 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication -- Turkey, 1994-1997In 1989, as part of the global poliomyelitis eradication initiative, Turkey adopted the goal of eliminating polio by 2000; since then, substantial progress has been made toward achieving this objective. Turkey is a priority country for the global polio eradication initiative because of its large population (1996 population: 60 million), strategic location between Europe and Asia, and proximity to countries with endemic polio. This report summarizes progress during 1994-1997 in Turkey toward implementing the four polio eradication strategies recommended by the World Health Organization (WHO) (1), reviews the cluster of polio cases in 1997, and suggests that recent poliovirus transmission might have resulted from suboptimal vaccination coverage in some areas of Turkey. Routine Vaccination Coverage. During 1989-1994, reported rates of vaccination coverage with three or more doses of oral poliovirus vaccine (OPV3) in children by age 1 year provided by the routine vaccination program ranged from 74% to 81% (Figure_1). OPV3 coverage declined to 66% in 1995 because of problems with vaccine procurement and increased to 83% in 1996. Based on preliminary data, in 1997 the reported vaccination coverage rate decreased to 75%. Rates differed substantially among the 80 provinces. Eighteen provinces, composing 15% of the total population and located primarily in southeastern and eastern Turkey, reported annual coverage rates of less than 80% (range: 8%-78%) for 1994-1997 (Figure_2). To improve routine vaccination coverage levels, vaccination activities with OPV, diphtheria-tetanus-pertussis vaccine, and measles vaccine were intensified in the 19 provinces in which coverage rates were low in 1996. As a result, OPV3 coverage among children by age 1 year in the targeted provinces increased from 46% to 60% by the end of 1996. National Immunization Days. Turkey conducted three National Immunization Days (NIDs) * in 1995, 1996, and 1997 as part of Operation MECACAR (Mediterranean, Caucasus, and Central Asian Republics) (2,3), in which 18 geographically contiguous countries in Asia, the Middle East, and Europe synchronized NIDs. NID coverage differed by province and was less than 80% in six to 11 provinces during 1995-1997. During this period, routine OPV3 coverage and NID coverage for any round did not reach 80% in seven provinces. In 1997, 73% of OPV doses given during NIDs were administered during house-to-house visits. Surveillance. Acute flaccid paralysis (AFP) surveillance was initiated in Turkey in 1989. Case-based information is passively reported by the provincial health departments to the Ministry of Health in Ankara. Stool specimens obtained from patients with AFP are evaluated at the national reference laboratory in Ankara. The national polio laboratory processes stool specimens to isolate poliovirus and identify poliovirus serotypes. Poliovirus isolates are sent to the regional reference laboratory in the Netherlands for intratypic differentiation of poliovirus as wild or vaccine-related; aliquots of primary stool specimens are shipped for confirmatory testing (4). In 1997, WHO began accrediting national polio laboratories in Europe to be formally recognized as members of the Global Laboratory Network. Accreditation includes a proficiency test panel of prepared specimens, with a target score of 80% (4). The Turkish national polio laboratory achieved a perfect score of 100% in this proficiency testing. Full accreditation by WHO, anticipated in 1998, will require additional technical improvements. An important performance indicator for adequate AFP surveillance is the annual reported rate of nonpolio AFP cases per 100,000 children aged less than 15 years (target: greater than or equal to 1 case per 100,000) (1). In Turkey, the nonpolio AFP rate was 0.3 in 1994, 0.5 in 1995, 0.4 in 1996, and 0.6 in 1997 (preliminary data). The increase in 1997 occurred primarily because a larger number of provinces (26 in 1997 versus 10 in 1996) achieved a rate of greater than or equal to 1 case. However, 39 (49%) of 80 provinces, constituting one third of the total population, did not report AFP cases. Four of these provinces are small, and an AFP case would not be expected every year; however, the remaining 35 provinces would be expected to report at least 63 nonpolio AFP cases annually. The second important surveillance quality indicator is the proportion of patients with AFP from whom two adequate stool specimens are obtained. ** In Turkey, the proportion of AFP cases for which two adequate stool specimens were evaluated was 16% in 1994, 45% in 1995, 36% in 1996, and 65% in 1997 (preliminary data). Other Supplementary Immunization Activities. Before conducting NIDs, vaccination campaigns were conducted in 1994 in two provinces following the detection of wild polioviruses. During these campaigns, in which children aged less than 5 years were targeted for vaccination regardless of their previous vaccination status, vaccination coverage rates were greater than or equal to 94%. In October and November 1997, Turkey conducted two rounds of "mopping-up" vaccination *** in 28 provinces with either low routine vaccination coverage (less than 80% OPV3 coverage since 1995), poor AFP surveillance (i.e., no reporting of cases since 1995), or increased risk for poliovirus importation from neighboring countries with endemic polio. Reported coverage in the first and second rounds of the mopping-up campaign, targeting 20% of the total population, was 84% and 84%, respectively. However, supplemental vaccination coverage for the first round was less than 80% in seven (25%) participating provinces. Polio Incidence In Turkey, the number of reported polio cases confirmed by the standard WHO clinical case definition **** has decreased under conditions of improved surveillance since 1994 (32 in 1994, 32 in 1995, and 19 in 1996) (Figure_1). In 1994, wild poliovirus type 1 (P1) was isolated from seven patients located in five provinces of the southeastern and western regions of the country. Two distinct genotypes of P1 were identified by genomic sequencing analysis. In 1995, wild poliovirus type 3 (P3) was isolated in a northwestern province. In 1996, no wild poliovirus was isolated. In 1997, a total of 141 AFP cases were reported from Turkey; six AFP cases were confirmed as polio by wild P1 isolation, the first wild P1 isolated since 1994. The virologically confirmed cases had onset of paralysis during July 23-October 10, 1997; these cases occurred in patients from Mardin province (Figure_2). All six patients were aged 9 months-2 years; four patients were unvaccinated, and two had received only one dose of OPV. Genomic sequencing of the viral isolates from the 1997 cluster indicated a distinct relation with wild P1 isolates obtained from eastern Turkey in 1994. Routine vaccination coverage in Mardin has been less than 50% since 1994, although reported coverage was greater than or equal to 78% for all NID rounds. Coverage rates for the two rounds of mopping-up vaccination in Mardin in 1997 were 80% and 65%, respectively. No additional polio cases have been detected from Mardin or other provinces in Turkey. Reported by: S Tumay, MD, N Satirlar, MD, O Afsar, MD, B Altay, MD, N Noyan, MD, A Ozkan, MD, S Caglayan, MD, Div of Primary Health Care Svcs; I Alaeddinoglu, C Artuk, E Ozkaya, MD, National Polio Laboratory, Ministry of Health, Turkey. Communicable Diseases and Immunization Unit, European Regional Office, World Health Organization, Copenhagen, Denmark; Global Programme for Vaccines and Immunization, World Health Organization, Geneva, Switzerland. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial NoteEditorial Note: Turkey has made substantial progress in polio eradication activities since 1989. The number of reported polio cases declined substantially after implementation of NIDs, and no wild polioviruses were isolated from June 1995 through June 1997. During July-October 1997, a cluster of virologically confirmed cases occurred in one province. This cluster emphasizes the importance of establishing effective AFP surveillance, maintaining high routine vaccination coverage, achieving high levels of coverage during NIDs, and implementing mopping-up campaigns in high-risk areas to limit poliovirus transmission. Wild poliovirus identified in this cluster was either indigenous virus, which may have continued to circulate undetected because of limited AFP surveillance, or wild virus originating from a neighboring country in which polio is endemic (5). The source of the wild poliovirus in this cluster could not be determined, and no virus isolates were available from recent cases in neighboring countries to enable molecular epidemiologic analysis of poliovirus circulation. As a result of this cluster and the outcome of past vaccination efforts, the Ministry of Health has strengthened all aspects of the national polio eradication effort. NIDs will be conducted in the spring of 1998, and mopping-up vaccination campaigns are being considered. Efforts are under way to improve routine vaccination coverage in the geographic area where wild polioviruses were detected and in all other provinces where coverage has been consistently low. Improving AFP surveillance in all provinces is a high priority. Efforts to improve AFP surveillance include 1) additional training of public health staff at the provincial and district levels; 2) establishing and maintaining active surveillance by regularly reviewing hospital records and contacting health-care providers in major clinics and hospitals; 3) establishing a strong relation with national and local organizations of health-care providers who are likely to treat patients with AFP; 4) augmenting laboratory equipment, supplies, and procedures; and 5) improving coordination between the laboratory and surveillance staff. In 1997, a year with a historically low number of reported cases in the European Region of WHO, Turkey was the only country in which wild poliovirus transmission was detected (3). The European Region can be certified as free of indigenous wild poliovirus transmission only after no wild poliovirus has been detected for at least 3 consecutive years in the presence of high-quality AFP surveillance. The WHO Regional Offices for Europe and the Eastern Mediterranean continue to coordinate polio eradication activities that began in 1995 with the synchronized mass vaccination activities of Operation MECACAR (3,5) and will include simultaneous "mopping-up" and catch-up vaccination campaigns. NIDs will be coordinated between several bordering countries in the Middle East, Caucasus, and Central Asian Republics during Operation MECACAR Plus, which will be held during March-May 1998. National governments are working in cooperation with the major partner agencies contributing to the polio-eradication initiative (e.g., WHO, Rotary International, United Nations Children's Fund {UNICEF}, U.S. Agency for International Development, and CDC) toward achieving the goal of global polio eradication by the year 2000. References
* Mass campaigns over a short period (days to weeks) in which two doses of OPV are administered to all children in the target age group, regardless of previous vaccination history, with an interval of 4-6 weeks between doses. ** Two stool specimens collected at an interval of at least 24 hours within 14 days of onset of paralysis. WHO recommends that greater than or equal to 80% of patients with AFP should have two adequate specimens collected (1). *** Focal mass campaign in high-risk areas over a short period (days to weeks) in which two doses of OPV are administered during house-to-house visits to all children in the target age group, regardless of previous vaccination history, with an interval of 4-6 weeks between doses. **** A confirmed case of polio is defined as AFP and at least one of the following: 1) laboratory-confirmed wild poliovirus infection, 2) residual paralysis at 60 days, 3) death, or 4) no follow-up investigation at 60 days. Figure_1 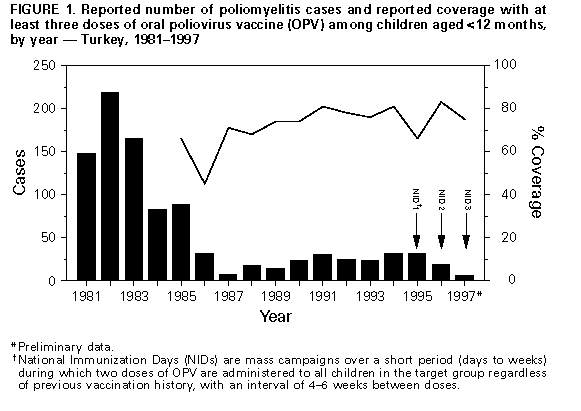 Return to top. Figure_2 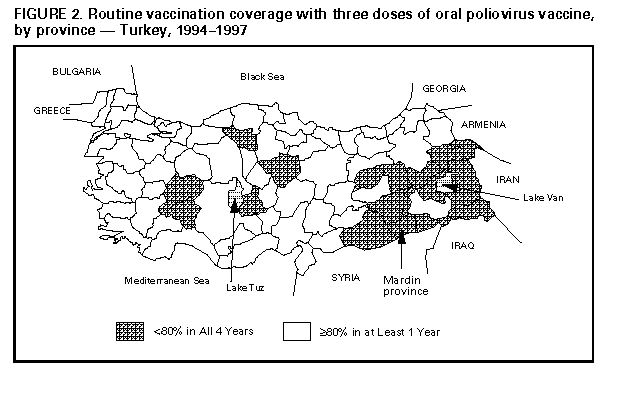 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|