 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication --- Ethiopia, 1997--August 2000In 1988, the World Health Assembly resolved to eradicate poliomyelitis globally by 2000 (1). Following the signing of the Yaounde Declaration on Polio Eradication in Africa in 1996, Ethiopia joined global efforts toward polio eradication (2). Since then, Ethiopia has accelerated implementation of polio eradication strategies. This report summarizes progress toward polio eradication in Ethiopia during 1997--August 2000 and highlights the remaining challenges toward achieving the goal. Routine Vaccination CoverageDuring 1990--1999, reported coverage of children aged 0--11 months with 3 doses of oral poliovirus vaccine (OPV3) ranged from 20%--90%. The last comprehensive coverage survey conducted in 1995 estimated OPV3 coverage at 36%. Preliminary data from the 2000 Ethiopia Demographic and Health Survey estimates average OPV3 coverage at 35%. Supplemental Vaccination ActivitiesIn 1996, Ethiopia conducted Subnational Immunization Days* (SNIDS) for the first time, targeting 2.5 million children aged <5 years in nine major cities. Since then, the country has conducted two rounds of National Immunization Days† (NIDs) annually. Implementation of NIDs during 1997--1999 and SNIDs in 2000 has reached >90% of the target population (Table 1), including areas with limited access to routine health services. In 1999, intensified campaigns with delivery of vaccine house-to-house were conducted in three regions (Afar, Benshangul, and Somali) that had performed poorly in previous years. As a result, 541,996 more children were reached in these regions compared with 1998 NIDs, which used only fixed-site vaccinations. Despite improvements in vaccine delivery, pockets of unvaccinated children remain. During the 2000 house-to-house SNIDs, efforts were made to detect the proportion of children never vaccinated by routine services or during NIDs. Of children vaccinated during the 2000 SNIDs, an average of 25% (range: 1%--100%) had never received vaccine. Acute Flaccid Paralysis SurveillanceSurveillance for acute flaccid paralysis (AFP) was initiated in 1997. During 1997--August 2000, the nonpolio AFP rate increased from 0.10 to 0.44 per 100,000 children aged <15 years (a sensitive system is defined as >1 per 100,000 children aged <15 years) (Table 2). Surveillance performance among the 11 regions of Ethiopia varies substantially. Wild poliovirus isolates have been isolated in zones (subregional administrative units) where AFP surveillance is improving and reached nonpolio AFP levels >0.5. However, only 26 of Ethiopia's 71 zones have achieved this level; the more densely populated zones in central Ethiopia have nonpolio AFP rates <0.5, and 25 zones have not reported any AFP cases during 2000. These 25 zones also have very low (<20%) routine OPV3 coverage. The proportion of adequate stool specimens from AFP case-patients (i.e., two stool specimens collected at an interval of at least 24 hours within 14 days of onset of paralysis and adequately shipped to the laboratory) has improved from 12% in 1998 to 44% in 2000 (Figure 1). All stool specimens routinely are split and tested in both the Ethiopia Health and Nutrition Research Institute (EHNRI) polio laboratory and the World Health Organization (WHO) accredited national laboratory in Uganda. The EHNRI polio labora tory is expected to attain WHO accreditation status by the end of 2000. Incidence of PolioUntil March 2000, AFP surveillance had not detected wild poliovirus in Ethiopia. In March 2000, the Johannesburg polio reference laboratory confirmed isolation of the first wild poliovirus type 1 (P1) in an AFP case from Oromia region with onset of paralysis in October 1999. A second isolate was reported in August 2000, with paralysis onset in March 2000. Neither of these virologically confirmed polio case-patients had received any doses of OPV. Genetic sequencing of polioviruses isolated from these cases revealed that they were indigenous to Ethiopia and unlike those polioviruses isolated in bordering countries. Reported by: Ministry of Health, Addis Ababa, Ethiopia. Vaccine Preventable Diseases Unit, Regional Office for Africa, World Health Organization, Hrare, Zimbabwe. Dept of Vaccines and Biologicals, World Health Organization, Geneva, Switzerland. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial Note:Rapid progress has been achieved in implementing polio eradication strategies in Ethiopia, one of the major polio reservoirs in the Africa Region (3). Recent improvements in AFP surveillance led to the detection of indigenous wild poliovirus transmission. In addition, the number of children reached by NIDs and SNIDs has increased annually. The house-to-house approach in parts of the country during 1999 and 2000 resulted in increased coverage of children aged <5 years, especially in hard-to-reach areas. Routine vaccination activities have been constrained by challenges related to program management, training, health sector reform, cold chain maintenance, a largely rural population, and difficult terrain. Low routine OPV3 coverage, suboptimal AFP surveillance, and indigenous wild poliovirus transmission underscore the need for continued high quality NIDs and extra SNIDs. House-to-house vaccination activities should continue to reach children residing in hard-to-reach areas who have never been vaccinated. A WHO-United Nations Children's Fund (UNICEF) technical review identified the need for an increased number of mid-level surveillance officers to assist in training, clinician sensitization, and supervision of active AFP surveillance in remote areas. The placement of mid-level surveillance officers in other countries has led to rapid improvement in AFP surveillance indicators. Polio eradication priorities in Ethiopia include 1) implementing high-quality NIDs (planned for November and December 2000 and tentatively planned for 2001), 2) ensuring high-quality house-to-house vaccination campaigns in hard-to-reach areas, 3) strengthening routine vaccination, 4) strengthening facility-based active AFP surveillance to reach certification standards (nonpolio AFP rate of >1.0) in all zones, 5) supporting the national laboratory to attain WHO accreditation, and 6) coordinating cross-border vaccination and surveillance activities to detect possible importation of wild poliovirus from neighboring countries. Meeting these challenges will require the continued support of polio eradication partners.§ References
*Focal mass campaigns in high-risk areas over a short period (days to weeks) in which two doses of OPV are administered to all children, usually aged <5 years, regardless of vaccination history, with an interval of 4--6 weeks between doses. † Mass campaigns over a short period (days to weeks) in which two doses of OPV are administered to all children, usually aged <5 years, regardless of vaccination history, with an interval of 4--6 weeks between doses. § Polio eradication efforts in Ethiopia are supported by WHO, UNICEF, Rotary International, U.S. Agency for International Development, the Japanese International Cooperation Agency, the United Kingdom Department of Foreign and International Development, and CDC. Table 1 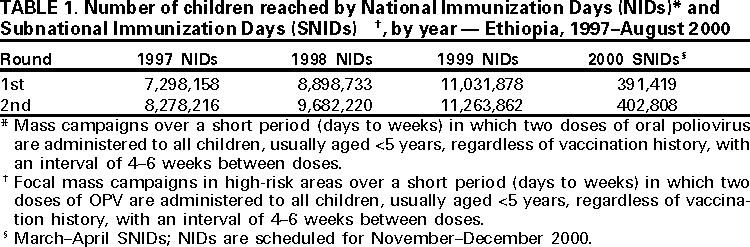 Return to top. Figure 1 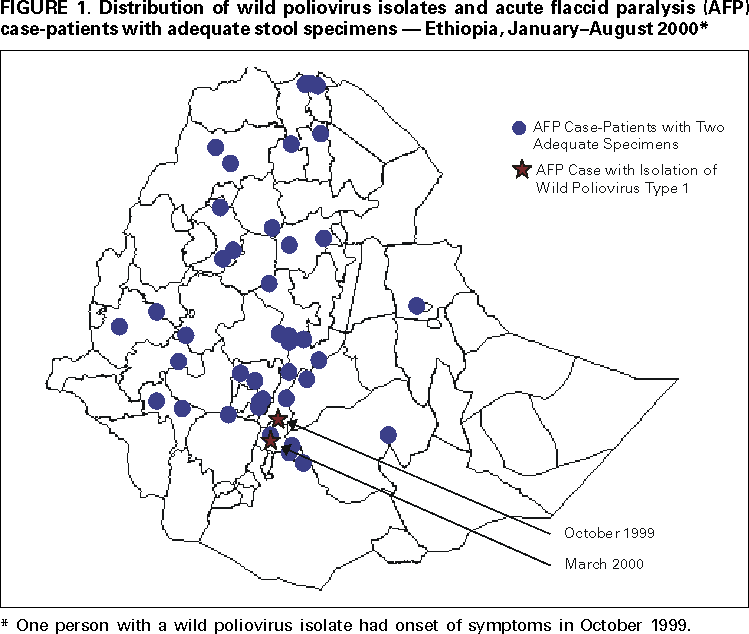 Return to top. Table 2 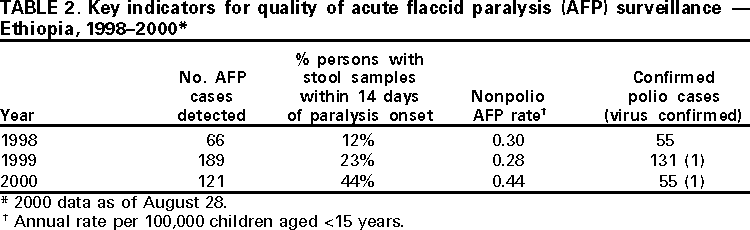 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 9/28/2000 |
|||||||||
This page last reviewed 5/2/01
|