 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Early-Onset Group B Streptococcal Disease --- United States, 1998--1999Despite recent declines, early-onset group B streptococcus (GBS) is a leading cause of neonatal sepsis, resulting in approximately 2200 infections each year among children aged <7 days in the United States (1). To identify opportunities for improved prevention, the Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network reviewed birth histories of infants with early-onset GBS disease. This report summarizes the results of this analysis and indicates that most mothers of infants with early-onset disease did not receive intrapartum antibiotics and that further declines in disease incidence are likely with better prevention efforts. To prevent perinatal GBS disease, two strategies are recommended: the risk-based and the screening-based approach (2--4). Under the risk-based approach, women in labor who have risk factors for GBS transmission (e.g., fever, prolonged rupture of the membranes, or preterm delivery) are offered intrapartum chemoprophylaxis. Under the screening-based approach, all pregnant women are tested for GBS carriage between 35--37 weeks' gestation by collecting vaginal and rectal combined swabs, and GBS carriers are offered intrapartum chemoprophylaxis. Birth histories of infants with early-onset GBS disease in 1998 and 1999 were evaluated to determine whether cases might have been prevented by either of these strategies. A case of early-onset GBS disease was defined as the isolation of group B streptococci from a normally sterile site from an infant aged <7 days born to a resident of the ABCs surveillance area (i.e., Connecticut, Maryland, Minnesota, and selected urban counties in California, Georgia, New York, Oregon, and Tennessee). To assess the quality of early-onset GBS disease intervention, surveillance staff reviewed prenatal GBS screening, risks for infection at the time of labor, receipt of intrapartum antibiotics, and infant outcome. In Connecticut, prenatal provider records also were reviewed. The incidence of early-onset disease was calculated using live birth data for 1997 from the National Center for Health Statistics. Surveillance reports indicated 190 cases of early-onset GBS disease in 1998 and 153 cases in 1999 (Table 1). Maternal labor and delivery records were available for 181 (96%) infants in 1998 and 141 (92%) infants in 1999. The case fatality ratio was 5%. In 1999, the incidence of disease was 0.7 per 1000 live births among black infants, 0.5 among Hispanic infants, and 0.3 among white infants. Prenatal GBS testing was documented in 104 (35%) of 322 women; 36 (35%) had a positive result (Table 2). Among the 82 women who had documented dates of screening and gestational age at delivery, 52 (63%) were screened after 33 weeks of pregnancy. GBS culture site was documented for 55 (53%) of 104 screened women; three women had vaginal and rectal combined swabs and 23 (22%) of 104 had vaginal swabs only. For 81 of 100 screened women, labor and delivery staff had access to GBS test results. Intrapartum antibiotics were administered to 68 (21%) of 322 women who had infants with early-onset disease. Fifty-one (40%) of 128 women with a positive screen or at least one risk factor and no GBS test results received prophylactic antibiotics (Table 2). Thirty-eight (56%) of 68 women treated had fever, the most common indication among women receiving chemoprophylaxis; 15 (39%) of 38 women with fever received an initial dose of antibiotics after presenting with fever. Intravenous ampicillin, clindamycin, and penicillin were the most commonly administered antibiotics (31%, 15%, and 14% respectively). The median time between admission and delivery among women receiving antibiotics was 12.5 hours (range: 0--846 hours), and the median time between administration of the first antibiotic dose and delivery was 3.5 hours (range: 0--299 hours). Twenty-three (34%) of 68 women first received antibiotics within 2 hours of delivery; 40 (59%) received one dose. Early-onset isolates were evaluated for antibiotic susceptibilities to penicillin, clindamycin, erythromycin, and cephalothin or cefazolin from 164 patients in Georgia, Maryland, Minnesota, and Oregon. All isolates were susceptible to penicillin; 32 (20%) isolates were resistant to erythromycin and 25 (15%) were resistant to clindamycin. Minimum inhibitory concentrations for cefazolin were <0.25 µg/mL. Reported by: K White, MPH, J Rainbow, MPH, S Johonson, B Juni, MS, J Besser, MS, C Olson, MD, R Lynfield, MD, R Danila, MD, Acting State Epidemiologist, Emerging Infections Program, Minnesota Dept of Health. G Rothrock, MPH, P Daily, MPH, Emerging Infections Program; A Reingold, MD, Emerging Infections Program and School of Public Health, Univ of California, Berkeley; D Vujia, MD, Div of Communicable Disease Control, California State Dept of Health Svcs. A Roome, PhD, H Linardos, MPH, J Hadler, MD, State Epidemiologist, Emerging Infections Program, Connecticut Dept of Health. W Baughman, MSPH, P Martell-Cleary, MSW, M Farley, MD, Emerging Infections Program, Veterans Administration Medical Center, Emory Univ School of Medicine, Atlanta, Georgia; P Blake, MD, Acting State Epidemiologist, Georgia Dept of Human Resources. M Pass, MS, L Harrison, MD, Emerging Infections Program, Johns Hopkins Univ School of Hygiene and Public Health, Baltimore, Maryland. J Roche, MD, Acting State Epidemiologist, Maryland Dept of Health and Mental Hygiene. B Damaske, S Zansky, PhD, NM Bennett, MD, Emerging Infections Program, New York Dept of Health; P Smith, MD, State Epidemiologist, New York Dept of Health; M Dragoon, MPH, J Donegan, Multnomah County Health Dept. M Cassidy, MT(ASCP)SM, K Stefonek, MPH, P Cieslak, MD, Emerging Infections Program, Dept of Human Svcs--Health Div, Portland, Oregon; M Kohn, MD, State Epidemiologist, Dept of Human Services--Health Div, Portland, Oregon. B Barnes, L Lefkowitz, MD, Emerging Infections Program and Vanderbilt Univ School of Medicine; A Craig, MD, W Moore, MD, State Epidemiologist, Tennessee Dept of Health. Respiratory Diseases Br, Div of Bacterial and Mycotic Diseases, the Active Bacterial Core Surveillance/Emerging Infections Program Network, National Center for Infectious Diseases; and an EIS Officer, CDC. Editorial Note:During 1993--1998, the incidence of GBS disease in the United States declined 65% (1). In this report, data from 1998--1999 indicated that the incidence of early-onset GBS disease further declined in the surveillance areas (Table 1); however, the data also indicated several areas where prevention could be improved. In 1998 and 1999, of mothers of infants with early-onset disease, 21% received intrapartum antibiotic prophylaxis. Prenatal screening often was not performed at the recommended time (35--37 weeks' gestation), and combined vaginal and rectal swabs were rarely documented (5). Approximately 70% of women who were unscreened and developed a risk factor did not receive intrapartum antibiotics. Many women were unscreened and did not present with risk factors at the time of labor. This suggests that some early-onset disease may have been prevented if the screening-based approach (versus the risk-based approach) had been used. Other women did not receive antibiotics until after they developed fever, suggesting that cases might have been prevented if antibiotics could have been administered earlier in the hospital admission (e.g., one dose of penicillin or ampicillin at least 4 hours before delivery). The findings in this report are subject to at least three limitations. First, early-onset GBS surveillance is limited to confirmed cases of invasive disease; therefore, generalizations cannot be made from the reported data about compliance with recommended guidelines or the effectiveness of intrapartum chemoprophylaxis. Second, because information was not available on whether health-care providers were attempting to follow the risk-based approach or the screening-based approach in specific cases, it is not possible in all instances to assess whether early-onset cases represented missed opportunities for prevention, protocol omissions, or antibiotic failures. Third, some information (e.g., GBS screening culture site) was poorly documented or missing from medical charts. Increased prevention of perinatal GBS disease has raised concern about potential adverse consequences of the increased use of intrapartum antibiotics. Penicillin resistance among GBS isolates has not been reported (6); however, erythromycin and clindamycin resistance has increased (6,7) and has occurred in 15--20% of early-onset cases for which isolates were available. For women with a history of severe penicillin allergy, clinicians should request that prenatal GBS screening include susceptibility testing of GBS isolates to determine an appropriate regimen for intrapartum prophylaxis. Cefazolin should be considered when erythromycin or clindamycin resistance occurs among women with penicillin allergy (8). This study indicates that further declines in disease incidence are likely with full implementation of the consensus guidelines for prevention of early-onset group B streptococcal GBS disease (2--4). Copies of the guidelines and educational materials for prenatal patients are available on the World-Wide Web, http://www.cdc.gov/ncidod/dbmd/gbs; copies may be obtained from CDC, Health Communications Activity (GBS information), Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, mailstop A-49, 1600 Clifton Rd., NE, Atlanta, GA 30333; after December 1, 2000, bulk orders may be obtained from Public Health Foundation, 1220 L St, NW, Suite 350, Washington, DC 20005, telephone (877) 252-1200, or by e-mail, www.phf.org. References
Table 1 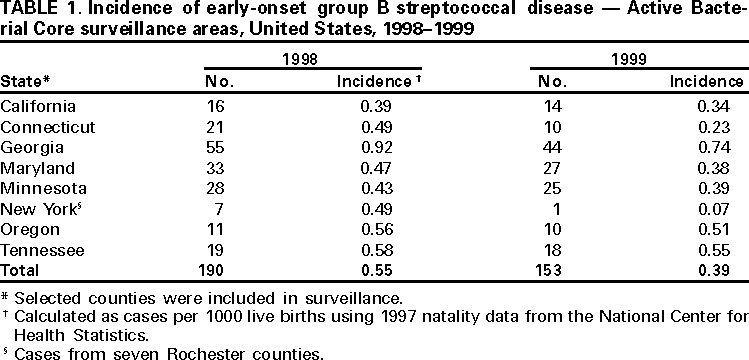 Return to top. Table 2 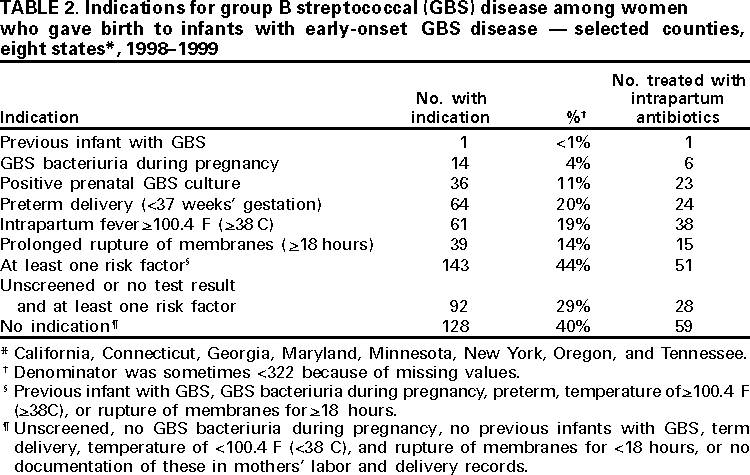 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 9/7/2000 |
|||||||||
This page last reviewed 5/2/01
|