 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Adverse Events Following Civilian Smallpox Vaccination --- United States, 2003During January 24--May 9, 2003, smallpox vaccine was administered to 36,217 civilian health-care and public health workers in 55 jurisdictions to prepare the United States for a possible terrorist attack using smallpox virus. This report updates information on vaccine-associated adverse events among civilians vaccinated since the beginning of the program and among contacts of vaccinees, received by CDC from the Vaccine Adverse Event Reporting System (VAERS) as of May 9. In this vaccination program, CDC, the Food and Drug Administration, and state health departments are conducting surveillance for vaccine-associated adverse events among civilian vaccinees (1). As part of the vaccination program, civilian vaccinees receive routine follow-up, and reported adverse events after vaccination receive follow-up as needed. The U.S. Department of Defense is conducting surveillance for vaccine-associated adverse events among military vaccinees and providing follow-up care to those persons with reported adverse events. Adverse events that have been associated with smallpox vaccination are classified on the basis of evidence supporting the reported diagnoses. Cases verified by virologic testing, or in some instances by other diagnostic testing, are classified as confirmed (Table 1). Cases are classified as probable if possible alternative etiologies are investigated and excluded and supportive information for the diagnosis is found. Patients are classified as suspected if they have clinical features compatible with the diagnosis, but either further investigation is required or investigation of the case did not provide supporting evidence for the diagnosis. All reports of events that follow vaccination are accepted (i.e., events associated temporally); however, reported adverse events are not necessarily associated causally with vaccination, and some or all of these events might be coincidental. This report includes cases reported as of May 9 that either are under investigation or have a reported final diagnosis. Because of ongoing discussions of final case definitions, numbers and classifications of adverse events might change and will be updated regularly in MMWR. In collaboration with the Smallpox Vaccine Safety Working Group of the Advisory Committee on Immunization Practices, a case definition for myo/pericarditis has been developed and will be described in a subsequent MMWR. Using this definition to categorize all reports received through May 9, a total of 24 cases are consistent with the definition of myo/pericarditis; one of these was a new report received during May 3--9 (Table 1). During May 3--9, no cases of eczema vaccinatum, erythema multiforme major, fetal vaccinia, or progressive vaccinia have been reported (Table 1). One case of suspected postvaccinial encephalomyelitis (PVE) was reported. A man aged 38 years with a history of heavy tobacco use had acute respiratory distress and hypoxia on April 18, a total of 10 days after primary smallpox vaccination. He had a diagnosis of acute epiglottitis and was hospitalized and treated with intravenous corticosteroids, bronchodilators, antibiotics, and intermittent lorazepam for agitation. He improved and was discharged on April 25 on an oral steroid taper and bupropion to aid in smoking cessation. On April 26, he had acute behavioral changes characterized by intense agitation, emotional lability, and confusion, and was readmitted. On examination, the patient was afebrile and oriented with no focal neurologic deficits, but with moderate difficulty with concentration. Computerized tomography (CT) of the head showed several punctate areas of deep white matter hypodensity. Magnetic resonance imaging (MRI) of the brain with and without gadolinium displayed multiple nonenhancing punctate areas of increased signal in the deep subcortical white matter seen mainly on fluid attenuation inversion recovery (FLAIR) sequences, a nonspecific finding potentially consistent with a history of heavy smoking, severe hypertension, or amphetamine use. Laboratory studies showed an elevated creatine phosphokinase (CPK) of 3,000 u/L (normal: <175 u/L), and a urine toxicology screen was positive for marijuana and benzodiazepines; other studies were normal. Cerebrospinal fluid (CSF) protein, glucose, cell counts, and markers of acute demyelination (oligoclonal banding, IgG indices) were normal; CSF polymerase chain reaction for herpes simplex virus and vaccinia virus were negative. An electroencephalogram (EEG) showed changes consistent with benzodiazepine effect. The patient's behavioral changes improved, CPK levels decreased to 278, and he was discharged on April 29 with a diagnosis of steroid-induced psychosis. On May 3, the patient suffered an uprovoked generalized tonic-clonic seizure. An MRI was unchanged from the previous scan. However, post-infectious demyelination was considered because of the patient's smallpox vaccination history. He was placed on phenytoin, steroids were increased, and he was discharged the following day with a diagnosis of post-infectious encephalomyelitis. As of May 8, the patient remained mildly confused and emotionally labile. During May 3--9, one other serious adverse event was reported for hospitalization and antibiotic administration, and 23 other nonserious events were reported (Table 2). Among the 488 vaccinees with reported other nonserious adverse events during January 24--May 9, the most common signs and symptoms were fever (n = 92), rash (n = 88), headache (n = 82), pain (n = 78), and fatigue (n = 74) (Table 2). All of these commonly reported events are consistent with mild expected reactions following receipt of smallpox vaccine. Some vaccinees reported multiple signs and symptoms. During this reporting period, no vaccinia immune globulin was released for civilian vaccinees. No cases of vaccine transmission from civilian vaccinees to their contacts have been reported during the vaccination program (Table 3). A total of 10 cases of transmission from military personnel to civilian contacts have been reported. Surveillance for adverse events during the civilian and military smallpox vaccination programs is ongoing; regular surveillance reports will be published in MMWR. Reported by: Smallpox vaccine adverse events coordinators. National Center for Infectious Diseases; National Immunization Program, CDC. Editorial Note:PVE is a rare adverse event associated with smallpox vaccination (2--5). Estimates have varied, but occurrence is thought to range from 2.4--12.3 cases per million vaccinees depending on age, vaccination status, and surveillance methods (2--4); approximately 15%--25% of PVE cases are fatal, and approximately 25% of survivors develop substantial neurologic sequelae (2). Although the exact pathogenesis is unknown, it is likely that both a direct, vaccinia-associated acute viral encephalomyelitis and an autoimmune-mediated inflammatory reaction resulting in postvaccination demyelination (acute disseminated encephalomyelitis [ADEM]) occur (6). Patients with PVE show signs of encephalitis (alteration of mental status and focal neurologic deficits), myelitis (upper- and lower-motor neuron dysfunction, sensory level and bowel and bladder dysfunction), or both. Rarely, vaccinia virus might be detected in CSF (7). Several features of this case are not typical of PVE. Examination on April 26 showed only difficulties with concentration; no focal deficits or encephalopathy were observed. Neurodiagnostic studies, including CSF examination and EEG, were normal. Consideration of PVE followed seizure. MRI findings before and after the seizure might indicate demyelination consistent with ADEM, but might also be consistent with the patient's heavy smoking history, although unusual in a person aged <50 years. Although neither MRI displayed the multifocal enhancing white matter lesions associated typically with ADEM, it is unknown whether very early treatment with high-dose steroids might impact MRI findings of ADEM. Other potential etiologies for development of a seizure in this patient include bupropion use (8,9). Investigation of this case is ongoing. This case highlights the difficulty in diagnosing PVE. The diagnosis is exclusionary. Temporal association with vaccination does not necessarily indicate causality because acute encephalomyelitis might be caused by many different metabolic, toxic, and infectious conditions (10), and appropriate diagnostic studies, including serum chemistry profile, neuroimaging, blood cultures, and CSF examination, should be pursued as indicated. In the setting of a recent smallpox vaccination, patients with acute mental status changes, focal neurologic deficits, or white matter lesions on MRI must be evaluated for other more common causes of encephalomyelitis and treatable etiologies such as herpes simplex encephalitis should be excluded. Neurologic adverse events following smallpox vaccination should be reported promptly to state health departments and to VAERS. References
Table 1 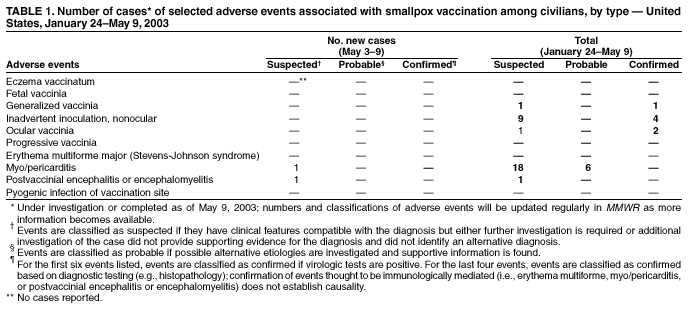 Return to top. Table 2 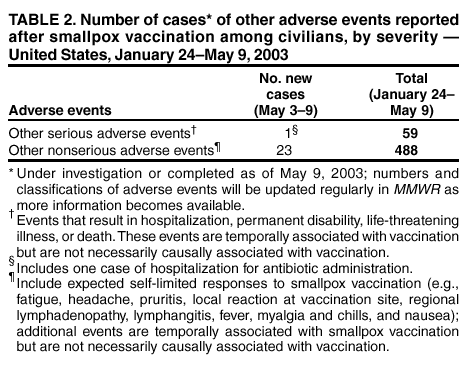 Return to top. Table 3 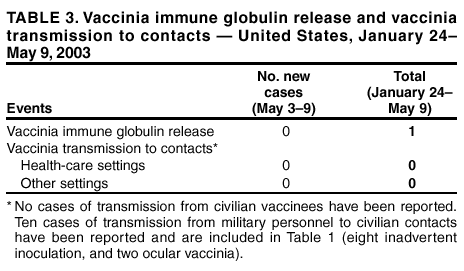 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 5/22/2003 |
|||||||||
This page last reviewed 5/22/2003
|