 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Bovine Spongiform Encephalopathy in a Dairy Cow --- Washington State, 2003On December 23, 2003, the U.S. Department of Agriculture (USDA) made a preliminary diagnosis of bovine spongiform encephalopathy (BSE) in a single "downer" (i.e., nonambulatory disabled) dairy cow in Washington state. On December 25, this diagnosis was confirmed by the BSE international reference laboratory in Weybridge, England. This report summarizes the findings of the initial investigation of this case and describes the public health prevention measures adopted by USDA to protect the human food supply. The occurrence of BSE in the United States reinforces the need for physicians to be aware of the clinical features of variant Creutzfeldt-Jakob disease (vCJD) and to arrange for brain autopsies in all decedents with suspected or probable CJD to assess the neuropathology of these patients. The BSE-positive cow was aged 6.5 years when it was slaughtered on December 9. Before slaughter, the cow was nonambulatory; its condition was attributed to complications from calving. The animal was examined by a USDA Food Safety and Inspection Service (FSIS) veterinary medical officer both before and after slaughter. After examination, the carcass was released for use as food for human consumption. Tissues (e.g., brain, spinal cord, and small intestine) considered to be at high risk for the transmission of the BSE agent were removed from the cow during slaughter and sent for inedible rendering (often used for nonruminant animal feed). Because the cow was nonambulatory at slaughter, brain tissue samples were taken by USDA's Animal and Plant Health Inspection Service (APHIS) as part of its targeted surveillance for BSE. On December 23, a presumptive diagnosis of BSE was made, and the herd to which this cow belonged was placed under a state hold order. USDA, in collaboration with state and other federal animal and public health agencies, industry representatives, and the Canadian Food Inspection Agency (CFIA), initiated investigations of potentially exposed cattle and regulated products. On December 24, FSIS recalled beef from cattle slaughtered in the same plant on the same day as the BSE-positive cow. Some of the beef subject to the recall had been shipped to several establishments, which processed it further. Meat products manufactured from the recalled meat were distributed primarily to locations in Oregon and Washington, with smaller quantities distributed to locations in California, Idaho, Montana, and Nevada. FSIS continues to verify the distribution and control of all recalled products. The U.S. Food and Drug Administration (FDA) and inspectors from Oregon and Washington have located all known potentially infectious rendered products from the BSE-positive cow. The rendering plants that processed this material have placed a voluntary hold on all known potentially infectious products, none of which had left the control of the companies or entered commercial distribution as of January 7, 2004. FDA continues its investigation of all regulated products related to the BSE-positive cow. APHIS, in collaboration with CFIA, traced the birth of the BSE-positive cow to a farm in Alberta, Canada. On January 6, USDA and CFIA announced that DNA evidence had confirmed this traceback to Canada with a high degree of certainty. This line of investigation indicates that the BSE-positive cow was one of 82 animals from a Canadian herd cleared for shipment to the United States; 81 of the cattle listed on the Canadian animal health certificate entered the United States on September 4, 2001, through Oroville, Washington. These cattle are being traced to determine their disposition or current location. The BSE-positive cow gave birth to two live calves while in the United States. The first is a yearling heifer on the same farm as the BSE-positive cow. The second, a bull calf, was in a group of calves at another location, a calf-feeding operation that also was under a state hold order. Because the bull calf could not be identified definitively, APHIS completed the elimination of all calves at this site on January 6. Since the epidemiologic investigation began, APHIS has developed criteria for determining additional cattle at risk for BSE that should be eliminated. On December 30, USDA announced additional safeguards to further minimize the risk for human exposure to BSE in the United States (Box. Beginning immediately, FSIS has prohibited the use of downer cattle for food for human consumption. Through its emergency rule-making powers, FSIS will take additional actions that will become effective on their publication. Planned actions include the required removal of "specified risk materials" (i.e., high-risk materials) from animals aged >30 months at the time of slaughter and withholding the USDA "inspected and passed" mark until negative BSE test results are received for any animal tested. To enhance the speed and accuracy of the response to animal health threats such as BSE, APHIS is working to implement a national identification system to track animals of various species through the livestock marketing chain. USDA also will appoint an international panel of scientists with BSE expertise to provide an objective review of the response to the identification of the BSE-positive cow described in this report and to identify areas for potential improvement of current BSE safeguards. Reported by: Animal and Plant Health Inspection Svc; Food Safety and Inspection Svc, U.S. Dept of Agriculture. U.S. Food and Drug Administration. Div of Vital Statistics, National Center for Health Statistics; Div of Viral and Rickettsial Diseases, National Center for Infections Diseases, CDC. Editorial Note:BSE is a progressive, fatal neurologic disorder of cattle and is classified as one of the transmissible spongiform encephalopathies, a group of diseases of animals and humans believed to be caused by abnormally folded proteins called prions. BSE was first identified in 1986 in the United Kingdom (UK), where it caused a large outbreak among cattle (1). Although the source of the BSE epizootic agent is uncertain, feeding cattle BSE-contaminated meat-and-bone meal is the major contributory factor to the amplification of BSE among cattle (2). Since 1986, BSE cases have been identified in 20 European countries, Japan, Israel, and Canada. Since BSE surveillance was initiated in the United States in 1990, USDA has tested brain tissue from approximately 57,000 cattle, targeting those at high risk for BSE (e.g., downer cattle and cattle with neurologic signs); the case described in this report represents the first identification of BSE in the United States. Whether an epidemiologic link exists between this BSE case traced to Canada and the previous case reported in Canada is not known. Epidemiologic and laboratory evidence suggests that the BSE agent has been transmitted to humans via consumption of BSE-contaminated cattle products, causing vCJD (1). However, the risk for acquiring vCJD from consumption of BSE-contaminated product is low, presumably because of a "species barrier" that provides substantial but incomplete protection against development of vCJD. In the UK, where an estimated one million or more cattle probably were infected with BSE, cases of vCJD continue to be reported; however, the number of cases of vCJD remains small, with 148 probable and confirmed vCJD cases identified as of January 7, including those of three persons residing in Ireland, Canada, and the United States who are believed to have been exposed to BSE in the UK (1,3). Seven additional cases not directly linked to the BSE outbreak in the UK also have been reported (six in France and one in Italy). In the United States, the feeding of rendered cattle products to other cattle has been prohibited since 1997, and the importation of cattle and cattle products from countries with BSE or considered to be at high risk for BSE has been prohibited since 1989; these measures have minimized the potential exposure of animals and humans to the BSE agent (4). The additional safeguards described in this report should further reduce the risk for acquiring vCJD. Substantial clinical and epidemiologic differences exist between vCJD and the more commonly occurring classic form of CJD recognized in the United States for decades before the emergence of BSE (Table). Although strong epidemiologic and laboratory evidence indicates that vCJD is linked causally with BSE, no exogenous source of infection has been identified for approximately 85% of classic CJD cases (5). The median age at death of classic CJD patients in the United States is 68 years, compared with 28 years for vCJD patients. The age distribution of these deaths illustrates that most vCJD occurs in age groups in which classic CJD is rare (Figure) (RG Will, M.D., National CJD Surveillance Unit, Edinburgh, Scotland, personal communication, 2004). In addition, the median duration of illness before death for classic CJD patients in the United States is 4--5 months, compared with 13--14 months for vCJD patients (6). Patients with vCJD often have prominent early behavioral or psychiatric manifestations and painful sensory symptoms, with neurologic signs such as myoclonus and extrapyramidal dysfunction being delayed for several months after illness onset (6). The characteristic electroencephalographic pattern of periodic sharp waves observed in classic CJD patients is absent in patients with vCJD. A characteristic high signal in the posterior thalamus on T2- and diffusion-weighted magnetic resonance imaging (the "pulvinar sign") is demonstrated in >75% of vCJD patients, and in the appropriate clinical context, is highly indicative of a vCJD diagnosis (7). Confirmatory diagnosis of vCJD and classic CJD requires pathologic examination of brain tissue obtained at autopsy or biopsy. The neuropathology in vCJD is distinguished by the presence of numerous deposits of kuru-type plaques surrounded by vacuoles (i.e., "florid plaques") in the cerebellum and cerebrum and the marked accumulation of the pathologic protease-resistant prion protein on immunohistochemical (IHC) analysis (8). Prions are detected readily by IHC analysis in lymphoid tissues (e.g., appendix, lymph nodes, spleen, and tonsils) of vCJD patients, but not in classic CJD patients (9). All persons with vCJD tested as of January 2004 have had methionine homozygosity at the polymorphic codon 129 of the prion protein gene, indicating that persons who do not carry this genotype (comprising the majority of the general population) appear to have increased resistance to vCJD. Since 1996, CDC has used several mechanisms to conduct surveillance for classic CJD and vCJD in the United States (10). CDC reviews national multiple cause-of-death data to monitor the epidemiology of CJD in the United States. CDC, in collaboration with state and local health departments, investigates CJD cases in persons aged <55 years to identify cases of possible vCJD. In addition, CDC assists routinely in the investigation of suspected cases of vCJD spontaneously reported by health-care providers. During 1996--1997, in collaboration with the American Association of Neuropathologists, CDC established the National Prion Disease Pathology Surveillance Center (NPDPSC) at Case Western Reserve University, Cleveland, Ohio. NPDPSC provides advanced neuropathologic and biochemical diagnostic services free of charge to U.S. physicians and state and local health departments. These surveillance efforts have not detected any cases of indigenous vCJD in the United States. The emergence of BSE in the United States reinforces the need for physicians to be aware of the clinical features of vCJD in all patients, regardless of age, who report with distinguishing characteristics (Table 2). Because testing brain tissue permits the most definitive diagnosis of all forms of CJD and identification of emerging forms of the disease, including vCJD, CDC encourages physicians to arrange for brain autopsies in all decedents with suspected or diagnosed CJD and to use the free services of NPDPSC to assess the neuropathology of these patients. Information about these services is available from NPDPSC at http://www.cjdsurveillance.com or from CDC, telephone 404-639-3091. References
Table 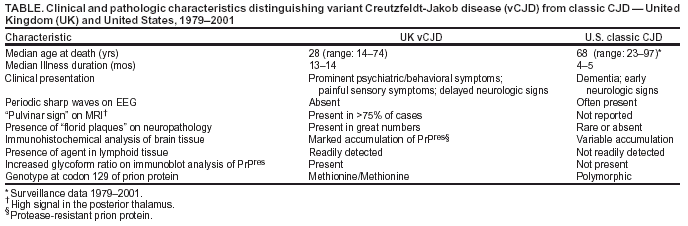 Return to top. Figure 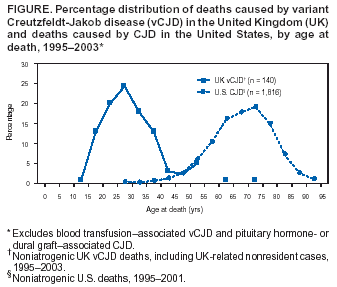 Return to top. Box  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 1/8/2004 |
|||||||||
This page last reviewed 1/8/2004
|