 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Creutzfeldt-Jakob Disease Associated with Cadaveric Dura Mater Grafts --- Japan, 1979--2003In 1997, a nongovernment surveillance group for Creutzfeldt-Jakob disease (CJD) in Japan supported financially by the Ministry of Health and Welfare* (MHW) reported 43 cases of CJD associated with receipt of cadaveric dura mater grafts (1). In all but one case, the most probable vehicle of transmission was a single brand of dural graft (LYODURA® [B. Braun Melsungen AG, Melsungen, Germany]) produced before May 1987. As of March 2003, ongoing surveillance in Japan had identified an additional 54 dura mater graft--associated cases. This report summarizes the investigation of the 97 cases, which indicated that during 1983--1987, the estimated minimum risk for CJD within 17 years of receipt of the implicated product in Japan was approximately one case per 1,250 grafts. No cases have been reported among patients who received their first dural graft after 1991; however, because of the long latency period between graft placement and symptom onset, additional cases of graft-associated CJD are likely to be reported. During 1996--2003, cases of CJD were identified in Japan by using 1) a mail survey of neurologic, psychiatric, and neuropathologic institutions (overall response rate: 74%) (1) and 2) subsequent reporting of CJD patients by clinicians to MHW. During this period, 97 cadaveric dura mater graft--associated CJD cases were identified. A case of dura mater--associated CJD was defined as a case in which a patient received a cadaveric dura mater graft and subsequently had CJD diagnosed by a physician and reviewed and accepted as CJD by a surveillance panel of neurologists. The 97 CJD patients had illness onset during September 1985--April 2002 (Figure 1). Median age at onset was 58 years (range: 15--80 years); mean age was 55 years. Mean age at onset was younger than that reported for sporadic CJD in Japan (66 years). A total of 58 (60%) patients were female. Neuropathologic confirmation of CJD diagnosis was obtained for 20 (21%) patients; 65 (84%) of the other 77 patients with physician-diagnosed CJD had an electroencephalogram with a periodic synchronous discharge pattern consistent with CJD. All 97 patients received dura mater grafts during 1978--1991 (Figure 2). Three patients received more than one dural graft during this period, including one patient reported previously (1). In all three cases, the first graft was considered to be the source of infection. Medical conditions leading to the use of dural grafts in these patients included tumor (n = 46), brain hemorrhage (n = 14), Jannetta procedure for facial palsy (n = 13) and for trigeminal neuralgia (n = six), intracranial aneurysm (n = eight), unspecified anomalies (n = five), hematoma (n = three), injury (n = one), and ossification of the spinal posterior longitudinal ligament (n = one). Latency periods ranged from 14 months (receipt in 1987 and onset in 1989) to 275 months (receipt in 1978 and onset in 2001). The median and mean latency periods were 122 and 125 months, respectively. A total of 93 patients received dural grafts during 1978--1987. In 1987, the manufacturer revised collection and processing procedures for the implicated product to reduce the risk for CJD transmission. Four patients received grafts during 1988--1991. No cases have been reported among patients who received their first dural graft after 1991. A total of 86 (89%) patients were documented to have received LYODURA®; the brand name of dural graft was unknown for 11 patients. A total of 81 (84%) of the 97 patients received their dural grafts during 1983--1987, during which time an estimated 100,000 patients received LYODURA® grafts in Japan. All 81 patients died from CJD within 17 years after receipt of the grafts. Lot numbers of the dura mater grafts used for the 97 patients could not be identified. As of September 2003, five additional cases were under investigation in Japan for suspected dural graft--associated CJD. Reported by: Y Nakamura, MD, M Watanabe, MD, K Nagoshi, MD, Dept of Public Health, Jichi Medical School, Minamikawachi; T Kitamoto, MD, Dept of Neuropathology, Tohuku Univ School of Medicine, Sendai; T Sato, MD, Kohnodai Hospital, National Center for Neurology and Psychiatry, Ichikawa; M Yamada, MD, Dept of Neurology, Kanazawa Univ Graduate School of Medical Science, Kanazawa; H Mizusawa, MD, Dept of Neurology, Tokyo Medical and Dental Univ School of Medicine, Tokyo, Japan. R Maddox, MPH, J Sejvar, MD, E Belay, MD, LB Schonberger, MD, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial Note:Dural graft--associated CJD cases continue to be identified in Japan. The estimated minimum risk within 17 years after receipt of LYODURA® is approximately one case per 1,250 recipients. The precise number of dura mater grafts used in Japan is unknown, but an estimated 20,000 grafts per year might have been used during 1983--1987. The widespread use of LYODURA® during neurosurgical procedures in Japan is the most probable source of the unusually high number of dural graft--associated CJD cases in Japan (2). Dural graft recipients have symptom onset at a younger age compared with age at onset in sporadic cases of CJD in Japan. The identification of additional cases over time has resulted in an expected increase in the latency period between dural graft placement and symptom onset. The mean and range for this latency of CJD from contaminated grafts is unknown, but the upper limit now exceeds 22 years. The occurrence of new cases, the increase in the mean and range of the latency period, and the identification of suspected cases under investigation all suggest that this outbreak is ongoing. No cases in Japan were reported to be related to receipt of a dural graft other than LYODURA®. For 11 cases, the manufacturer brand name was unknown. Although LYODURA®, or in one case either LYODURA® or a dural graft from another manufacturer (Tutoplast® [Pfrimmer-Viggo GmbH & Co., Erlangen, Germany]), was suspected in these cases, documentation of a specific source was unavailable. Four patients received dural grafts after collection and processing procedures were revised by the manufacturer in 1987, but whether the implicated dural grafts were LYODURA® produced before 1987 is unknown. That all LYODURA®-associated CJD cases to date occurred among patients who received grafts before 1992 suggests that all implicated grafts likely were processed before 1987; the implicated product's expiration date is 5 years after processing. LYODURA® never was produced by the manufacturer for distribution in the United States, and relatively few LYODURA® grafts were used in this country. In May 1987, after identification of the first dural graft--associated CJD case in a U.S. patient who had received the implicated product, the manufacturer revised its procedures for collecting and processing dura mater grafts to reduce the risk for CJD transmission (e.g., by discontinuing the commingling of dura and disinfecting them with sodium hydroxide) (3,4). Subsequently, numerous other dura mater graft--associated cases were identified worldwide; nearly all patients had received the implicated product, including one additional U.S. patient. In 1997, the report of 43 cases of dura mater graft--associated CJD in Japan represented the largest cluster of such cases in any one country (1). In one of the CJD cases reported in Japan, the implicated graft was used in a spinal (not an intracranial) procedure. This case suggests that transmission from contaminated dura might occur in areas of the neuraxis outside of the cranial vault. In 1997, the Food and Drug Administration's Transmissible Spongiform Encephalopathy Advisory Committee (TSEAC) recognized that the use of human dura mater in the United States carries an inherent risk for transmitting CJD. However, the committee recommended that the use of such grafts be left to the discretion of the treating neurosurgeon, provided that the human dura mater is procured and processed according to appropriate safety measures (5). In 1997, an estimated 4,500 dural grafts were distributed for use in the United States (6). After the TSEAC recommendations were issued, the number of dural grafts distributed for use in the United States declined to an estimated 900 grafts in 2002 (B.E. Buck, M.D., Miami Tissue Bank, personal communication, 2003). The cases described in this report indicate that recipients of contaminated dura mater grafts might remain at risk for CJD for >22 years after receiving grafts. CDC continues to conduct surveillance for cases of CJD in the United States. Patients with a rapidly progressive dementia consistent with CJD and a history of dural graft implantation should be reported through local or state health departments to CDC, telephone 404-639-3091. References
* Subsequently named the Ministry of Health, Labor, and Welfare.
Figure 1 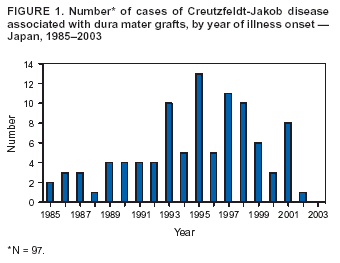 Return to top. Figure 2 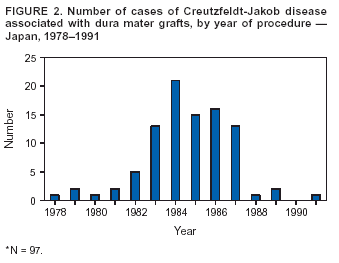 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 12/4/2003 |
|||||||||
This page last reviewed 12/4/2003
|