 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Public Health Service Guidelines for the Management of Health-Care Worker Exposures to HIV and Recommendations for Postexposure ProphylaxisPlease note: An update has been published for this report. To view the update, please click here. Summary This report updates and consolidates all previous PHS recommendations for the management of health-care workers (HCWs) who have occupational exposure to blood and other body fluids that may contain human immunodeficiency virus (HIV); it includes recommendations for HIV postexposure prophylaxis (PEP) and discusses the scientific rationale for PEP. The decision to recommend HIV postexposure prophylaxis must take into account the nature of the exposure (e.g., needlestick or potentially infectious fluid that comes in contact with a mucous membrane) and the amount of blood or body fluid involved in the exposure. Other considerations include pregnancy in the HCW and exposure to virus known or suspected to be resistant to antiretroviral drugs. Assessments of the risk for infection resulting from the exposure and of the infectivity of the exposure source are key determinants of offering PEP. Systems should be in place for the timely evaluation and management of exposed HCWs and for consultation with experts in the treatment of HIV when using PEP. Recommendations for PEP have been modified to include a basic 4-week regimen of two drugs (zidovudine and lamivudine) for most HIV exposures and an expanded regimen that includes the addition of a protease inhibitor (indinavir or nelfinavir) for HIV exposures that pose an increased risk for transmission or where resistance to one or more of the antiretroviral agents recommended for PEP is known or suspected. An algorithm is provided to guide clinicians and exposed health-care workers in deciding when to consider PEP. Occupational exposures should be considered urgent medical concerns to ensure timely administration of PEP. Health-care organizations should have protocols that promote prompt reporting and facilitate access to postexposure care. Enrollment of HCWs in registries designed to assess side effects in HCWs who take PEP is encouraged. INTRODUCTION Although preventing blood exposures is the primary means of preventing occupationally acquired human immunodeficiency virus (HIV) infection, appropriate postexposure management is an important element of workplace safety. In January 1990, CDC issued a statement on the management of HIV exposures that included considerations for zidovudine (ZDV) use for postexposure prophylaxis (PEP) (1). At that time, data were insufficient to assess the efficacy of ZDV as a prophylactic agent in humans or the toxicity of this drug in persons not infected with HIV. Although there are still only limited data to assess safety and efficacy, additional information is now available that is relevant to this issue. In December 1995, CDC published a brief report of a retrospective case-control study of health-care workers (HCWs) from France, the United Kingdom, and the United States exposed percutaneously to HIV; the study identified risk factors for HIV transmission and documented that the use of ZDV was associated with a decrease in the risk for HIV seroconversion (2). This information, along with data on ZDV efficacy in preventing perinatal transmission (3) and evidence that PEP prevented or ameliorated retroviral infection in some studies in animals (4), prompted a Public Health Service (PHS) interagency working group *, with expert consultation (5), in June 1996 to issue provisional recommendations for PEP for HCWs after occupational HIV exposure (6). Since the provisional recommendations were released, several new antiretroviral drugs have been approved by the Food and Drug Administration (FDA), and more information is available about the use and safety of antiretroviral agents in exposed HCWs (7-10). In addition, questions have been raised about the use of chemoprophylaxis in situations not fully addressed in the 1996 recommendations, including when not to offer PEP, what to do when the source of exposure or the HIV status of the source person is unknown, how to approach PEP in HCWs who are or may be pregnant, and considerations for PEP regimens when the source person's virus is known or suspected to be resistant to one or more of the antiretroviral agents recommended for PEP. In May 1997, a meeting of expert consultants, convened by CDC to consider the new information, prompted a PHS interagency working group ** decision to issue updated recommendations. This document addresses the management of occupational exposure to HIV, including guidance in assessing and treating exposed HCWs, updates previous recommendations for occupational postexposure chemoprophylaxis, and updates and replaces all previous PHS guidelines and recommendations for occupational HIV exposure management for HCWs. Included in this document is an algorithm to guide decisions regarding the use of PEP for HIV exposures. The algorithm and these recommendations together address most issues that may be encountered during postexposure follow-up. As relevant information becomes available, updates of these recommendations will be published. Recommendations for nonoccupational (e.g., sexual or pediatric) exposures are not addressed in these guidelines. DEFINITIONS OF HEALTH-CARE WORKERS AND EXPOSURE In this report, "health-care worker" (HCW) is defined as any person (e.g., an employee, student, contractor, attending clinician, public-safety worker, or volunteer) whose activities involve contact with patients or with blood or other body fluids from patients in a health-care or laboratory setting. An "exposure" that may place an HCW at risk for HIV infection and therefore requires consideration of PEP is defined as a percutaneous injury (e.g., a needlestick or cut with a sharp object), contact of mucous membrane or nonintact skin (e.g., when the exposed skin is chapped, abraded, or afflicted with dermatitis), or contact with intact skin when the duration of contact is prolonged (i.e., several minutes or more) or involves an extensive area, with blood, tissue, or other body fluids. Body fluids include a) semen, vaginal secretions, or other body fluids contaminated with visible blood that have been implicated in the transmission of HIV infection (11,12); and b) cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic fluids, which have an undetermined risk for transmitting HIV (11). In addition, any direct contact (i.e., without barrier protection) to concentrated HIV in a research laboratory or production facility is considered an "exposure" that requires clinical evaluation and consideration of the need for PEP. Although one nonoccupational episode of HIV transmission has been attributed to contact with blood-contaminated saliva (13), this incident involved intimate kissing between sexual partners and is not similar to contact with saliva that may occur during the provision of health-care services. Therefore, in the absence of visible blood in the saliva, exposure to saliva from a person infected with HIV is not considered a risk for HIV transmission; also, exposure to tears, sweat, or nonbloody urine or feces does not require postexposure follow-up. *** Human breast milk has been implicated in perinatal transmission of HIV. However, occupational exposure to human breast milk has not been implicated in HIV transmission to HCWs. Moreover, the contact HCWs may have with human breast milk is quite different from perinatal exposure and does not require postexposure follow-up. BACKGROUND The rationale is provided here for the postexposure management and prophylaxis recommendations given at the end of the document. Additional details concerning the risk for occupational HIV transmission to HCWs and management of occupational HIV exposures are available elsewhere (16-18). Risk for Occupational Transmission of HIV to HCWs Prospective studies of HCWs have estimated that the average risk for HIV transmission after a percutaneous exposure to HIV-infected blood is approximately 0.3% (95% confidence interval {CI}=0.2%-0.5%) (16) and after a mucous membrane exposure is 0.09% (95% CI=0.006%-0.5%) (19). Although episodes of HIV transmission after skin exposure have been documented (20), the average risk for transmission by this route has not been precisely quantified because no HCWs enrolled in prospective studies have seroconverted after an isolated skin exposure. The risk for transmission is estimated to be less than the risk for mucous membrane exposures (21). The risk for transmission after exposure to fluids or tissues other than HIV-infected blood also has not been quantified. As of June 1997, CDC has received reports of 52 U.S. HCWs with documented HIV seroconversion temporally associated with an occupational HIV exposure. An additional 114 episodes in HCWs are considered possible occupational HIV transmissions; these workers reported that their infection was occupationally acquired and no other risk for HIV infection was identified, but transmission of infection after a specific exposure was not documented (22). Of the 52 documented episodes, 47 HCWs were exposed to HIV-infected blood, one to a visibly bloody body fluid, one to an unspecified fluid, and three to concentrated virus in a laboratory. Forty-five exposures were percutaneous, and five were mucocutaneous; one HCW had both a percutaneous and a mucocutaneous exposure. The route of exposure for one person exposed to concentrated virus is uncertain. Of the percutaneous exposures, the objects involved included a hollow-bore needle (41), a broken glass vial (two), a scalpel (one), and an unknown sharp object (one) (CDC, unpublished data, 1998). Epidemiologic and laboratory studies suggest that several factors may affect the risk for HIV transmission after an occupational exposure. The one retrospective case-control study of HCWs who had percutaneous exposure to HIV found that the risk for HIV transmission was increased with exposure to a larger quantity of blood from the source patient as indicated by a) a device visibly contaminated with the patient's blood, b) a procedure that involved a needle placed directly in a vein or artery, or c) a deep injury (23). (A laboratory study that demonstrated that more blood is transferred by deeper injuries and hollow-bore needles lends further support for the observed variation in risk related to blood quantity {24}). The risk also was increased for exposure to blood from source patients with terminal illness, possibly reflecting either the higher titer of HIV in blood late in the course of AIDS or other factors (e.g., the presence of syncytia-inducing strains of HIV). It was estimated that the risk for HIV transmission from exposures that involve a larger volume of blood, particularly when the source patient's viral load is probably high, exceeds the average risk of 0.3% (23). The utility of viral load measurements from a source patient as a surrogate for estimating the viral titer for assessing transmission risk is not known. Plasma viral load measurement (e.g., HIV RNA) reflects only the level of cell-free virus in the peripheral blood. This measurement does not reflect the level of cell-associated virus in the peripheral blood or the level of virus in other body compartments (e.g., lymphatic tissue). Although a lower viral load, or results that are below the limits of viral quantification, in the peripheral blood probably indicates a lower titer exposure, it does not rule out the possibility of transmission; HIV transmission from persons with a plasma viral load below the limits of viral quantification (based on the assay used at the time) has been reported in instances of mother-to-infant transmission (25,26) and in one HCW seroconversion (J.L. Gerberding, San Francisco General Hospital, unpublished data, May 1997). There is some evidence that host defenses also may influence the risk for HIV infection. In one small study, HIV-exposed but uninfected HCWs demonstrated an HIV-specific cytotoxic T-lymphocyte (CTL) response when peripheral blood mononuclear cells were stimulated in vitro with HIV mitogens (27). Similar CTL responses have been observed in other populations with repeated HIV exposure without resulting infection (28-33). Among several possible explanations for this observation, one is that the host immune response sometimes may be able to prevent establishment of HIV infection after a percutaneous exposure; another is that the CTL response simply may be a marker for exposure. HIV Seroconversion in HCWs Data on the timing and clinical characteristics of seroconversion in HIV-exposed HCWs are limited by the infrequency of infection following occupational exposure, variations in postexposure testing intervals, and differences over time in the sensitivity of HIV-antibody testing methods. Among the HCWs with documented seroconversions reported to CDC for whom data are available, 81% experienced a syndrome compatible with primary HIV infection a median of 25 days after exposure (CDC, unpublished data, 1998). In a recent analysis of 51 seroconversions in HCWs, the estimated median interval from exposure to seroconversion was 46 days (mean: 65 days); an estimated 95% seroconverted within 6 months after the exposure (34). These data suggest that the time course of HIV seroconversion in HCWs is similar to that in other persons who have acquired HIV through nonoccupational modes of transmission (35). Three instances of delayed HIV seroconversion occurring in HCWs have been reported; in these instances, the HCWs tested negative for HIV antibodies greater than 6 months postexposure but were seropositive within 12 months after the exposure (36,37; J.L. Gerberding, San Francisco General Hospital, unpublished data, May 1997). DNA sequencing confirmed the source of infection in one instance. Two of the delayed seroconversions were associated with simultaneous exposure to hepatitis C virus (HCV) (37; J.L. Gerberding, San Francisco General Hospital, unpublished data, May 1997). In one case, co-infection was associated with a rapidly fatal HCV disease course (37); however, it is not known whether HCV directly influences the risk for or course of HIV infection or is a marker for other exposure-related factors. Rationale for PEP Considerations that influence the rationale and recommendations for PEP include the pathogenesis of HIV infection, particularly the time course of early infection; the biologic plausibility that infection can be prevented or ameliorated by using antiretroviral drugs and direct or indirect evidence of the efficacy of specific agents used for prophylaxis; and the risk/benefit of PEP to exposed HCWs. The following discussion considers each of these issues. Role of Pathogenesis in Considering Antiretroviral Prophylaxis Information about primary HIV infection indicates that systemic infection does not occur immediately, leaving a brief "window of opportunity" during which postexposure antiretroviral intervention may modify viral replication. Data from studies in animal models and in vitro tissue studies suggest that dendritic cells in the mucosa and skin are the initial targets of HIV infection or capture and have an important role in initiating HIV infection of CD4+ T-cells in regional lymph nodes (38). In a primate model of simian immunodeficiency virus (SIV) infection, infection of dendritic-like cells occurred at the site of inoculation during the first 24 hours following mucosal exposure to cell-free virus. During the subsequent 24-48 hours, migration of these cells to regional lymph nodes occurred, and virus was detectable in the peripheral blood within 5 days (39). HIV replication is rapid (generation time: 2.5 days) and results in bursts of up to 5,000 viral particles from each replicating cell (40; M.S. Saag, University of Alabama, personal communication, September 1997). The exponential increase in viral burden continues unless controlled by the immune system or other mechanisms (e.g., exhaustion of available target CD4+ T-cells). Theoretically, initiation of antiretroviral PEP soon after exposure may prevent or inhibit systemic infection by limiting the proliferation of virus in the initial target cells or lymph nodes. Efficacy of Antiretrovirals for PEP Studies in animals and humans provide direct and indirect evidence of the efficacy of antiretroviral drugs as agents for postexposure prophylaxis. In human studies and in most animal studies, ZDV was the antiretroviral agent used for prophylaxis (26,41-54). However, in more recent animal studies, newer agents also have been reported to be effective (55,56). Data from animal studies have been difficult to interpret, in part because of problems identifying a comparable animal model for humans. Most studies use a higher inoculum for exposure than would be expected in needlestick injuries. Among the animal studies, differences in controlled variables (e.g., choice of viral strain {based on the animal model used}, inoculum size, route of inoculation, time of prophylaxis initiation, and drug regimen) make attempts to apply these results to humans difficult. In the animal studies that showed efficacy of pre-exposure and/or postexposure prophylaxis, reported outcomes (4,57) have included
In studies of HIV-2 or SIV in nonhuman primates in which ZDV or 3'-fluorothymidine was used, suppression or delay of antigenemia was the most common outcome; prevention of infection was infrequent (43,52,58-60). However, two other antiretroviral agents, 2',3'-dideoxy-3'-hydroxymethyl cytidine (BEA-005) and (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA), used to study PEP in primates have been more effective in preventing infection. When PMPA was administered 48 hours before, 4 hours after, or 24 hours after intravenous SIV inoculation to long-tailed macaques, a 4-week regimen prevented infection in all treated animals (55). A 3-day regimen of BEA-005 prevented SIV infection in 12 of 12 pigtailed macaques when administered 1-8 hours after intravenous inoculation; infection also was prevented in four of four animals that received 3 days of BEA-005 within 10 minutes after HIV-2 inoculation (56). Animal studies have demonstrated that early initiation of PEP and small inoculum size are correlates of successful PEP. ZDV initiated 1 hour or 24 hours after intravenous exposure to a rapidly lethal variant of SIV in pigtailed macaques prevented infection in one of three animals and modified SIV disease in three of six animals, respectively; PEP initiated at 72 hours had no effect (54). In macaques administered ZDV or BEA-005 1 to 72 hours after SIV intravenous challenge, earlier initiation of PEP was correlated with delayed onset and peak of antigenemia, decreased duration of antigenemia, and reduction in SIV serum titer; the most potent effect was evident when PEP was initiated within 8 hours of exposure (43,56). Studies in primates and murine and feline animal models have demonstrated that larger inocula decrease prophylactic efficacy (47,48,53,60). In addition, delaying initiation, shortening the duration, or decreasing the antiretroviral dose of PEP, individually or in combination, decreased prophylactic efficacy (42,43,45,47,50,55). There is little information with which to assess the efficacy of PEP in humans. Seroconversion is infrequent after an occupational exposure to HIV-infected blood; therefore a prospective trial would need to enroll many thousands of exposed HCWs to achieve the statistical power necessary to directly demonstrate PEP efficacy. During 1987-1989, the Burroughs-Wellcome Company sponsored a prospective placebo-controlled clinical trial among HCWs to evaluate 6 weeks of ZDV prophylaxis; however, this trial was terminated prematurely because of low enrollment (61). Because of current indirect evidence of PEP efficacy, it is unlikely that a placebo-controlled trial in HCWs would ever be feasible. In the retrospective case-control study of HCWs, after controlling for other risk factors for HIV transmission, the risk for HIV infection among HCWs who used ZDV as PEP was reduced by approximately 81% (95% CI=43%-94%) (23). In addition, in a randomized, controlled, prospective trial (AIDS Clinical Trial Group {ACTG} protocol 076) in which ZDV was administered to HIV-infected pregnant women and their infants, the administration of ZDV during pregnancy, labor, and delivery and to the infant reduced transmission by 67% (3). Only 9%-17% (depending on the assay used) of the protective effect of ZDV was explained by reduction of the HIV titer in the maternal blood, suggesting that ZDV prophylaxis in part involves a mechanism other than the reduction of maternal viral burden (26). The limitations of all of these studies must be considered when reviewing evidence of PEP efficacy. The extent to which data from animal studies can be extrapolated to humans is largely unknown, and the exposure route for mother-to-infant HIV transmission is not similar to occupational exposures; therefore these findings may not reflect a similar mechanism of ZDV prophylaxis in HCWs. Although the results of the retrospective case-control study of HCWs suggest PEP efficacy, the limitations of that study include the small number of cases studied and the use of cases and controls from different cohorts. Failure of ZDV PEP to prevent HIV infection in HCWs has been reported in at least 14 instances (62-64; G. Ippolito, AIDS Reference Center, Rome, Italy, and J. Heptonstall, Communicable Disease Surveillance Center, London, United Kingdom, personal communication, 1997). Although eight of the 13 source patients had taken ZDV, laboratory assessment for ZDV resistance of the virus from the source patient was performed in only three instances, two of which demonstrated reduced susceptibility to ZDV. In addition to possible exposure to a ZDV-resistant strain of HIV, other factors that may have contributed to the apparent failures in these instances may include a high titer and/or large inoculum exposure, delayed initiation and/or short duration of PEP, and possible factors related to the host (e.g., cellular immune system responsiveness) and/or to the source patient's virus (e.g., presence of syncytia-forming strains) (62). Antiretroviral Agents for PEP Several antiretroviral agents from at least three classes of drugs are available for the treatment of HIV disease. These include the nucleoside analogue reverse transcriptase inhibitors (NRTIs), nonnuceloside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) (See Appendix). Among these drugs, ZDV (an NRTI) is the only agent shown to prevent HIV transmission in humans (2,3). Although there are theoretical concerns that the increased prevalence of resistance to ZDV may diminish its utility for PEP (65), no data are available to assess whether this is a factor for consideration. Clinical data from the ACTG protocol 076 study documented that despite genotypic evidence of maternal ZDV resistance, ZDV prevented perinatal transmission (66). Thus, based on the available information, it is still reasonable that ZDV should continue to be the first drug of choice for PEP regimens. There are no data to directly support the addition of other antiretroviral drugs to ZDV to enhance the effectiveness of the PEP regimen. However, in HIV-infected patients, combination regimens have proved superior to monotherapy regimens in reducing HIV viral load (67,68). Thus, theoretically a combination of drugs with activity at different stages in the viral replication cycle (e.g., NRTIs with a PI) could offer an additive preventive effect in PEP, particularly for occupational exposures that pose an increased risk for transmission. Determining which agents and how many agents to use or when to alter a PEP regimen is largely empiric. Guidelines for the treatment of early HIV infection recommend the use of three drugs (two NRTIs and a PI) (69); however, the applicability of these recommendations to PEP remains unknown. In addition, the routine use of three drugs for all occupational HIV exposures may not be needed. Although the use of a highly potent regimen can be justified for exposures that pose an increased risk for transmission, it is uncertain whether the potential additional toxicity of a third drug is justified for lower-risk exposures. For this reason, the recommendations at the end of this report provide guidance for two- and three-drug PEP regimens that are based on the level of risk for HIV transmission represented by the exposure. NRTIs that can be considered for use with ZDV for PEP are lamivudine (3TC), didanosine (ddI), and zalcitabine, each of which has been included in recommended regimens that include ZDV (69). In previous CDC recommendations, 3TC was recommended as a second agent for PEP based on greater antiretroviral activity of the ZDV/3TC combination and its activity against many ZDV-resistant HIV strains without substantially increased toxicity (6). Also, data suggest that ZDV-resistant mutations develop more slowly in patients receiving the ZDV/3TC combination than those receiving ZDV alone (70), and in vitro studies indicate that the mutation associated with 3TC resistance may be associated with reversal of ZDV phenotypic resistance (71). No additional information has emerged to warrant altering the original recommendation of 3TC as the second agent for PEP. In addition, because ZDV and 3TC are available in a combination formulation (CombivirTM, manufactured by Glaxo Wellcome, Inc., Research Triangle Park, NC), the use of 3TC may be more convenient for HCWs. However, individual clinicians may prefer other NRTIs or combinations of other antiretroviral agents based on local knowledge and experience in treating HIV infection and disease. The addition of a PI as a third drug for PEP following high-risk exposures is based on the site of activity in the replication cycle (i.e., after viral integration has occurred) and demonstrated effectiveness in reducing viral burden. Previously, indinavir (IDV) was recommended as the PI for PEP because of its increased bioavailability when compared with saquinavir and its more favorable immediate toxicity profile compared with ritonavir (72). In addition, requirements for dose escalation when initiating ritonavir make it less practical for use in PEP. Since the 1996 PEP recommendations were published, nelfinavir (NEL) was approved for use by FDA and is now included in regimens recommended for the treatment of primary HIV infection (69). Also, FDA recently approved a soft-gel formulation of saquinavir (FortovaseTM, manufactured by Hoffmann-LaRoche, Inc., Nutley, New Jersey) that has improved bioavailability relative to its hard-gel formulation (InviraseTM, manufactured by Hoffmann-LaRoche, Inc.). However, the recommended dose of soft-gel saquinavir (1200 mg three times a day) is twice that of the hard-gel formulation (600 mg three times a day) and necessitates taking 18 pills a day, a factor that may influence HCW compliance if used for PEP. Based on these considerations, either IDV or NEL is recommended as first choice for inclusion in an expanded PEP regimen. If saquinavir is preferred by the prescribing physician, the soft-gel formulation (FortovaseTM) should be used. Also, differences in the side effects associated with IDV and NEL, discussed below, may influence which of these agents is selected in a specific situation. The NNRTIs (i.e., nevirapine and delavirdine) have not been included in these recommended regimens for PEP. As a class of antiretroviral agents, the NNRTIs are fast-acting and very potent, making them appealing in concept for PEP. In addition, there is some evidence of prophylactic efficacy (73). However, concerns about side effects and the availability of alternative agents argue against routinely using this class of drugs for initial PEP, although with expert consultation, an NNRTI might be considered. Side Effects and Toxicity of Antiretroviral Agents An important goal of PEP is to encourage and facilitate compliance with a 4-week PEP regimen. Therefore, the toxicity profile of antiretroviral agents, including the frequency, severity, duration, and reversibility of side effects, is a relevant consideration. All of the antiretroviral agents have been associated with side effects (See Appendix). However, studies of adverse events have been reported primarily for persons with advanced disease (and longer treatment courses) and therefore may not reflect the experience of persons with less advanced disease or those who are uninfected (74). Side effects associated with many of the NRTIs (e.g., ZDV or ddI) are chiefly gastrointestinal (e.g., nausea or diarrhea), and in general the incidence of adverse effects has not been greater when these agents are used in combination (72). All of the approved PIs may have potentially serious drug interactions when used with certain other drugs, requiring careful evaluation of concomitant medications being used by an HCW before prescribing a PI and close monitoring for toxicity when an HCW is receiving one of these drugs (See Appendix). PIs may inhibit the metabolism of nonsedating antihistamines and other hepatically metabolized drugs; NEL and ritonavir may accelerate the clearance of certain drugs, including oral contraceptives (requiring alternative or additional contraceptive measures for women taking these drugs). The use of PIs also has been associated with new onset of diabetes mellitus, hyperglycemia, diabetic ketoacidosis, and exacerbation of pre-existing diabetes mellitus (75-77). Nephrolithiasis has been associated with IDV use (including in HCWs using the drug for PEP) (8); however, the incidence of this potential complication may be limited by drinking at least 48 oz (1.5 L) of fluid per 24-hour period (e.g., six 8 oz glasses of water throughout the day) (72). Rare cases of hemolytic anemia also have been associated with the use of IDV. NEL, saquinavir, and ritonavir have been associated with the development of diarrhea; however, this side effect usually responds to treatment with antimotility agents that can be prescribed for use, if necessary, at the time any one of these drugs is prescribed for PEP. The manufacturer's package insert should always be consulted for questions about potential drug interactions. Among HCWs receiving ZDV PEP, usually at doses of 1,000-1,200 mg per day (i.e., higher than the currently recommended dose), 50%-75% reported one or more subjective complaints and approximately 30% discontinued the drug because of symptoms (7,78,79). Common symptoms included nausea, vomiting, malaise or fatigue, headache, or insomnia. Mild decreases in hemoglobin and absolute neutrophil count also were observed. All side effects were reversed when PEP was discontinued. Preliminary information about HCWs receiving combination drugs for PEP (usually ZDV plus 3TC with or without a PI) suggests that approximately 50%-90% of HCWs report subjective side effects that caused 24%-36% to discontinue PEP (8-10). One study documented that combination regimens that included ZDV at a lower dose (600 mg per day) were better tolerated than high-dose ZDV used alone (1,000-1,200 mg per day) (10). However, serious side effects, including nephrolithiasis, hepatitis, and pancytopenia, have been reported with the use of combination drugs for PEP (9,80; J.L. Gerberding, San Francisco General Hospital, personal communication, May 1997). Resistance to Antiretroviral Agents Known or suspected resistance of the source virus to antiretroviral agents, particularly to one or more agents that might be included in a PEP regimen, is a concern for those making decisions about PEP. Resistance of HIV has been reported with all available antiretroviral agents (65). However, the relevance of exposure to a resistant virus is not understood. Although transmission of resistant strains has been reported (81-85), in the perinatal clinical trial that studied vertical HIV transmission (ACTG protocol 076), ZDV prevented perinatal transmission despite genotypic resistance of HIV to ZDV in the mother (66). In addition, patients generally take more than one antiretro-viral drug and, unless testing is performed, often it is difficult to know to which drug(s) resistance exists. The complexity of this issue is further compounded by the frequency of cross-resistance within drug classes. Resistance should be suspected in source patients when there is clinical progression of disease or a persistently increasing viral load and/or a decline in CD4 T-cell count despite therapy, or a lack of virologic response to a change in therapy. Nevertheless, in this situation it is unknown whether a modification in the PEP regimen is necessary or will influence the outcome of an occupational exposure. Antiretroviral Drugs in Pregnancy Considerations for the use of antiretroviral drugs in pregnancy include their potential effect on the pregnant woman and on her fetus or neonate. The pharmacokinetics of antiretroviral drugs has not been completely studied in pregnant women. Some of the antiretroviral drugs are known to cross the placenta, but data for humans are not yet available for others (particularly the PIs). In addition, data are limited on the potential effects of antiretroviral drugs on the developing fetus or neonate (86). Decisions on the use of specific drugs in pregnancy also are influenced by whether a drug has specific adverse effects or might further exacerbate conditions associated with pregnancy, (e.g., drugs that cause nausea may be less tolerated when superimposed on the nausea normally associated with pregnancy). There are data on both ZDV and 3TC from clinical trials in HIV-infected pregnant women. The most extensive experience has been with the use of ZDV after 14 weeks of gestation in pregnant HIV-infected women in phase I studies and the perinatal ACTG protocol 076 (4,87). The dose of ZDV for pregnant women is the same as that in nonpregnant persons, and ZDV appears safe and well tolerated in both women and their infants who have had a follow-up period of several years (88-90). Data from the Antiretroviral Pregnancy Registry have not documented an increased risk for birth defects in infants with in utero exposure to ZDV (91). There are limited data on use of 3TC alone or in combination with ZDV in late gestation in pregnant HIV-infected women. As with ZDV, the pharmacokinetics and dose of 3TC appear to be similar to those for nonpregnant persons. The drug appears safe during pregnancy for women and infants, although long-term safety is not known (92,93). Carcinogenicity and/or mutagenicity is evident in several in vitro screening tests for ZDV and all other FDA-licensed nucleoside antiretroviral drugs. In some in vivo rodent studies, high-dose lifetime continuous ZDV exposure (94) or very high dose in utero ZDV exposure has been associated with the development of tumors in adult females or their offspring (95,96). The relevance of these animal data to humans is unknown. However, in 1997 an independent panel reviewed these data and concluded that the known benefits of ZDV in preventing perinatal transmission, where the risk for transmission without ZDV is 25%-30%, outweigh the hypothetical concerns about transplacental carcinogenesis (97). No data are available regarding pharmacokinetics, safety, or tolerability of any of the PIs in pregnant women. The use of PIs in HIV-infected persons has been associated with hyperglycemia; it is unknown whether the use of these agents during pregnancy will exacerbate the risk for pregnancy-associated hyperglycemia. Therefore, close monitoring of glucose levels and careful instruction regarding symptoms related to hyperglycemia are recommended for pregnant HCWs receiving a PI for PEP. IDV is associated with infrequent side effects in adults (i.e., hyperbilirubinemia and renal stones) that could be problematic for the newborn. As the half-life of IDV in adults is short, these concerns may be relevant only if the drug is administered shortly before delivery. RECOMMENDATIONS FOR THE MANAGEMENT OF POTENTIALLY EXPOSED HCWs Health-care organizations should make available to their workers a system that includes written protocols for prompt reporting, evaluation, counseling, treatment, and follow-up of occupational exposures that may place HCWs at risk for acquiring any bloodborne infection, including HIV. Employers also are required to establish exposure-control plans, including postexposure follow-up for their employees, and to comply with incident reporting requirements mandated by the Occupational Safety and Health Administration (15). Access to clinicians who can provide postexposure care should be available during all working hours, including nights and weekends. Antiretroviral agents for PEP should be available for timely administration (i.e., either by providing access to PEP drugs on site or creating links with other facilities or providers to make them available offsite). Persons responsible for providing postexposure counseling should be familiar with evaluation and treatment protocols and the facility's procedures for obtaining drugs for PEP. HCWs should be educated to report occupational exposures immediately after they occur, particularly because PEP is most likely to be effective if implemented as soon after the exposure as possible (41,55,56). HCWs who are at risk for occupational exposure to HIV should be taught the principles of postexposure management, including options for PEP, as part of job orientation and ongoing job training. Exposure Report If an occupational exposure occurs, the circumstances and postexposure management should be recorded in the HCW's confidential medical record (usually on a form the facility designates for this purpose). Relevant information includes
details about the exposure source (i.e., whether the source material contained HIV or other bloodborne pathogen{s}), and if the source is an HIV-infected person, the stage of disease, history of antiretroviral therapy, and viral load, if known; and
Exposure Management Treatment of an Exposure Site Wounds and skin sites that have been in contact with blood or body fluids should be washed with soap and water; mucous membranes should be flushed with water. There is no evidence that the use of antiseptics for wound care or expressing fluid by squeezing the wound further reduces the risk for HIV transmission. However, the use of antiseptics is not contraindicated. The application of caustic agents (e.g., bleach) or the injection of antiseptics or disinfectants into the wound is not recommended. Assessment of Infection Risk After an occupational exposure, the source-person and the exposed HCW should be evaluated to determine the need for HIV PEP. Follow-up for hepatitis B virus and hepatitis C virus infections also should be conducted in accordance with previously published CDC recommendations (98,99). Evaluation of exposure. The exposure should be evaluated for potential to transmit HIV based on the type of body substance involved and the route and severity of the exposure. Exposures to blood, fluid containing visible blood, or other potentially infectious fluid (including semen; vaginal secretions; and cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic fluids) or tissue through a percutaneous injury (i.e., needlestick or other penetrating sharps-related event) or through contact with a mucous membrane are situations that pose a risk for bloodborne transmission and require further evaluation (Figure_1 and Figure_1C). In addition, any direct contact (i.e., personal protective equipment either was not used or was ineffective in protecting skin or mucous membranes) with concentrated HIV in a research laboratory or production facility is considered an exposure that requires clinical evaluation to assess the need for PEP. For skin exposures, follow-up is indicated if it involves direct contact with a body fluid listed above and there is evidence of compromised skin integrity (e.g., dermatitis, abrasion, or open wound). However, if the contact is prolonged or involves a large area of intact skin, postexposure follow-up may be considered on a case-by-case basis or if requested by the HCW. For human bites, the clinical evaluation must consider possible exposure of both the bite recipient and the person who inflicted the bite. HIV transmission only rarely has been reported by this route (100,101; CDC, unpublished data, 1998). If a bite results in blood exposure to either person involved, postexposure follow-up, including consideration of PEP, should be provided. Evaluation and testing of an exposure source. The person whose blood or body fluids are the source of an occupational exposure should be evaluated for HIV infection. Information available in the medical record at the time of exposure (e.g., laboratory test results, admitting diagnosis, or past medical history) or from the source person may suggest or rule out possible HIV infection. Examples of information to consider when evaluating an exposure source for possible HIV infection include laboratory information (e.g., prior HIV testing results or results of immunologic testing {e.g., CD4+ count}), clinical symptoms (e.g., acute syndrome suggestive of primary HIV infection or undiagnosed immunodeficiency disease), and history of possible HIV exposures (e.g., injecting-drug use, sexual contact with a known HIV-positive partner, unprotected sexual contact with multiple partners {heterosexual and/or homosexual}, or receipt of blood or blood products before 1985). If the source is known to have HIV infection, available information about this person's stage of infection (i.e., asymptomatic or AIDS), CD4+ T-cell count, results of viral load testing, and current and previous antiretroviral therapy, should be gathered for consideration in choosing an appropriate PEP regimen. If this information is not immediately available, initiation of PEP, if indicated, should not be delayed; changes in the PEP regimen can be made after PEP has been started, as appropriate. If the HIV serostatus of the source person is unknown, the source person should be informed of the incident and, if consent is obtained, tested for serologic evidence of HIV infection. If consent cannot be obtained (e.g., patient is unconscious), procedures should be followed for testing source persons according to applicable state and local laws. Confidentiality of the source person should be maintained at all times. HIV-antibody testing of an exposure source should be performed as soon as possible. Hospitals, clinics, and other sites that manage exposed HCWs should consult their laboratories regarding the most appropriate test to use to expedite these results. An FDA-approved rapid HIV-antibody test kit should be considered for use in this situation, particularly if testing by enzyme immunoassay (EIA) cannot be completed within 24-48 hours. Repeatedly reactive results by EIA or rapid HIV-antibody tests are considered highly suggestive of infection, whereas a negative result is an excellent indicator of the absence of HIV antibody. Confirmation of a reactive result by Western blot or immunofluorescent antibody is not necessary for making initial decisions about postexposure management but should be done to complete the testing process. If the source is HIV seronegative and has no clinical evidence of acquired immunodeficiency syndrome (AIDS) or symptoms of HIV infection, no further testing of the source is indicated. It is unclear whether follow-up testing of a source who is HIV negative at the time of exposure, but recently (i.e., within the last 3-6 months) engaged in behaviors that pose a risk for HIV transmission, is useful in postexposure management of HCWs; HCWs who become infected generally seroconvert before repeat testing of a source would normally be performed. If the exposure source is unknown, information about where and under what circumstances the exposure occurred should be assessed epidemiologically for risk for transmission of HIV. Certain situations, as well as the type of exposure, may suggest an increased or decreased risk; an important consideration is the prevalence of HIV in the population group (i.e., institution or community) from which the contaminated source material is derived. For example, an exposure that occurs in a geographic area where injecting-drug use is prevalent or on an AIDS unit in a health-care facility would be considered epidemiologically to have a higher risk for transmission than one that occurs in a nursing home for the elderly where no known HIV-infected residents are present. In addition, exposure to a blood-filled hollow needle or visibly bloody device suggests a higher-risk exposure than exposure to a needle that was most likely used for giving an injection. Decisions regarding appropriate management should be individualized based on the risk assessment. HIV testing of needles or other sharp instruments associated with an exposure, regardless of whether the source is known or unknown, is not recommended. The reliability and interpretation of findings in such circumstances are unknown. Clinical Evaluation and Baseline Testing of Exposed HCWs Exposed HCWs should be evaluated for susceptibility to bloodborne pathogen infections. Baseline testing (i.e., testing to establish serostatus at the time of exposure) for HIV antibody should be performed. If the source person is seronegative for HIV, baseline testing or further follow-up of the HCW normally is not necessary. If the source person has recently engaged in behaviors that are associated with a risk for HIV transmission, baseline and follow-up HIV-antibody testing (e.g., 3 and/or 6 months postexposure) of the HCW should be considered. Serologic testing should be made available to all HCWs who are concerned that they may have been exposed to HIV. For purposes of considering HIV PEP, the evaluation also should include information about medications the HCW may be taking and any current or underlying medical conditions or circumstances (i.e., pregnancy, breast feeding, or renal or hepatic disease) that may influence drug selection. Pregnancy testing should be offered to all nonpregnant women of childbearing age whose pregnancy status is unknown. HIV PEP The following recommendations apply to situations where an HCW has had an exposure to a source person with HIV or where information suggests that there is a likelihood that the source person is HIV-infected. These recommendations are based on the risk for HIV infection after different types of exposure and limited data regarding efficacy and toxicity of PEP. Because most occupational HIV exposures do not result in the transmission of HIV, potential toxicity must be carefully considered when prescribing PEP. When possible, these recommendations should be implemented in consultation with persons having expertise in antiretroviral therapy and HIV transmission. Explaining PEP to HCWs Recommendations for chemoprophylaxis should be explained to HCWs who have sustained occupational HIV exposures (Figure_1 and Figure_1C). For exposures for which PEP is considered appropriate, HCWs should be informed that a) knowledge about the efficacy and toxicity of drugs used for PEP are limited; b) only ZDV has been shown to prevent HIV transmission in humans; c) there are no data to address whether adding other antiretroviral drugs provides any additional benefit for PEP, but experts recommend combination drug regimens because of increased potency and concerns about drug-resistant virus; d) data regarding toxicity of antiretroviral drugs in persons without HIV infection or in pregnant women are limited for ZDV and not known regarding other antiretroviral drugs; and e) any or all drugs for PEP may be declined by the HCW. HCWs who have HIV occupational exposures for which PEP is not recommended should be informed that the potential side effects and toxicity of taking PEP outweigh the negligible risk of transmission posed by the type of exposure. Factors in Selection of a PEP Regimen Selection of the PEP regimen should consider the comparative risk represented by the exposure and information about the exposure source, including history of and response to antiretroviral therapy based on clinical response, CD4+ T-lymphocyte counts, viral load measurements, and current disease stage. Most HIV exposures will warrant only a two-drug regimen, using two NRTIs, usually ZDV and 3TC. The addition of a third drug, usually a PI (i.e., IDV or NEL), should be considered for exposures that pose an increased risk for transmission or where resistance to the other drugs used for PEP is known or suspected. Timing of PEP Initiation PEP should be initiated as soon as possible. The interval within which PEP should be started for optimal efficacy is not known. Animal studies have demonstrated the importance of starting PEP within hours after an exposure (43,54,56). To assure timely access to PEP, an occupational exposure should be regarded as an urgent medical concern and PEP started as soon as possible after the exposure (i.e., within a few hours rather than days). If there is a question about which antiretroviral drugs to use, or whether to use two or three drugs, it is probably better to start ZDV and 3TC immediately than to delay PEP administration. Although animal studies suggest that PEP probably is not effective when started later than 24-36 hours postexposure (42,55,56), the interval after which there is no benefit from PEP for humans is undefined. Therefore, if appropriate for the exposure, PEP should be started even when the interval since exposure exceeds 36 hours. Initiating therapy after a longer interval (e.g., 1-2 weeks) may be considered for exposures that represent an increased risk for transmission; even if infection is not prevented, early treatment of acute HIV infection may be beneficial (69). The optimal duration of PEP is unknown. Because 4 weeks of ZDV appeared protective in HCWs (2), PEP probably should be administered for 4 weeks, if tolerated. PEP if Serostatus of Source Person is Unknown If the source person's HIV serostatus is unknown at the time of exposure (including when the source is HIV negative but may have had a recent HIV exposure), use of PEP should be decided on a case-by-case basis, after considering the type of exposure and the clinical and/or epidemiologic likelihood of HIV infection in the source (Figure_1 and Figure_1C). If these considerations suggest a possibility for HIV transmission and HIV testing of the source is pending, it is reasonable to initiate a two-drug PEP regimen until laboratory results have been obtained and later modify or discontinue the regimen accordingly. PEP if Exposure Source is Unknown If the exposure source is unknown, use of PEP should be decided on a case-by-case basis. Consideration should include the severity of the exposure and the epidemiologic likelihood that the HCW was exposed to HIV. PEP for Pregnant HCWs If the HCW is pregnant, the evaluation of risk and need for PEP should be approached as with any other HCW who has had an HIV exposure. However, the decision to use any antiretroviral drug during pregnancy should involve discussion between the woman and her health-care provider regarding the potential benefits and potential risks to her and her fetus. Follow-up of HCWs Exposed to HIV Postexposure Testing HCWs with occupational exposure to HIV should receive follow-up counseling, postexposure testing, and medical evaluation regardless of whether they receive PEP. HIV-antibody testing should be performed for at least 6 months postexposure (e.g., at 6 weeks, 12 weeks, and 6 months). It is unclear whether an extended follow-up period (e.g., 12 months) is indicated in certain circumstances. Although rare instances of delayed HIV seroconversion have been reported (36,37, J.L. Gerberding, San Francisco General Hospital, unpublished data, May 1997), the infrequency of this occurrence does not warrant adding to HCWs' anxiety by routinely extending the duration of postexposure follow-up. Circumstances for which extending the duration of follow-up have been suggested include the use of highly potent antiretroviral regimens (i.e., more than two drugs) because of theoretical concerns that HIV seroconversion could be delayed, or simultaneous exposure to HCV. Data are insufficient for making a general recommendation in these situations. However, this should not preclude a decision to extend follow-up in an individual situation based on the clinical judgement of the HCW's health-care provider. HIV testing should be performed on any HCW who has an illness that is compatible with an acute retroviral syndrome, regardless of the interval since exposure. HIV-antibody testing using EIA should be used to monitor for sero-conversion. The routine use of direct virus assays (e.g., HIV p24 antigen EIA or polymerase chain reaction for HIV RNA) to detect infection in exposed HCWs generally is not recommended (34). Although direct virus assays may detect HIV infection a few days earlier than EIA, the infrequency of HCW seroconversion and increased costs of these tests do not warrant their routine use in this setting. Also, HIV RNA is approved for use in established HIV infection; its reliability in detecting very early infection has not been determined. Monitoring and Management of PEP Toxicity If PEP is used, drug-toxicity monitoring should be performed at baseline and again 2 weeks after starting PEP. Clinical judgement, based on medical conditions that may exist in the HCW and any toxicity associated with drugs included in the PEP regimen, should determine the scope of testing. Minimally these should include a complete blood count and renal and hepatic chemical function tests. Monitoring for evidence of hyperglycemia should be included for HCWs whose regimen includes any PI; if the HCW is receiving IDV, monitoring for crystalluria, hematuria, hemolytic anemia, and hepatitis also should be included. If toxicity is noted, modification of the regimen should be considered after expert consultation; further diagnostic studies may be indicated. HCWs who fail to complete the recommended regimen often do so because of the side effects they experience (e.g., nausea and diarrhea). These symptoms often can be managed without changing the regimen by prescribing antimotility and antiemetic agents or other medications that target the specific symptoms. In other situations, modifying the dose interval (i.e., administering a lower dose of drug more frequently throughout the day, as recommended by the manufacturer), may help promote adherence to the regimen. Counseling and Education Although HIV infection following an occupational exposure occurs infrequently, the emotional impact of the exposure often is substantial (102,103). In addition, HCWs are given seemingly conflicting information. Although HCWs are told that there is a low risk for HIV transmission, a 4-week regimen of PEP is recommended and they are asked to commit to behavioral measures (i.e., sexual abstinence or condom use) to prevent secondary transmission, all of which influence their lives for several weeks to months (102). Therefore, access to persons who are knowledgeable about occupational HIV transmission and who can deal with the many concerns an HIV exposure may raise for the HCW is an important element of postexposure management. HIV-exposed HCWs should be advised to use the following measures to prevent secondary transmission during the follow-up period, especially during the first 6-12 weeks after the exposure when most HIV-infected persons are expected to seroconvert: use sexual abstinence or condoms to prevent sexual transmission and to avoid pregnancy; and refrain from donating blood, plasma, organs, tissue, or semen. If the exposed HCW is breastfeeding, she should be counseled about the risk for HIV transmission through breast milk, and discontinuation of breastfeeding should be considered, especially following high-risk exposures. If the HCW chooses to receive PEP, temporary discontinuation of breastfeeding while she is taking PEP should be considered to avoid exposing the infant to these agents. NRTIs are known to pass into breast milk; it is not known whether this also is true for PIs. There is no need to modify an HCW's patient-care responsibilities to prevent transmission to patients based solely on an HIV exposure. If HIV seroconversion is detected, the HCW should be evaluated according to published recommendations for HIV-infected HCWs (104). Exposed HCWs should be advised to seek medical evaluation for any acute illness that occurs during the follow-up period. Such an illness, particularly if characterized by fever, rash, myalgia, fatigue, malaise, or lymphadenopathy, may be indicative of acute HIV infection but also may be due to a drug reaction or another medical condition. Exposed HCWs who choose to take PEP should be advised of the importance of completing the prescribed regimen. Information should be provided about potential drug interactions and the drugs that should not be taken with PEP, the side effects of the drugs that have been prescribed (See Appendix), measures to minimize these effects, and the methods of clinical monitoring for toxicity during the follow-up period. They should be advised that the evaluation of certain symptoms should not be delayed (e.g., back or abdominal pain, pain on urination or blood in the urine, or symptoms of hyperglycemia {i.e., increased thirst and/or frequent urination}). RECOMMENDATIONS FOR THE SELECTION OF DRUGS FOR PEP The selection of a drug regimen for HIV PEP must strive to balance the risk for infection against the potential toxicity of the agent(s) used. Because PEP is potentially toxic, its use is not justified for exposures that pose a negligible risk for transmission (Figure_1 and Figure_1C). Also, there is insufficient evidence to recommend a highly active regimen for all HIV exposures. Therefore, two regimens for PEP are provided (Table_1): a "basic" two-drug regimen that should be appropriate for most HIV exposures and an "expanded" three-drug regimen that should be used for exposures that pose an increased risk for transmission (Figure_1 and Figure_1C) or where resistance to one or more antiretroviral agents is known or suspected. When possible, the regimens should be implemented in consultation with persons having expertise in antiretroviral treatment and HIV transmission. Situations That Require Special Consideration Resistance of the Source Virus to Antiretroviral Drugs It is unknown whether drug resistance influences transmission risk; however, transmission of drug-resistant HIV has been reported (81,82) and is therefore a theoretical concern when choosing PEP regimens. If the source-person's virus is known or suspected to be resistant to one or more of the drugs included in the PEP regimen, the selection of drugs to which the source person's virus is unlikely to be resistant is recommended (69). If the resistance is to one class of antiretroviral drugs, the addition to the basic PEP regimen of a drug from another class might be considered (e.g., addition of a PI when a source patient has not been treated with a PI but has virus resistant to one or more NRTIs). It is strongly recommended that PEP be started regardless of the resistance status in the source virus; if resistance is known or suspected, a third or fourth drug may be added to the regimen until consultation with a clinical expert in the treatment of HIV infection or disease can be obtained. Known or Suspected Pregnancy in the HCW Pregnancy should not preclude the use of optimal PEP regimens, and PEP should not be denied to an HCW solely on the basis of pregnancy. However, as discussed previously, an occupationally exposed pregnant HCW must be provided with full information about what is known and not known regarding the potential benefits and risks associated with use of the antiretroviral drugs to her and her fetus for her to make an informed decision regarding the use of PEP. The choice of antiretroviral drugs to use for PEP in pregnant HCWs is complicated by the potential need to alter dosing because of physiologic changes associated with pregnancy and the potential for short- or long-term effects on the fetus and newborn. Thus, considerations that should be discussed with a pregnant HCW include the potential risk for HIV transmission based on the type of exposure; the stage of pregnancy (the first trimester being the period of maximal organogenesis and risk for teratogenesis); and what is known about the pharmacokinetics, safety, and tolerability of the drug or combination of drugs in pregnancy. POSTEXPOSURE REGISTRIES Health-care providers in the United States are encouraged to enroll HCWs who receive PEP in a confidential registry developed by CDC, Glaxo Wellcome Inc., and Merck & Co., Inc., to assess toxicity; telephone (888) 737-4448 ({888} PEP-4HIV), or write the HIV PEP Registry, 1410 Commonwealth Drive, Suite 215, Wilmington, NC 28405. Unusual or serious and unexpected toxicity from antiretroviral drugs should be reported to the manufacturer and/or FDA, telephone (800) 332-1088. Health-care providers also should report instances of prenatal exposure to antiretroviral agents to the Antiretroviral Pregnancy Registry. The registry is an epidemiologic project to collect observational, nonexperimental data on antiretroviral drug exposure during pregnancy to assess potential teratogenicity. Referrals should be directed to the Antiretroviral Pregnancy Registry, 1410 Commonwealth Drive, Suite 215, Wilmington, NC 28405; telephone (800) 258-4263 or (800) 722-9292, ext. 39437; fax (800) 800-1052. A protocol has been developed to evaluate HIV seroconversion in an HCW who received PEP. These events can be reported to CDC, telephone (404) 639-6425. RESOURCES FOR CONSULTATION Clinicians who seek consultation on HIV PEP for assistance in managing an occupational exposure should access local experts in HIV treatment as much as possible. In addition, the "National Clinicians' Post-Exposure Prophylaxis Hotline (PEP-Line)" has been created to assist clinicians with these issues; telephone (888) 448-4911. Other resources and registries include the HIV Postexposure Prophylaxis Registry, the Antiretroviral Pregnancy Registry, FDA, and CDC (Table_2). ADMINISTRATIVE CONSIDERATIONS Effective implementation of the elements of postexposure management detailed in these recommendations may require various types of expertise. The assessment of the severity of an exposure generally requires clinical training and experience (i.e., medical or nursing). However, the assessment of HIV infection risk and initiation of a basic PEP regimen necessitates knowledge or experience in clinical epidemiology, infection control, occupational health, or the clinical treatment of HIV. Decisions about HIV PEP are particularly complex if PIs are used or there is concern about drug-resistant virus. Thus, expert consultation when prescribing PEP is strongly encouraged. PEP protocols should list the names of readily available resources for consultation and could include policies that require infectious disease evaluation before prescribing an expanded antiretroviral regimen. However, these efforts should not delay initial implementation of PEP where it is appropriate. Acknowledgments Drafts of this document have been reviewed by many experts in the treatment of HIV infection and disease. We thank these consultants for their thoughtful comments, suggestions, and assistance. References
This interagency working group comprised representatives of CDC, the Food and Drug Administration, the Health Resources and Services Administration, and the National Institutes of Health. ** This interagency working group comprised representatives of CDC, FDA, and the National Institutes of Health. Information included in these recommendations may not represent FDA approval or approved labeling for the particular product or indications in question. Specifically the terms "safe" and "effective" may not be synonymous with the FDA-defined legal standards for product approval. *** Although exposure to these body substances generally is not considered a risk for occupational HIV transmission, this does not negate the importance of handwashing and appropriate glove use when contacting these body substances. Handwashing and appropriate glove use are part of standard precautions for infection control to prevent transmission of nosocomial and community-acquired pathogens and are required for compliance with the Occupational Safety and Health Administration bloodborne pathogen standard (14,15). In addition, postexposure evaluation for hepatitis B (and possibly hepatitis C) should be provided if contact with saliva includes a possible portal of entry (i.e., nonintact skin, mucous membrane, or percutaneous injury). Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Basic and expanded postexposure prophylaxis regimens
=============================================================================
Regimen category Application Drug regimen
-----------------------------------------------------------------------------
Basic Occupational HIV exposures 4 weeks (28 days) of both
for which there is a zidovudine 600 mg every
recognized transmission day in divided doses (i.e.
risk (Figure 1). 300 mg twice a day, 200 mg
three times a day, or 100
mg every 4 hours) and
lamivudine 150 mg twice a
day.
Expanded Occupational HIV exposures Basic regimen plus either
that pose an increased risk indinavir 800 mg every 8
for transmission (e.g. hours or nelfinavir 750 mg
larger volume of blood three times a day.*
and/or higher virus titer
in blood) (Figure 1).
-----------------------------------------------------------------------------
* Idinavir should be taken on an empty stomach (i.e. without food or
with a light meal) and with increased fluid consumption (i.e. drinking
six 8oz glasses of water throughout the day); nelfinavir should be taken
with meals.
=============================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. HIV postexposure prophylaxis resources and registries
=============================================================================
Resource or registry Contact Information
-----------------------------------------------------------------------------
National Clinicians' Telephone: (888) 448-4911
Postexposure Hotline Telephone: (888) 737-4448
(888) PEP4HIV
Write: 1410 Commonwealth Drive
Suite 215
Wilmington, NC 28405
Antiretroviral Pregnancy Telephone: (800)258-4263
Registry Fax: (800)800-1052
Write: 1410 Commonwealth Drive
Suite 215
Wilmington, NC 28405
Food and Drug Administration Telephone: (800)332-1088
(for reporting unusual or
severe toxicity to anti-
retroviral agents)
CDC (for reporting HIV Telephone: (404)639-6425
seroconversions in health-
care workers who received
PEP)
=============================================================================
Return to top. Figure_1 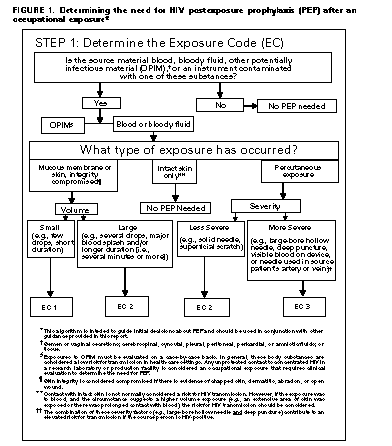 Return to top. Figure_1C 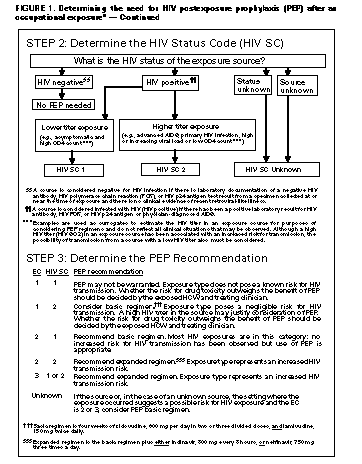 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|