complications of measles were reported in 2201 (32%) of 6971 cases: 1519 (22%) persons were hospitalized for treatment of measles, 579 (8%) had pneumonia, four (0.1%) had convulsions, three (less than 0.1%) had encephalopathy, and 96 (1.4%) had other complications (e.g., otitis media and bronchitis). The median age of the 13 persons who died was 2 years (range: 4 months-18 years); seven persons who died were unvaccinated, and six were reported to have received one dose of measles vaccine. Measures to control the epidemic were initiated by MOH in January 1997. These measures focused on persons potentially exposed to measles diagnosed in institutional settings (e.g., orphanages, day care centers, schools, hospitals, and military camps). In these settings, measles vaccination was recommended for unvaccinated persons aged 6 months-23 years and vaccinated persons aged less than or equal to 23 years in whom greater than or equal to 6 years had elapsed since vaccination. No mass vaccination efforts were undertaken because of limited vaccine supplies. Reported by: N Ion-Nedelcu, D Craciun, G Molnar, Ministry of Health, Romania. Expanded Programme on Immunization, World Health Organization Regional Office for Europe, Copenhagen, Denmark. Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial NoteEditorial Note: The measles vaccination program in Romania achieved a greater than 90% reduction in measles incidence (Figure_1), compared with the incidence during the prevaccine era. Following the introduction of measles vaccination in 1979, epidemics of measles occurred in 1982, 1986, 1993, and 1997. These epidemics have been of progressively smaller magnitude, while the median age of persons with cases increased from 6.5 years in 1986 to 10.8 years in 1997. Unlike previous epidemics, the 1997 epidemic involved predominantly school-aged children who were vaccinated. The large outbreak in Romania during 1996-1997 occurred despite maintenance of high coverage with the first dose of measles vaccine for approximately 15 years and the introduction of a second dose of measles vaccine in 1994. However, this pattern is consistent with that in other countries with well established vaccination programs (e.g., Hungary and the United States) in which large numbers of susceptible persons accumulated and outbreaks occurred (3,4). In Romania, susceptible persons included school-aged children who either failed to respond to the first dose of vaccine or whose immunity from the first dose had waned and young preschool children who have not yet been vaccinated. The incidence was lowest among children aged 8-9 years, suggesting high efficacy of the two-dose schedule used among this age group. The increased incidence among persons born during 1980-1987 may reflect reduced vaccine efficacy, increased contact rates, or a combination of these factors. The findings in this report underscore the potential for large outbreaks of measles in countries that have achieved high coverage with a single dose of measles vaccine unless such countries provide a second dose of vaccine to at least all persons born since the introduction of vaccine. A routine second dose (e.g., administered at school entry) may result in the elimination of measles if coverage levels of greater than 95% can be achieved and maintained for an extended period (5). Alternatively, catch-up campaigns (e.g., those conducted in Canada, Latin America, and United Kingdom that targeted children across a broad age range regardless of previous vaccination status) have been effective in preventing outbreaks and interrupting transmission (6-8). The selection of a specific strategy may be determined by levels of resources available and by the commitment of the country to accelerate measles control. In Romania, the optimal approach may be to implement a catch-up campaign targeting school-aged children (aged less than or equal to 18 years) in conjunction with efforts to increase routine vaccination coverage with the first dose to 90% in all districts and to maintain high routine coverage with the second dose. Ongoing studies to evaluate vaccine efficacy during the outbreak and to determine age-specific susceptibility to measles will guide the development of a measles-elimination strategy for Romania. References
Figure_1 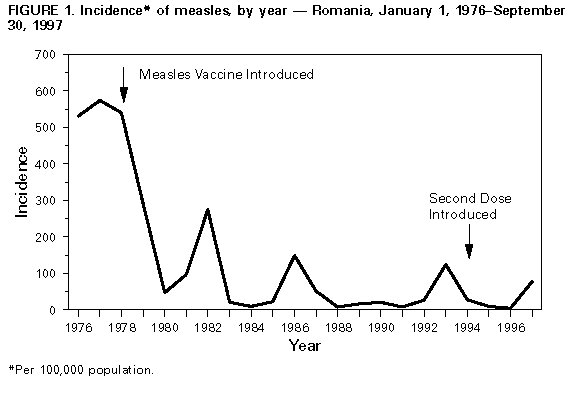 Return to top. Figure_2 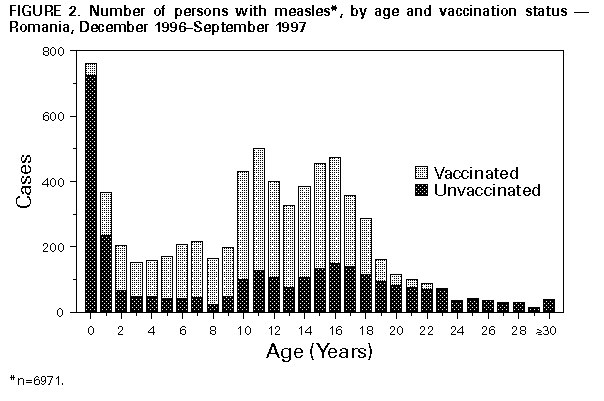 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|