 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress in Development of Immunization Registries ---United States, 2000Immunization registries are confidential, population-based, computerized information systems that attempt to collect vaccination data about all children within a geographic area (1). Registries are an important tool to increase and sustain high vaccination coverage by consolidating vaccination records of children from multiple providers, generating reminder and recall vaccination notices for each child, and providing official vaccination forms and vaccination coverage assessments. One of the national health objectives for 2010 is to increase to 95% the proportion of children aged <6 years who participate in fully operational population-based immunization registries (objective 14.26) (2). To assess the status of immunization registry development, CDC analyzed self-reported data from 62 immunization grantees on the basis of data from the 2000 Immunization Registry Annual Report (IRAR). This report summarizes the results of this analysis, which indicate that approximately half of the grantees are operating population-based immunization registries that target their entire catchment areas; however, approximately 75% of children aged <6 years still need to be included in an immunization registry to reach the national health objective. The 2000 IRAR was a self-administered questionnaire distributed to immunization program managers or immunization registry managers that requested information on the enrollment status of a registry's target population and the implementation of 13 functional standards (Table 1) considered essential for immunization registry operation (3). Key elements for each of the 13 standards were defined by the Immunization Registry Technical Working Group (IRTWG) and are used to measure registry development. The 2000 IRAR also collected data on provider participation and other electronic information systems that shared data with the registry. In April 2000, CDC's 64 immunization grantees (50 states; the District of Columbia; Chicago, Illinois; Houston, Texas; New York, New York; Philadelphia, Pennsylvania; San Antonio, Texas; American Samoa; Guam; Marshall Islands; Micronesia; Northern Mariana Islands; Puerto Rico; Republic of Palau; and the U.S. Virgin Islands) were asked to complete the 2000 IRAR; 62 (97%) responded. Thirty-two (52%) of the 62 grantees (26 states, four cities, and two territories/commonwealths) reported operating population-based immunization registries that targeted their entire catchment areas. Of the remaining 30 (48%) grantees, seven operated population-based registries in regions or counties as demonstrations or pilot projects, and 23 were planning to develop population-based registries. Data from 31 of the 32 grantees operating population-based registries indicated that approximately 46% of the estimated 10.4 million target children aged <6 years in these catchment areas had received at least two doses of vaccine. The two doses typically included one vaccine dose in addition to the dose of hepatitis B vaccine given at birth and recorded in a population-based registry's database (Figure 1). The 32 grantees also reported that an average of 74% of public vaccination provider sites and 44% of private provider sites participated in a population-based registry during the 6 months preceding completion of the 2000 IRAR. All 32 grantees implemented at least one key element on nine of the 13 functional standards (Table 1). Six (19%) of the 32 grantees reported implementing at least one key element in each standard. However, none had implemented fully all key elements of the 13 functional standards. Thirty-one of the 32 grantees reported electronic linkages (sending and/or receiving electronic data) between immunization registries and at least one other information system. Of these, 28 were linked electronically to their vital records department (Figure 2). Reported by: Systems Development Br, Data Management Div, National Immunization Program, CDC. Editorial Note:The findings in this report indicate that an estimated 21% of children aged <6 years have their immunization histories included in a population-based immunization registry. Four major issues may limit registry participation and development: protecting the privacy of persons and the confidentiality of registry information, ensuring provider participation, overcoming technical and operational challenges, and determining resources needed to develop and maintain immunization registries (1). To protect the privacy of patients, providers, and other participants of these systems, CDC developed privacy specifications and implementation guidelines in 2000 (4). Ensuring provider participation in registries is critical to attaining complete and accurate electronic immunization records. By age 2 years, approximately 23% of children have seen more than one immunization provider (5). When most or all immunization providers in a registry's catchment area participate in a registry, scattered records can be consolidated and appropriate vaccination decisions can be made based on accurate and complete information. Data from San Bernardino, California, indicate that in 1999, approximately 2000 children received at least one unneeded dose of vaccine because of incomplete immunization records (San Bernardino Department of Public Health, unpublished data, 2000). A national survey in 1997 indicated that an estimated $26.5 million could have been saved by avoiding unneeded doses (6). Because registry development initially was targeted at the public sector, the proportion of public vaccination provider sites participating in registries is considerably higher than that of private provider sites. Increasing private provider recruitment efforts will be critical as immunization services continue to shift to the private sector (7). CDC and IRTWG are finalizing criteria to measure the progress being made toward achieving the national health objective for 2010 (2). Progress toward reaching these criteria will be evaluated through annual National Immunization Program on-site visits, and recommendations and feedback will be provided. Although developing and operating immunization registries can be expensive (CDC, unpublished data, 2000), a fully operational population-based registry offsets many other costs by avoiding duplicate immunizations, limiting the cost of missed appointments through the use of reminder/recall notices, reducing vaccine waste, and reducing the staff time required to find and/or produce immunization records or certificates. Registries also can play an important role in assisting vaccine safety efforts and can be used for vaccine ordering, inventory control, and vaccine use monitoring. The findings in this report are subject to at least two limitations. First, because IRAR 2000 relied on self-reported information, some bias is expected. On-site verifications of these data are being conducted. Second, because only immunization grantees were surveyed, these data underestimate the degree of registry activity in the United States. Survey respondents reported an additional 22 population-based registries operating in local communities. Additional information on immunization registries is available from CDC's immunization registry World-Wide Web site, http://www.cdc.gov/nip/registry; by telephone, (800) 799-7062; or e-mail, [email protected]. References
Table 1 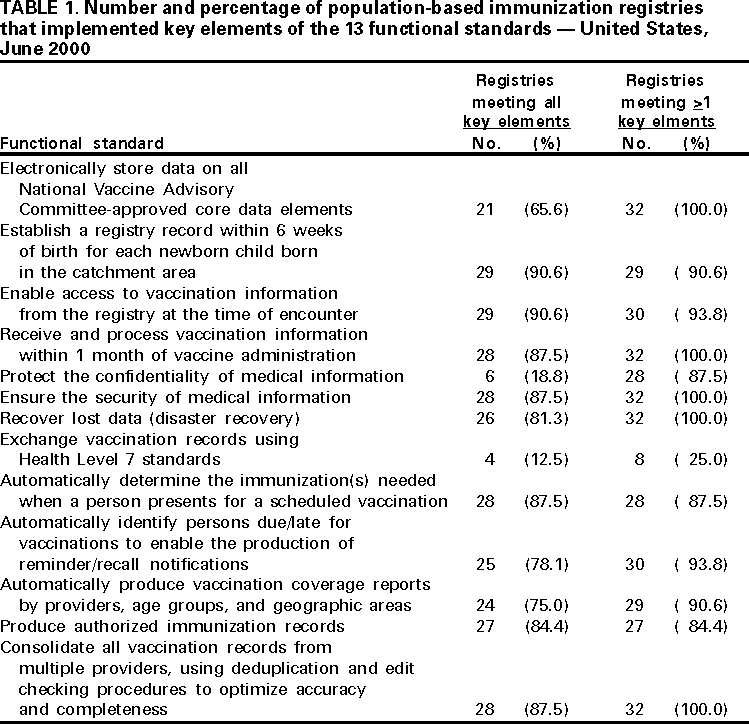 Return to top. Figure 1 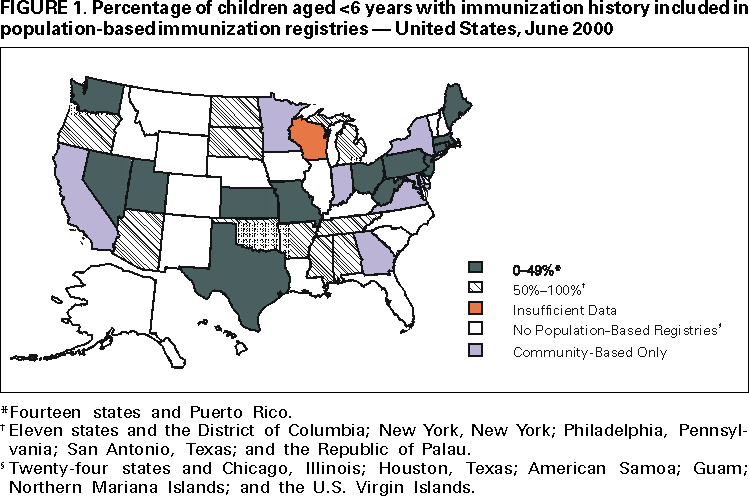 Return to top. Figure 2 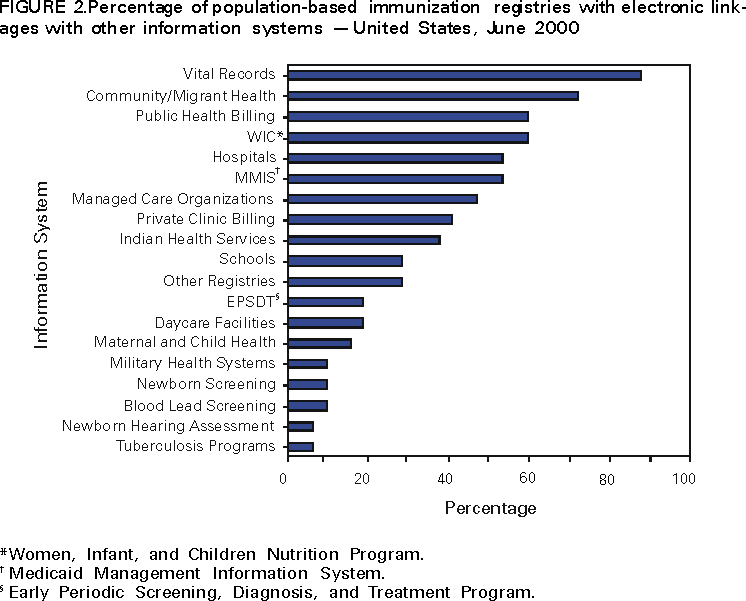 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 1/10/2001 |
|||||||||
This page last reviewed 5/2/01
|