 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevalence of Hepatitis C Virus Infection Among Clients of HIV Counseling and Testing Sites --- Connecticut, 1999Hepatitis C virus (HCV) is a common chronic bloodborne virus infection that affects an estimated 2.7 million persons in the United States (1,2). HCV infection causes an estimated 8,000--10,000 deaths each year from cirrhosis and hepatocellular carcinoma and is the leading reason for liver transplantation. Because injection drug use is a major risk factor for both human immunodeficiency virus (HIV) and HCV transmission, publicly funded HIV counseling and testing sites (HIV CTS) may have a role in HCV prevention (3,4). To evaluate the need for HCV services at these sites, the Connecticut Department of Public Health (CDPH) conducted an anonymous HCV seroprevalence study among clients of HIV CTS. This report summarizes the results of this analysis, which indicate that, among clients of these HIV CTS, the prevalence of antibody to HCV (anti-HCV) was 9.8%, compared with 1.3% for HIV, with significantly higher prevalence among clients of substance abuse treatment sites (40.2%), compared with other sites (6.9%). HCV counseling and testing should be integrated into all HIV CTS, especially those associated with substance abuse treatment. CDPH supports HIV CTS in various public health settings: 12 sites in local health departments, 12 in sexually transmitted disease clinics, 10 in community health centers, and four in family planning clinics. CDPH also supports HIV counseling and testing services for their enrolled clients in 24 substance abuse treatment programs. In all sites, blood specimens are sent to the CDPH virology laboratory for HIV testing. Blood specimens submitted for HIV testing from HIV CTS over 60 days during April--October 1999 were tested for anti-HCV using an enzyme immunoassay (EIA 2.0, Abbot Laboratories, Abbott Park, Illinois); repeatedly reactive specimens were confirmed by recombinant immunoblot assay (RIBA™ Chiron Corporation, Emeryville, California). Results were linked to information collected as part of HIV counseling, including demographics, HIV infection risk, site of service, and history of previous HIV testing. Clients who were tested for HIV using oral fluid or blood collected on filter paper were not included in the study. Multivariate analysis was performed using the Proc Logistic function of SAS. CDPH's Human Investigations Committee approved this project. Of 2801 specimens submitted for HIV testing during the study period, 2133 (76.2%) peripheral venous blood samples were tested for anti-HCV. Of these, 210 (9.8%) were confirmed positive for anti-HCV, 27 (1.3%) for HIV, and seven (0.3%) for both HCV and HIV. Risk factor data were missing for 87 samples (four were anti-HCV positive), and were excluded from further analysis. Among 1852 persons tested at HIV CTS not associated with substance abuse treatment, 128 (6.9%) had specimens positive for anti-HCV (Table 1), compared with 78 (40.2%) of 194 persons tested at HIV CTS associated with substance abuse treatment (Table 2). Among persons tested at HIV CTS not associated with substance abuse treatment (Table 1), the prevalence of HCV infection was highest (65.3%) among injection drug users (IDUs) (i.e., persons reporting that they had self-injected or received an injection with a needle of a nonprescription drug or substance since 1978). IDUs composed 5.5% of persons tested and accounted for 51.6% of HCV-infected persons in these settings. Among non-IDUs, those aged >40 years had the highest prevalence of HCV infection (9.2%). HCV infection among clients of these sites was associated independently with injection drug use, previous HIV testing, older age, not graduating from high school, and low income (<$10,000 per year). No significant association was found between HCV infection and race/ethnicity, sex, or HIV status. Among persons tested in HIV CTS associated with substance abuse treatment, the prevalence of HCV infection was highest among IDUs (67.8%). Non-IDUs in substance abuse treatment, many with a history of polysubstance abuse, including alcohol, still had a substantially higher HCV infection rate (16.3%) than expected in the general population (2), especially among those aged >40 years (36.0%). HCV infection among these clients was associated independently only with IDUs and older age groups. Reported by: R Melchreit, MD, R Baume, PhD, AIDS Div; K Carley, DrPH, A Roome, PhD, J Hadler, MD, Infectious Diseases Div; C Brinkman, D Vance, PhD, D Mayo, PhD, Bur of Laboratories, Connecticut Dept of Public Health; H Birden, MPH, New Britain Health Dept, New Britain, Connecticut. National Center for HIV, STD, and TB Prevention; Div of Viral Hepatitis (proposed), National Center for Infectious Diseases, CDC. Editorial Note:The findings in this report indicate that HIV CTS may be important settings to identify persons with risk factors for HCV. More persons seeking services in these programs in Connecticut had HCV infection than HIV infection. The high prevalence of HCV infection among both IDUs and non-IDUs, especially those aged >40 years, seeking HIV testing in HIV CTS associated with substance abuse treatment indicate that HCV counseling and testing should be offered to all clients, regardless of their risk factors. The high rate of HCV infection among non-IDUs could represent an undisclosed history of injection drug use or use only before 1978. Infections in this group may be the result of known risk factors that were not ascertained. A similar finding was observed in a cross-sectional study of persons tested for HCV in drug treatment centers in seven U.S. cities during 1993--1994 (CDC, unpublished data, 2000). The prevalence of HCV among persons seeking HIV testing in HIV CTS not associated with substance abuse treatment in Connecticut indicates that testing in this setting primarily be guided by a history of risk factors. Among non-IDUs seeking HIV testing in these settings, older age or history of HIV testing may be useful indicators of whether some non-IDUs might benefit from HCV counseling and testing. However, indicators such as age and previous HIV testing might vary across the country and require further study. The findings in this report are subject to at least three limitations. First, because information collected on persons from whom blood samples were taken was based on HIV risk factors, use of injection drugs only after 1978 was considered. Injection drug use before 1978 probably is a risk factor for HCV infection. Second, other potential risk factors (e.g., receipt of a blood transfusion before 1992) were not ascertained. Finally, persons seeking HIV counseling and testing in publicly funded sites in Connecticut may not be representative of persons seeking such services in other states. The rate of HCV infection among IDUs may vary by population and geographic area (4--7). CDC recommends identifying persons at increased risk for HCV infection to provide them with the opportunity for counseling and testing to determine their infection status, for medical evaluation to determine their disease status if infected, and for antiviral therapy if appropriate. Identification of infected persons also provides them with the opportunity to obtain information about preventing further hepatic injury (e.g., not drinking alcohol and getting vaccinated for hepatitis A and B), preventing HCV transmission, and reducing their risk for infection with HIV and hepatitis B virus (HBV). This study documents the potential for integrating services to prevent major bloodborne and sexually transmitted virus infections into existing public HIV CTS. Risk factors for transmission of these viruses are shared by populations seeking public health services in such sites. Offering HCV counseling and testing as part of existing programs may attract new clients primarily interested in hepatitis screening but who also are at risk for and might accept prevention services for HIV. In addition, HIV CTS can provide hepatitis B vaccination to persons at increased risk for HBV infection (8). Because of the well-established infrastructure for HIV counseling and testing in public health programs, expanding these services to include prevention of HCV and HBV infection should be feasible. Health-care providers in HIV CTS should be trained to screen actively for risk factors for HIV, HBV, and HCV and to offer prevention education, counseling, and hepatitis B vaccine to clients with risk factors. In substance abuse treatment settings, data from Connecticut indicate that counseling and testing for HIV and HCV should be provided to all clients. References
Table 1 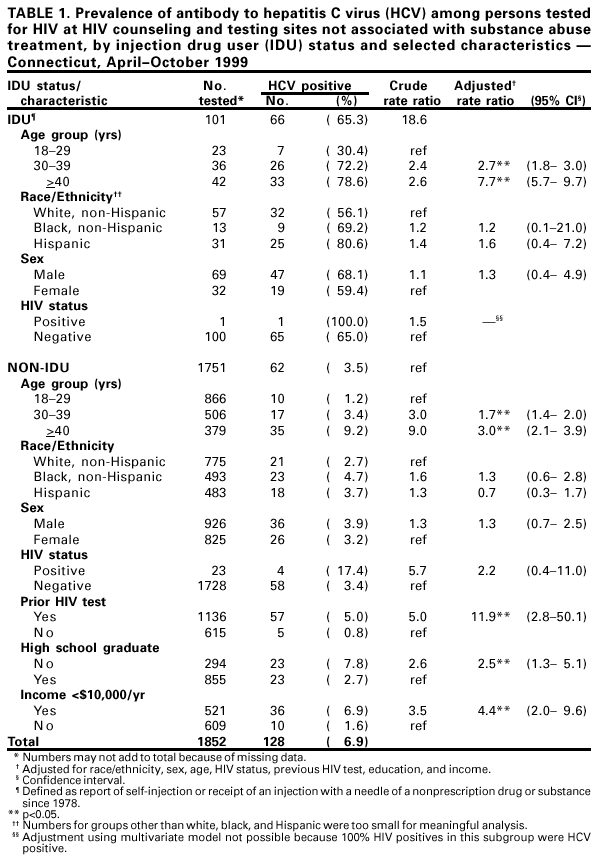 Return to top. Table 2 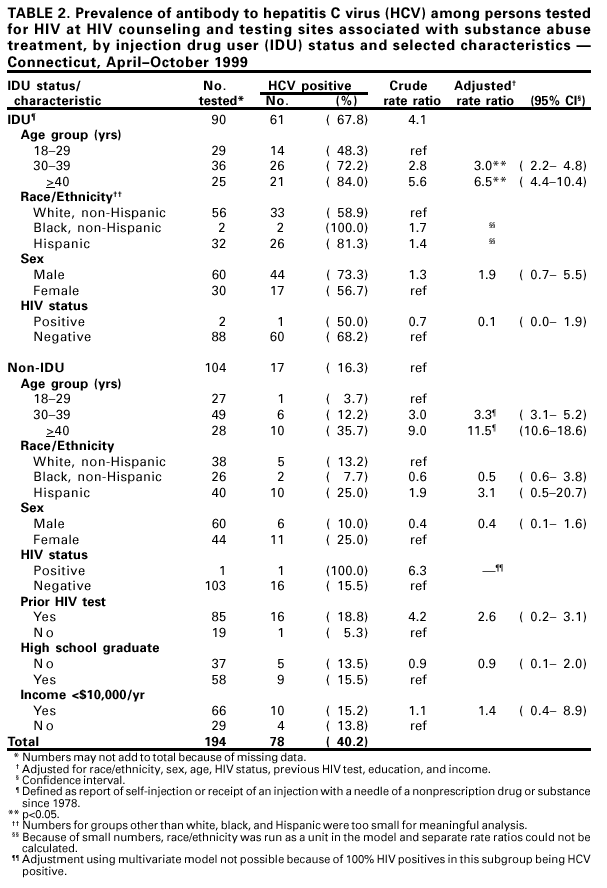 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 7/13/2001 |
|||||||||
This page last reviewed 7/13/2001
|