 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Outbreak of Varicella Among Vaccinated Children --- Michigan, 2003On November 18, 2003, the Oakland County Health Division alerted the Michigan Department of Community Health (MDCH) to a varicella (chicken pox) outbreak in a kindergarten--third grade elementary school. On December 11, MDCH and Oakland County public health epidemiologists, with the technical assistance of CDC, conducted a retrospective cohort study to describe the outbreak, determine varicella vaccine effectiveness (VE), and examine risk factors for breakthrough disease (i.e., varicella occurring >42 days after vaccination). This report summarizes the results of that study, which indicated that 1) transmission of varicella was sustained at the school for nearly 1 month despite high vaccination coverage, 2) vaccinated patients had substantially milder disease (<50 lesions), and 3) a period of >4 years since vaccination was a risk factor for breakthrough disease. These findings highlight the importance of case-based reporting of varicella and the exclusion of patients from school until all lesions crust or fade away. Information about recognizing vaccinated patients with mild cases should be disseminated to health-care providers, school administrators, and parents. Self-administered standard questionnaires were sent to parents of all students to collect data on students' vaccination and disease history. Parents of patients were interviewed by telephone to ascertain detailed information about potential exposures to varicella and clinical characteristics of disease. A case was defined as an acute generalized maculopapulovesicular rash, without other apparent cause, in a student who attended the school during September 1--December 19. Disease was categorized as mild (<50 lesions), moderate (50--500 lesions), or severe (>500 lesions or presence of complications or hospitalization). Vaccination status was confirmed for students by reviewing school vaccination records and contacting health-care providers. VE was calculated by comparing attack rates among vaccinated and unvaccinated students. The following were excluded from VE calculations and analyses of risk factors for breakthrough disease: 1) students with previous or unknown varicella disease history, 2) recipients of invalid doses (i.e., doses administered before age 12 months), 3) those with unverified vaccination, 4) those vaccinated <42 days before the start of the outbreak, and 5) those whose parents did not return surveys. The elementary school had 580 students; 73 (12.6%) had illness consistent with the case definition. Testing for varicella zoster virus DNA using polymerase chain reaction (PCR) on lesion specimens collected from two patients yielded one positive result. Cases were concentrated in first and third graders; grade-specific attack rates* were as follows: kindergarten, 5.6% (seven of 125); first grade, 15.7% (21 of 134); second grade, 6.7% (nine of 134); and third grade, 26.6% (29 of 109) (Figure). Male students accounted for 54.5% (36 of 66) of the patients†. The earliest rash onset date was November 5 in a previously vaccinated third grader. Her rash consisted of two pruritic, vesicular lesions of 5 days' duration on her neck and stomach. She did not appear clinically ill, was afebrile, and missed no days of school. Her parents did not recall any potential exposure to varicella in the weeks before rash onset. The outbreak peaked 7 days after this onset; however, a source case was not identified. Date of rash onset was unknown for four patients whose parents were not interviewed; however, their clinical course was inconsistent with rash onset occurring before November 5. Students did not attend school during November 12--14 because of parent-teacher conferences and during November 26--28 because of the Thanksgiving holiday. These breaks in attendance might have interrupted disease transmission. Eight secondary cases outside the school were identified among six siblings and two adults (one father and one aunt). All had rash within 2 weeks of exposure. Survey response rate was 95.5% (554 of 580). Among respondents, 62 reported no vaccination history. Among these, 47 reported varicella disease history before the outbreak, 13 reported no previous disease history, and two had an unknown disease history. Among the 507 respondents with no disease history, 492 reported vaccination history, and vaccination was verified for 485 students, resulting in a vaccination coverage of 95.7% (485 of 507); 43 of the 485 verified vaccinees were excluded from further analyses because they either reported a previous or unknown varicella disease history (n = 32), were vaccinated before age 12 months (n = five), had an unknown age at vaccination (n = two), or were vaccinated <42 days before the start of the outbreak (n = four), resulting in 442 children who were vaccinated appropriately with no known disease history. Attack rates were 11.8% (52 of 442) for vaccinated and 76.9% (10 of 13) for unvaccinated students. VE was 84.7% (95% confidence interval [CI] = 77.4%--89.7%) in preventing varicella of any severity and 97.6% (95% CI = 95.0%--98.9%) in preventing moderate to severe varicella. Vaccinated patients were more likely to have mild disease than unvaccinated patients (84.6% versus 20.0%; p<0.01), were less likely to have fever (44.2% versus 88.9%; p<0.05), and missed fewer days of school (1.3 versus 3.5 median days; p<0.01). Children vaccinated >4 years before the outbreak were nearly five times more likely to acquire varicella than children vaccinated within the previous 4 years (relative risk = 4.65; 95% CI = 1.48--14.61). Age at vaccination, sex, and preexisting conditions (e.g., asthma and eczema) were not associated with vaccine failure. Vaccine lot numbers were identified for 30 patients; vaccine from 26 different lot numbers was administered on multiple dates by multiple providers, indicating that breakdown in vaccine storage or handling procedures was not a likely risk factor for vaccine failure. Reported by: R Renas, MPH, S Bies, MPH, C Bird, MD, Oakland County Health Div; J Blostein, MPH, M Boulton, MD, Michigan Dept of Community Health. A Lopez, MHS, A Jumaan, PhD, Epidemiology and Surveillance Div, National Immunization Program; DK El Reda, DrPH, EIS Officer, CDC. Editorial Note:Varicella is a highly infectious disease that, in the prevaccine era, resulted in approximately 4 million illnesses, 11,000 hospitalizations, and 100 deaths annually in the United States (1--3). In 1995, a live, attenuated varicella vaccine was licensed for use in the United States, and the majority of studies of vaccine performance have demonstrated an overall VE of 70%--90% (4,5). Since vaccine licensure, the United States has experienced a steady decline in the incidence of varicella disease, attributed to increasing vaccination coverage (6). The findings in this report are consistent with those of recently published studies on VE and the association between longer time since vaccination and breakthrough disease (5,7). Cases of mild disease, not recognized as varicella before detection of the outbreak, might have played an important role in virus transmission in this highly vaccinated population. All patients with chicken pox should be excluded from schools or day care centers until all lesions have crusted. However, breakthrough disease usually is mild and might not include vesicular lesions that crust. To help prevent disease spread in schools and day care centers, health-care providers, school administration, and parents must learn to recognize students with vaccine-modified varicella and exclude them from schools until lesions fade away or no new lesions appear. Local varicella surveillance consists of passive reporting of aggregate case counts to state health departments. Timely reporting of individual varicella cases and appropriate follow-up might have ensured exclusion of patients from school and reduced the size of this outbreak. As vaccination coverage increases, the proportion of breakthrough cases also will increase. Health departments can begin to evaluate the impact of varicella vaccination programs through case-based surveillance that collects information about age, vaccination status, and severity of disease. These data can help to detect changes in epidemiology of varicella disease over time, such as a potential shift to older age groups or changes in disease severity among breakthrough cases. The Council of State and Territorial Epidemiologists has recommended that states implement case-based surveillance of varicella by 2005 (8). The findings in this report indicate that varicella vaccine was effective (85%) in preventing varicella of any severity and highly effective (98%) in preventing moderate to severe disease. Although longer time since vaccination was identified as a potential risk factor for vaccine failure, prospective follow-up studies are needed to examine the importance of individual risk factors for breakthrough disease, after controlling for the effects of other factors (e.g., risk for exposure). In addition, these findings underscore the importance of continuing to increase vaccination rates nationwide, ensuring that vaccination remains the cornerstone of efforts to control varicella. References
* Totals differ from school census (n = 580) and total cases identified (n = 73) because students with varicella disease history or unknown history before this outbreak were excluded (n = 78) from calculations. † Limited to 66 patients with no varicella disease history before this outbreak (four had unknown history, and three reported previous history). Figure 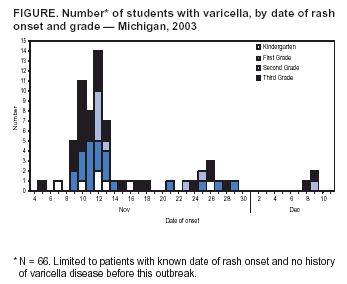 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 5/12/2004 |
|||||||||
This page last reviewed 5/12/2004
|