 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Simultaneous Administration of Varicella Vaccine and Other Recommended Childhood Vaccines --- United States, 1995--1999Live attenuated varicella vaccine (Var) is recommended in the United States for children aged 12--18 months and for susceptible older children, adolescents, and adults (1). The Advisory Committee on Immunization Practices recommends that Var be administered either simultaneously with measles-mumps-rubella (MMR) vaccine or separately by >30 days (1). This report summarizes an evaluation of these recommendations, which found that a decrease in Var effectiveness occurred when Var was administered <30 days after MMR; therefore, as currently recommended, physicians should administer Var simultaneously with MMR or wait at least 30 days if the vaccines are administered separately. Using the Vaccine Safety Datalink (VSD) project, the effectiveness of Var was assessed when administered simultaneously with or within 30 days of administering MMR; diphtheria and tetanus toxoids and pertussis vaccine (DTP); Haemophilus influenzae type B vaccine (Hib); oral poliovirus vaccine (OPV); inactivated poliovirus vaccine (IPV); and hepatitis B vaccine (HepB). VSD links computerized vaccination records to clinic and hospital discharge records of children from several large health maintenance organizations (HMOs) in the United States (2). VSD has expanded from four to seven HMOs and includes an estimated 2.5% of the U.S. population. A retrospective cohort study was conducted among children from the two HMOs in the VSD project with the earliest available automated clinic data and the highest uptake of Var. Children included in the study cohort were those who received Var at age >12 months during January 1995--December 1999 at HMO A and during January 1996--December 1999 at HMO B. The effectiveness (or failure) of Var can be measured by the proportion of vaccinated children who develop varicella breakthrough infections (i.e., cases of varicella that occur following exposure to wild-type virus) >42 days after Var; each recommended vaccine was compared with the incidence of breakthrough varicella in children who received Var simultaneously with the vaccine, children who received Var <30 days after the vaccine, and control children who received Var >30 days before or after the vaccine. To identify breakthrough disease, clinic and hospital discharge records from both HMOs were screened for having the same International Classification of Diseases, Ninth Revision, (ICD-9) codes* for varicella. Automated telephone contact records available at HMO B also were screened for reports of varicella. Cox proportional hazards models were used to estimate the relative risks (RRs) for breakthrough disease between children receiving Var and other recommended childhood vaccines at different intervals, group-matched on year of birth, year and month of vaccination, and HMO membership. A cohort was identified of 104,192 children vaccinated with Var from HMO A and 10,482 from HMO B. The median age of children receiving Var was 15 months (range: 12--71 months). The median follow-up time after Var was administered was 20 months (range: 1 day--4.5 years). The number of children aged >12 months receiving other vaccines simultaneously with Var, receiving Var before 30 days following other vaccines, and receiving Var >30 days before or after other vaccines also were identified (Table 1). The median age and age range were not available for vaccines other than Var. The simultaneous administration with Var of the vaccines studied did not increase the risk for breakthrough disease (Table 2). Receipt of Var <30 days following MMR was associated with a 2.5-fold increase in the incidence of breakthrough disease (95% confidence interval [CI]=1.3--4.9). Receipt of Var <30 days following any of the other vaccines did not increase the risk for breakthrough disease. Reported by: J Mullooly, PhD, Northwest Kaiser Permanente, Portland, Oregon. S Black, MD, Northern California Kaiser Permanente, Oakland and San Francisco, California. Child Vaccine Preventable Disease Br and Vaccine Safety and Development Activity, Epidemiology and Surveillance Div, National Immunization Program; and an EIS Officer, CDC. Editorial Note:No adverse effects have been reported of simultaneous administration of DTP, Hib, MMR, and OPV on the immunogenicity of Var (3--6), and the absence of increased risk for breakthrough varicella among children receiving MMR, DTP, Hib, OPV, IPV or HepB simultaneously with Var confirms these findings. Recommendations that caution against the use of Var and MMR within 30 days of each other (1) are based on the reported reduction in responsiveness to smallpox vaccine following measles vaccine (7). Findings in this report indicate an increased risk for breakthrough disease in children who received Var <30 days after MMR. No increase in breakthrough disease was noted in children who were administered Var <30 days after any of the other vaccines. The findings in this report are subject to at least two limitations. First, the VSD database contains only information on medical encounters. The number of cases of breakthrough varicella, which is usually mild and not brought to medical attention (8), may be underestimated; however, this underestimation is not likely to differ by vaccine administration schedules. Second, misclassification of cases might have occurred during the assignment of ICD-9 codes. No evidence was found that simultaneous administration of MMR, DTP, Hib, OPV, IPV, or HepB and Var increases the risk for breakthrough disease. To minimize the number of visits needed for immunization, Var should be administered simultaneously with these vaccines or should follow administration of MMR by >30 days. References
* Code 052. Table 1 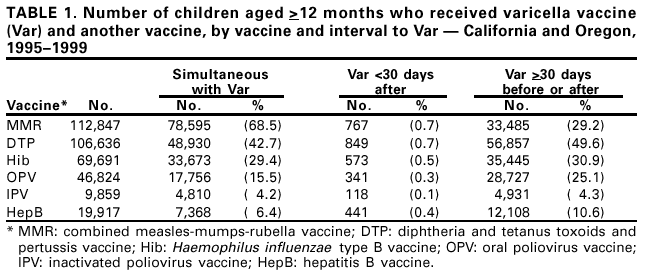 Return to top. Table 2 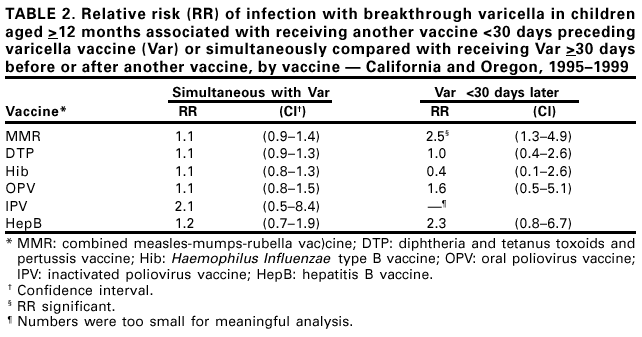 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 11/30/2001 |
|||||||||
This page last reviewed 11/30/2001
|