 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Effectiveness of a Seventh Grade School Entry Vaccination Requirement -- Statewide and Orange County, Florida, 1997-1998Vaccine-preventable diseases continue to occur among adolescents (i.e., persons aged 11-21 years) (1). In 1996, the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics, the American Academy of Family Physicians, and the American Medical Association published joint recommendations emphasizing appropriate vaccination of adolescents aged 11-12 years who have not been vaccinated with hepatitis B vaccine, a second dose of measles, mumps, and rubella vaccine (MMR), varicella vaccine (if indicated), a booster dose of tetanus and diphtheria toxoids (Td), and other vaccines that may be indicated for certain adolescents (2). School entry requirements are an effective mechanism for ensuring high vaccination coverage among children. At the start of the 1997-98 school year, an amendment to the Florida Administrative Code (64D-3.011, F.A.C.) was instituted that requires all persons entering seventh grade to be vaccinated with three doses of hepatitis B vaccine, a second dose of MMR, and a Td booster, or to be on schedule for vaccination (i.e., having received at least one dose of hepatitis B vaccine, one dose of MMR, and a Td booster). To determine vaccination coverage among students entering seventh grade in Florida and in Orange County in 1997, CDC, in collaboration with the Florida Department of Health, analyzed state vaccination coverage data. This report summarizes the results of the analysis and indicates that a vaccination requirement for middle school entry can be effective in ensuring vaccination of adolescents. Florida At the start of the 1997-98 school year, 196,074 students entered the seventh grade in 1286 public and private schools in Florida. By November 30, 1997, 121,219 (61.8%) of these students were fully vaccinated with three doses of hepatitis B vaccine, a second dose of MMR, and a Td booster. An additional 72,275 (36.9%) students lacked one or more required vaccinations but were on schedule and therefore in compliance with the requirement, and 763 (0.4%) were exempted for medical or religious reasons. The percentage of seventh-grade students fully vaccinated varied among the 67 Florida counties (Figure_1), ranging from 36.0% in Charlotte County to 97.2% in Franklin County. Coverage varied in the six counties with greater than or equal to 10,000 seventh graders: Broward (74.1%), Dade (43.1%), Duval (42.8%), Hillsborough (55.5%), Orange (55.1%), and Palm Beach (77.9%) (less than or equal to 0.01). Statewide coverage among the 177,903 Florida seventh graders enrolled in 617 public schools was substantially lower (59.6%) than that among the 18,171 enrolled in 669 private schools (83.8%) (less than or equal to 0.01). From 1995 through 1997, the number of vaccinations administered to children aged 10-14 years by Florida public health facilities (i.e., school-based, county, or city clinics) increased substantially (Figure_2). In Florida, vaccines mandated by law must be made available to children free of charge by the Florida Department of Health regardless of a child's insurance status. Orange County, Florida To ensure vaccination of seventh graders, Orange County Health Department (OCHD) officials teamed with a community coalition consisting of private and public health-care providers, local businesses, nongovernment organizations, and local colleges. The Orange County strategy included the vaccination of adolescents by private providers, public health department clinics, and school-based vaccination programs. At the start of the 1997-98 school year, 11,122 students entered seventh grade in Orange County. Of these students, 10,166 (91.4%) were enrolled in 33 public schools and 956 (8.6%) were enrolled in 24 private schools. In anticipation of the law, during the 1996-97 school year, OCHD sent pamphlets home with all sixth graders explaining the new requirement. In January 1997, the "Cool School Shots Campaign" was initiated that included local media announcements and a public school-based vaccination program targeting sixth graders. Three sessions were scheduled to allow students to receive all required vaccinations, including the three doses of hepatitis B vaccine, at school. Overall, 3739 (34%) students received at least one vaccination during the first of three school-based vaccination events conducted during January 1997 (Table_1). Hepatitis B vaccine accounted for 35.7% of the vaccine doses administered during the first session, 92.7% during the second, and 100% during the third. However, 44% fewer third doses of hepatitis B vaccine (1886) than first doses (3329) were administered. Based on anecdotal information from OCHD officials, lack of parental knowledge regarding school entry vaccination requirements was a key barrier to achieving higher participation and completion by students in the program. During July-September 1997, immediately before implementation of the seventh grade entry requirement and after the school-based vaccination campaign, the OCHD administered 9087 total vaccine doses, including 5015 doses of hepatitis B vaccine, 1700 doses of MMR, and 2372 doses of Td booster to children aged 10-14 years, representing a 380% increase from the 2379 total doses administered during the same period in 1996. By November 30, 1997, 6123 (55.1%) Orange County seventh graders entering school were fully vaccinated. A total of 4988 (44.9%) students lacked one or more required vaccinations but were considered in compliance with the requirement, eight were exempted for either medical or religious reasons, and three lacked documentation. Seventh graders enrolled in private schools were more likely to be fully vaccinated than seventh graders enrolled in public schools (86.4% vs. 52.1%) (less than or equal to 0.01). Reported by: HT Janowski, MPH, Florida Bur of Immunization, Flordia Dept of Health; D Deloach, CJ Keough, Orange County Health Dept; SF Morrison, PhD, Orange County Public Schools, Orlando, Florida. N Smith, MPH, Council of State and Territorial Epidemiologists, Atlanta, Georgia. Health Svcs Research and Evaluation Br, Immunization Svcs Div, National Immunization Program; and an EIS Officer, CDC. Editorial NoteEditorial Note: The findings in this report indicate that a middle school vaccination entry requirement in Florida was effective in ensuring that most seventh-grade students were appropriately vaccinated after the law was enacted. Other successful programs to vaccinate adolescents in schools and in provider settings have been previously described (3,4). Many older children and adolescents may require additional doses of vaccine when new vaccines are introduced or recommendations for existing vaccines are revised. For example, hepatitis B vaccine has been recommended for all infants since 1991. However, in 1997, ACIP revised its recommendations to include all persons aged 0-18 years; vaccine is available through the Vaccines for Children (VFC) program for persons who are eligible for VFC. The lifetime risk for hepatitis B virus (HBV) infection is 4.2% for persons aged greater than or equal to 6 years, and approximately 70% of HBV infections occur in late adolescence and early adulthood (5). In the United States, failure to vaccinate a single cohort of adolescents will result in an estimated 160,000 HBV infections, 10,000 chronic HBV infections, and 1400 deaths (6). Without vaccination, an estimated 8157 cases of hepatitis B infection (4.2% of population lifetime risk for infection for persons aged greater than or equal to 6 years), 489 chronic HBV infections (6% of HBV infections) and 69 hepatitis-related deaths (14% of chronic HBV infections) will occur among this single cohort of 196,074 Florida adolescents during their lifetimes. Immediate action is needed to ensure that adolescents receive hepatitis B vaccine along with other recommended vaccinations. The findings in this report are subject to at least three limitations. First, because data collected for Florida consisted of regional reports sent from schools to the department of health during November 1997, no mechanism was in place to determine the total number of fully vaccinated seventh-grade students at the end of the school year. Second, the number of vaccinations and other recommended preventive services received by these adolescents from their primary-care provider or managed-care organization is unknown. Finally, data were not available to determine the rate of vaccine coverage in previous years; however, the increase in vaccine administered by public clinics suggest that vaccination rates in previous years among persons aged 10-14 years was lower. In 1997, four states (Colorado, Florida, Oklahoma and Wisconsin) implemented middle school vaccination entry requirements for hepatitis B vaccine. The number of states with vaccination entry requirements for middle school students will increase to 14 by 2006, when an estimated 75% of adolescents aged 11-12 years in the United States will be subject to hepatitis B vaccination requirements through both elementary and middle school requirements (6). Because of current successes in the infant vaccination program, most adolescents will be appropriately vaccinated against hepatitis B by the year 2010. References
Figure_1 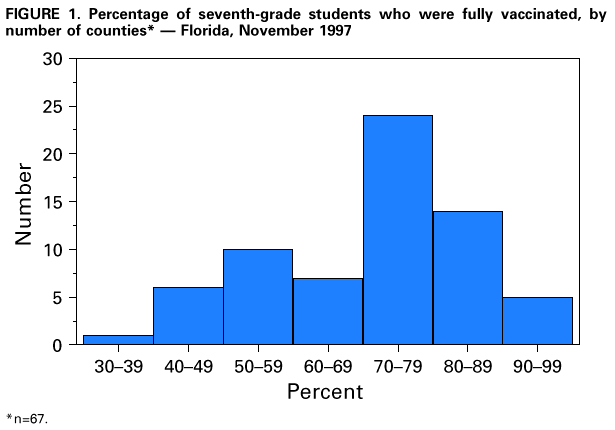 Return to top. Figure_2 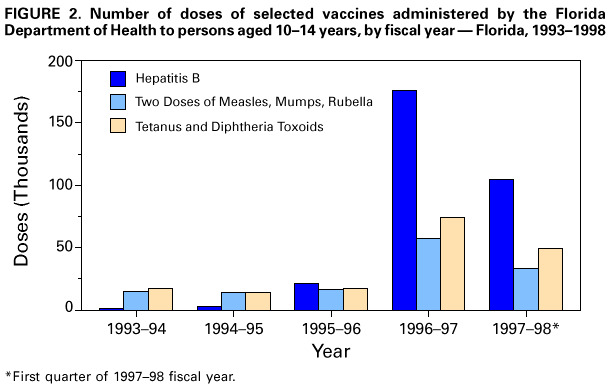 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Number of vaccine doses administered to persons aged 10-14 years during
three school-based vaccination events, by vaccine -- Orange County, Florida, 1997
===================================================================================
Vaccination session
------------------------------
Vaccine January February May
----------------------------------------------------------
Hepatitis B-dose 1 3329 92 24
Hepatitis B-dose 2 70 2538 348
Hepatitis B-dose 3 24 19 1886
MMR * dose 2 2959 106 N/A +
Td booster & 3191 103 N/A
Total participants 3739 2665 2258
Total vaccine doses 9573 2858 2258
----------------------------------------------------------
* Measles, mumps, and rubella vaccine.
+ Not available.
& Tetanus and diphtheria toxoids booster.
===================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|