 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Influenza Activity --- United States and Worldwide, 1999--2000 Season, and Composition of the 2000--01 Influenza VaccineInfluenza A (H3N2) viruses were the predominant viruses isolated in the United States and worldwide during 1999--2000. This was the third consecutive year that influenza A/Sydney/05/97-like (H3N2) viruses were the most prevalent viruses isolated in the United States. Influenza activity in the United States was similar to the previous two seasons, although mortality measurements attributed to pneumonia and influenza (P&I) were unusually high. Overall, the 1999--2000 influenza vaccine was well matched to circulating influenza viruses. The 2000--01 influenza season will be the first for which influenza vaccination is recommended for all persons aged >50 years. This report summarizes surveillance for influenza in the United States and worldwide during the 1999--2000 influenza season, describes the composition of the 2000--01 influenza vaccine, and highlights changes in the recommendations for prevention and control of influenza. United StatesInfluenza activity began to increase in mid-December 1999 and peaked during the weeks ending December 25, 1999 (week 51), and January 15, 2000 (week 2). Widespread* influenza activity was first reported during the week ending December 18, 1999 (week 50). The number of state and territorial epidemiologists reporting widespread or regional influenza activity peaked at 44 during the week ending January 15 (week 2) (Figure 1). No state has reported widespread or regional influenza activity since the week ending March 25 (week 12). The percentage of patient visits to U.S. sentinel physicians for influenza-like illness (ILI) increased above baseline levels (0--3%) to 4% during the week ending December 25, 1999 (week 51), and remained elevated for 4 consecutive weeks. The proportion of visits for ILI peaked at 6% during the week ending January 1 (week 52) and returned to baseline levels in all surveillance regions by the week ending February 5 (week 5) (Figure 2). The proportion of deaths attributed to P&I reported by 122 U.S. cities exceeded the epidemic threshold† for 22 consecutive weeks beginning the week ending November 27 (week 47) through the week ending April 22 (week 16). The percentage of deaths attributed to P&I peaked at 11.2% during the week ending January 22 (week 3) (1). From October 3, 1999, through April 22, 2000, the predominant viruses isolated were influenza A (H3N2) with sporadic isolations of influenza B viruses throughout the season. Influenza A (H1N1) viruses were isolated sporadically throughout the season and increased in frequency during February--March. From October 3 through April 22, World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System collaborating laboratories tested 88,429 specimens for respiratory viruses; 13,623 (15%) were positive for influenza. The percentage of respiratory specimens positive for influenza peaked at 33% during the week ending December 25 (week 51) (Figure 3). Of the specimens testing positive for influenza, 13,561 (99.5%) were influenza type A, and 62 (0.5%) were influenza type B. Of the 3742 influenza A viruses that were subtyped, 3622 (97%) were A (H3N2) viruses, and 120 (3%) were A (H1N1) viruses (Figure 3). The 1999--2000 influenza vaccine strains were well matched to the circulating influenza virus strains. CDC antigenically characterized 593 influenza viruses received from U.S. laboratories since October 1. Of the 484 influenza A (H3N2) viruses tested, 469 (97%) were similar to the vaccine strain A/Sydney/05/97, and 15 (3%) showed somewhat reduced hemagglutination inhibition (HI) titers to ferret antiserum produced against the A/Sydney/05/97 virus. Of the 81 A (H1N1) viruses antigenically characterized, one (1%) was similar to the vaccine strain A/Beijing/262/95, 54 (67%) were related more closely to the recent antigenic variant A/New Caledonia/20/99, and 26 (32%) were similar to the A/Bayern/07/95 virus (2). A/Beijing/262/95 and A/Bayern/07/95 are antigenically distinct viruses, but vaccines containing the A/Beijing/262/95 strain induce high titers of antibody that cross-react with A/Bayern/07/95-like viruses (2). All 28 of the influenza type B viruses antigenically characterized were similar to the recommended B/Beijing/184/93-like virus represented in the 1999--2000 vaccine by the B/Yamanashi/166/98 virus. WorldwideFrom October 3 through April 28, moderate to severe influenza outbreaks were reported in the Americas, Asia, and Europe. Influenza A (H3N2) viruses were associated with outbreaks in Africa (Tunisia), Asia (China, Hong Kong Special Administrative Region [SAR] of China, Iran, and Japan), Europe (Albania, Austria, Belarus, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Finland, France, Germany, Hungary, Iceland, Ireland, Israel, Italy, Latvia, Netherlands, Norway, Poland, Portugal, Romania, Russian Federation, Slovakia, Spain, Sweden, Switzerland, Ukraine, and United Kingdom), and North America (United States and Canada). Influenza A (H3N2) isolates from sporadic cases were reported from Argentina, Australia, Brazil, Republic of Cyprus, Egypt, Greece, Guam, French Guyana, India, Republic of Korea, Malaysia, Mauritius, Mexico, New Zealand, Peru, Philippines, Senegal, Singapore, South Africa, Syria, Taiwan, Thailand, Turkey, and the Federal Republic of Yugoslavia. Of the A (H3N2) isolates that were characterized antigenically, most were similar to the reference strains A/Moscow/10/99 (2) and A/Panama/2007/99 viruses. Many isolates also were closely related to the A/Sydney/05/97-like (H3N2) virus. Influenza A (H1N1) outbreaks were reported in the Hong Kong SAR of China, Japan, and Spain. Isolates of influenza A (H1N1) from sporadic cases were reported from Argentina, Australia, Belgium, Brazil, Canada, Chile, China, France, Germany, Iceland, Italy, Latvia, Philippines, Portugal, Russian Federation, Saudi Arabia, Singapore, South Africa, Spain, Thailand, United Kingdom, United States, and Vietnam. Of the A (H1N1) isolates that were characterized antigenically, most were similar to the A/New Caledonia/20/99 virus. Influenza B viruses circulated at low levels, and isolates from sporadic cases were reported from Argentina, Australia, Brazil, Canada, China, Croatia, Czech Republic, Egypt, Finland, France, Germany, Hong Kong SAR of China, Hungary, Iceland, Israel, Italy, Japan, Republic of Korea, Madagascar, Malaysia, New Caledonia, New Zealand, Norway, Philippines, Russian Federation, Singapore, Senegal, South Africa, Spain, Sweden, Syria, Taiwan, Thailand, Tunisia, United Kingdom, United States, Vietnam, and the Federal Republic of Yugoslavia. Of the influenza B isolates that were characterized antigenically, most were related to B/Beijing/184/93 and B/Yamanashi/166/98 viruses. Composition of the 2000--01 Influenza VaccineThe Food and Drug Administration's Vaccines and Related Biologic Products Advisory Committee (VRBPAC) recommended A/New Caledonia/20/99-like (H1N1), A/Panama/2007/99-like (H3N2), and B/Yamanashi/166/98-like viruses for the 2000--01 U.S. trivalent influenza vaccine.§ This recommendation was based on antigenic and molecular analyses of recently isolated influenza viruses, epidemiologic data, and postvaccination serologic studies in humans. Although A/Sydney/05/97-like (H3N2) viruses have predominated in the United States for the last three influenza seasons, an increasing proportion of antigenically characterized A (H3N2) isolates worldwide were more similar to the A/Moscow/10/99 and A/Panama/2007/99 reference strains, two antigenically equivalent viruses. Vaccination with the 1999--2000 A/Sydney/05/97-like (H3N2) strain stimulated HI antibodies that were lower in titer and frequency to some recent A (H3N2) isolates (2). Therefore, VRBPAC recommended changing the A (H3N2) vaccine strain to A/Panama/2007/99-like (H3N2) virus. Worldwide, most antigenically characterized influenza A(H1N1) virus isolates were similar to A/New Caledonia/20/99. Both A/New Caledonia/20/99 and A/Bayern/7/95 (A/Johnnesburg/82/96-like) (H1N1) viruses were isolated in the United States. The 1999-- 2000 vaccine contained an A/Beijing/262/95-like strain that induced a cross-reactive antibody response to A/Bayern/7/95-like viruses but induced lower titers of antibodies to A/New Caledonia/20/99-like strains (2). Therefore, VRBPAC recommended changing the A (H1N1) vaccine strain to A/New Caledonia/20/99-like (H1N1) virus. Influenza type B viruses were isolated sporadically in the United States and worldwide and were antigenically similar to the 1999--2000 vaccine strain B/Beijing/184/93 and to the widely used equivalent vaccine strain B/Yamanashi/166/98. Therefore, VRBPAC recommended retaining B/Beijing184/93-like virus for the 2000--01 vaccine. Manufacturers will use the B/Yamanashi/16/98 strain as the 2000--01 influenza B vaccine component because of its growth properties and antigenic similarity to B/Beijing184/93-like viruses. Reported by: Participating state and territorial epidemiologists and state public health laboratory directors. A Hay, PhD, World Health Organization (WHO) Collaborating Center for Reference and Research on Influenza, National Medical Institute for Medical Research, London, England. I Gust, MD, A Hampson, WHO Collaborating Center for Reference and Research on Influenza, Parkville, Australia. K Nerome, PhD, M Tashiro, MD, WHO Collaborating Center for Reference and Research on Influenza, National Institute of Infectious Diseases, Tokyo, Japan. L Canas, Armstrong Laboratory, Brooks Air Force Base, Texas. D Lavanchay, MD, Div of Emerging and Other Communicable Diseases Surveillance and Control, WHO National Influenza Centers, Geneva, Switzerland. National Respiratory Enteric Virus Surveillance System collaborating laboratories. WHO collaborating laboratories. Sentinel Physicians Influenza Surveillance System. R Levandowski, MD, Div of Virology, Center for Biologics Evaluation and Research, Food and Drug Administration. WHO Collaborating Center for Reference and Research on Influenza, Influenza Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; and an EIS Officer, CDC. Editorial Note:During the 1999--2000 influenza season, influenza A (H3N2) viruses predominated in the United States and worldwide. This was the third consecutive season that influenza A/Sydney/05/97-like (H3N2) viruses were the most frequently isolated influenza viruses in the United States. Typically, influenza seasons in which influenza A (H3N2) viruses predominated have been more severe than seasons in which influenza A (H1N1) and influenza B viruses predominated (4). This season's influenza activity was similar to the previous two influenza seasons as indicated by reports from state and territorial epidemiologists, the percentage of respiratory specimens positive for influenza, and the proportion of visits for ILI. This was the fifth consecutive year in which the percentage of deaths attributed to P&I reported by 122 U.S. cities was above projected epidemic thresholds for a prolonged period, and this season's peak was higher than the peaks of the previous four seasons. However, whether this season's unusually high percentage of P&I deaths resulted from influenza activity, other respiratory pathogens, or changes in the surveillance reporting case definition is unknown (1). Overall, the 1999--2000 influenza vaccine strains were well matched to the circulating influenza virus strains. Beginning with the 2000--01 influenza season, the Advisory Committee on Immunization Practices (ACIP) recommends that all persons aged >50 years receive annual influenza vaccination (5). This recommendation reduces the age for annual universal vaccination from 65 years to 50 years. The policy change was made to increase influenza vaccination among persons aged 50--64 years because a substantial proportion of persons in this age group (24%--32%) have chronic medical conditions that place them at high risk for influenza-related hospitalization and death (5). Vaccination levels of high-risk persons aged 50--64 years have been low, and age- based vaccination strategies have been more successful than risk-based vaccination strategies (5). No other changes have been made to the list of groups targeted for influenza vaccination. However, ACIP also recommended that persons planning large organized vaccination campaigns may consider scheduling these events after mid-October because the availability of vaccine in any location cannot be assured consistently in the early fall (5). Although influenza activity typically peaks during December--March in temperate regions of the Northern Hemisphere, sporadic isolated outbreaks and large outbreaks of influenza during summer months have occurred (6--8). In temperate regions of the Southern Hemisphere, influenza activity peaks during May--August. In tropical regions, influenza viruses may circulate year-round. During the past two summers, large outbreaks of respiratory disease attributed to influenza occurred among persons traveling in organized overland groups and among passengers on cruise ships in Alaska and the Yukon Territory (7,8). Influenza outbreaks aboard cruise ships also have been reported during other times of the year worldwide (9,10). Persons at high risk for complications of influenza who will be traveling in large tour groups this summer 1) should consider receiving influenza vaccine if not vaccinated during the preceding fall or winter; and 2) might wish to consult their physicians to discuss the symptoms and risks for influenza and the advisability of carrying antiviral medications for either prophylaxis or treatment of influenza. This is particularly important if the group includes travelers from areas where influenza viruses are circulating or if travel will be to the Southern Hemisphere or the tropics. Physicians who evaluate persons who have traveled to these regions should consider influenza in the differential diagnosis of febrile respiratory illness during the summer. Use of rapid diagnostic tests can facilitate a diagnosis of influenza; however, such tests have lower sensitivity than viral culture. Additional information about influenza, influenza vaccine, and influenza in travelers is available on the World-Wide Web at http://www.cdc.gov/ncidod/diseases/flu/fluvirus.htm. References
* Levels of activity are 1) no activity; 2) sporadic---sporadically occurring influenza-like illness (ILI) or culture-confirmed influenza with no outbreaks detected; 3) regional---outbreaks of ILI or culture-confirmed influenza in counties with a combined population of <50% of the state's total population; and 4) widespread---outbreaks of ILI or culture-confirmed influenza in counties with a combined population of >50% of the state's total population. † The epidemic threshold is 1.645 standard deviations above the seasonal baseline. The expected seasonal baseline is projected using a robust regression procedure in which a periodic regression model is applied to observed percentages of deaths from P&I since 1983. § The influenza A (H3N2) vaccine component recommended by WHO is an A/Moscow/10/99-like strain (3). The A/Panama/2007/99-like strain will be used by vaccine manufacturers in Europe and North America. Figure 1 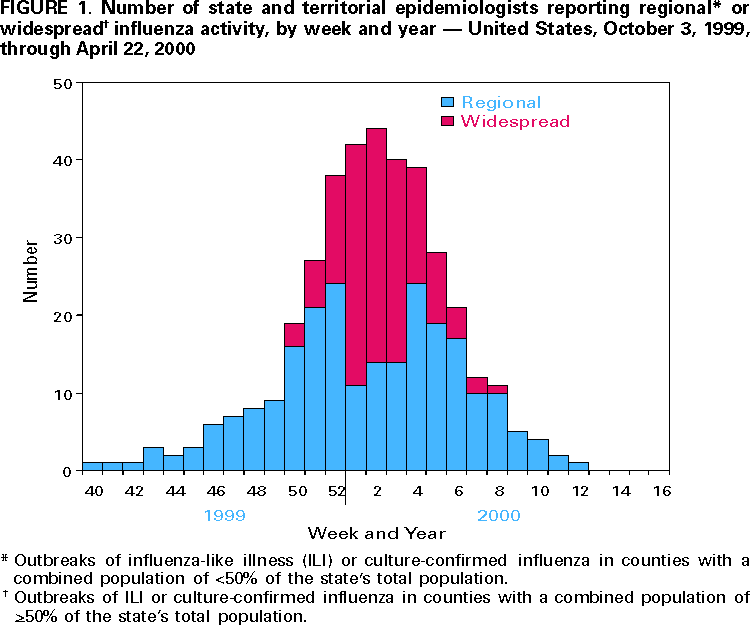 Return to top. Figure 2 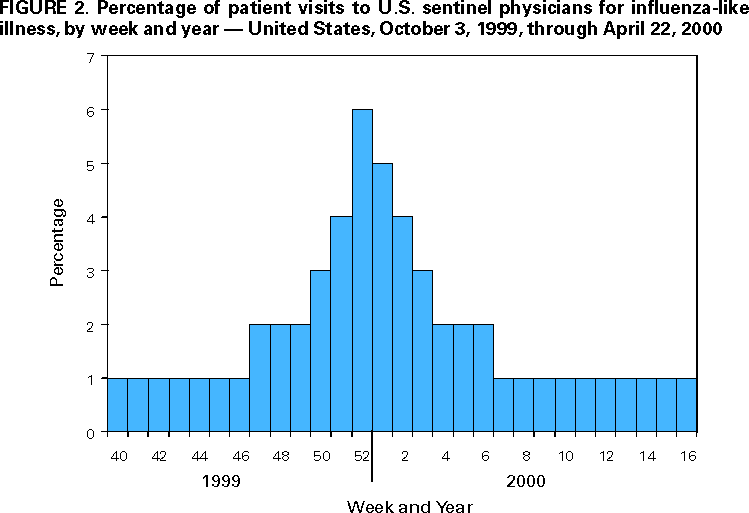 Return to top. Figure 3 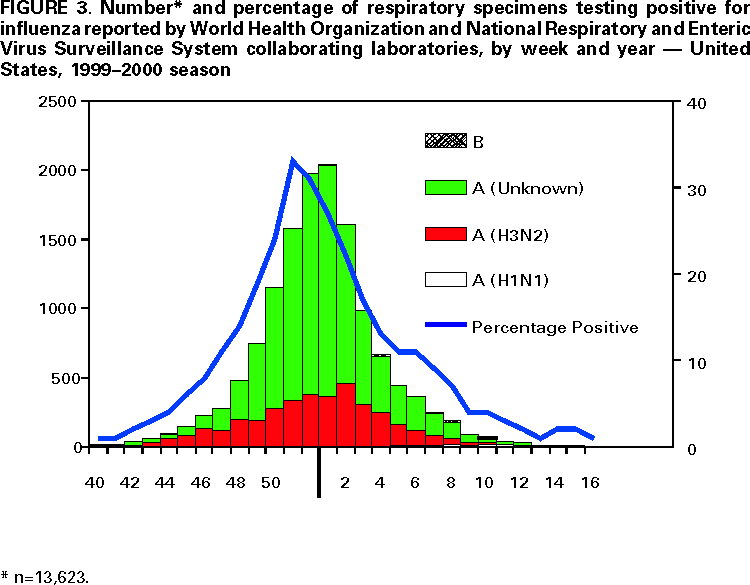 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 5/4/2000 |
|||||||||
This page last reviewed 5/2/01
|