Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Tularemia --- Missouri, 2000--2007
Tularemia is an uncommon but potentially fatal zoonotic disease caused by the gram-negative coccobacillus Francisella tularensis. Approximately 40% of all tularemia cases reported to CDC each year occur in Arkansas, Oklahoma, and Missouri (1). To define the epidemiologic and clinical features of tularemia in Missouri, the Missouri Department of Health and Senior Services (MDHSS) analyzed surveillance data and conducted a retrospective clinical chart review of cases that occurred during 2000--2007. This report describes the results of that analysis, which identified 190 cases (87 confirmed and 103 probable), for an average annual incidence of 0.4 cases per 100,000 population statewide. Most cases occurred during the summer months (78%) and among males (66%). Analysis of 121 clinical charts revealed that children were more likely than adults to be diagnosed with glandular tularemia, whereas adults were more likely to be diagnosed with pneumonic tularemia. Sixty-three (52%) patients were hospitalized; one patient died. Among 78 cases with a documented exposure source, 72% were associated with tick bite. In 33 (85%) of 39 culture-confirmed cases, the laboratory received specimens without any indication of suspicion of a tularemia diagnosis. Clinicians should 1) be aware of the range of tularemia symptoms, 2) consider the diagnosis in patients reporting fever and tick or animal exposure, and 3) initiate empiric antimicrobial therapy while awaiting laboratory confirmation. Laboratory staff should take appropriate precautions when processing culture specimens from tularemia-endemic regions, even if suspicion of tularemia is not noted when the specimen is submitted.
Tularemia is a nationally notifiable disease. Although tularemia was removed from the list of nationally notifiable diseases in 1994, it was reinstated in 2000 because of increased concern about potential use of F. tularensis as a biologic weapon (1,2). In Missouri, since 2000, clinicians and laboratories have been required to report to MDHSS cases of illness that are clinically compatible with tularemia and have presumptive or confirmed laboratory evidence of infection. The clinical presentation of tularemia ranges from cutaneous ulcers to pneumonia and depends on the mode of transmission and site of inoculation (3). Routes of F. tularensis transmission to humans include arthropod bites, contact with infected animal tissues, ingestion of contaminated food or water, and inhalation of contaminated aerosols (e.g., aerosols generated by mowing over infected animal carcasses and through improper handling of laboratory cultures).
To define the epidemiologic and clinical features of tularemia in Missouri, MDHSS analyzed 190 tularemia case reports from the period 2000--2007 and conducted an independent review of 121 available clinical records (including clinician notes, laboratory results, and drug administration records) using an abstraction form modified from the CDC case report form.* Reports were included in this analysis if the diagnosis of tularemia met the National Notifiable Disease Surveillance System case definition.† The primary clinical form of the disease was classified according to health-care provider diagnosis and documented clinical features. For the purpose of this analysis, patients with tularemia who presented with undifferentiated febrile illness or sepsis without localizing signs (often referred to as typhoidal tularemia) were categorized as pneumonic tularemia, because these cases frequently have evidence of respiratory disease (3). Data on exposures occurring within 3 weeks of illness onset were abstracted from clinical notes; aerosol exposure was defined as exposure through inhalation of agricultural grains or dusts, or aerosols created by mowing over animal carcasses. MDHSS reviewed clinical notes of all culture-confirmed cases to determine whether the provider had documented suspicion of tularemia by the time specimens were submitted to the laboratory. Appropriate antibiotic therapy was defined as treatment with an aminoglycoside or a fluoroquinolone for at least 10 days or a tetracycline for at least 15 days (4). The county of residence and 2000 census data were used for county incidence calculations. Continuous variables were analyzed by Student's t-tests, and categorical variables were analyzed using chi-square or Fischer's exact tests, as appropriate.
During 2000--2007, a total of 190 cases of tularemia (87 confirmed and 103 probable) were reported to MDHSS, yielding a statewide average annual incidence of 0.4 cases per 100,000 population. No increase or decrease was observed in annual trend (range: 13--32 cases per year). The majority of cases were reported from central and southwestern Missouri. The total number of cases by county for the 8-year period ranged from zero to 14, yielding average annual incidence rates that ranged up to 5.25 cases per 100,000 population. Males accounted for 125 (66%) patients; median patient age was 37 years (range: 6 months--93 years), with a distinct bimodal distribution among males (Figure 1).
Clinical records were available for 121 (64%) patients, including 59 (49%) with confirmed and 62 (51%) with probable tularemia. For the 107 (88%) cases with data on primary clinical form, ulceroglandular tularemia was the most common overall (42%). The distribution of clinical form differed significantly between children and adults (p<0.01). Children were diagnosed with glandular tularemia more than twice as often as adults, whereas adults were diagnosed with the pneumonic form 10 times as often as children (Table).
For the 26 cases categorized as pneumonic tularemia based on clinical features, 12 (46%) had recorded exposures, of which six were inhalational (four patients worked with grain or hay; two mowed over dead animals) and six were tick exposures (without lesions or lymphadenopathy). Ten (38%) patients had cough, and seven (27%) had shortness of breath or chest pain. The mean initial temperature documented in clinical record was 100.7°F (38.2°C) (range: 98.0--105.0°F [36.7--40.6°C]). Among the 16 patients for whom initial chest radiograph reports were available, six (38%) reports were normal, six (38%) noted unilateral pulmonary infiltrates, and four (25%) noted pleural effusions. Two (13%) patients developed empyema, and two (13%) developed generalized sepsis.
Eighty (66%) of the 121 patients had an uneventful clinical course with full recovery, 40 (33%) patients had a complicated clinical course, and one patient died of sepsis (Table). Sixty-three (52%) of the 121 patients were hospitalized (median duration: 4 days [range: 1--27 days]). Three patients with pneumonic and one patient with ulceroglandular tularemia were admitted to an intensive-care unit. Six patients with glandular and two with pneumonic tularemia were rehospitalized because of relapse or other complications. Among 17 (14%) patients who required surgical intervention, 15 had suppurated lymph nodes requiring incision and drainage, and two developed a loculated empyema requiring thoracotomy and decortication.
Information on antimicrobial treatment was available for 109 patients; 97 (89%) received at least one appropriate antibiotic to treat tularemia (4) (Table), and the remaining 12 (11%) were treated with combinations of antibiotics that are considered ineffective against tularemia. Among 14 patients initially treated with 10 days of ciprofloxacin monotherapy, 12 (86%) recovered completely, whereas two (14%) experienced persistence of symptoms. Of 73 patients for whom sufficient data were available, the median interval between onset of symptoms and commencement of an effective antimicrobial was 14 days (range: 0--82 days). The incidence of complications was not related to age, sex, or the timing of effective therapy.
The total number of specimens submitted for culture and serology could not be determined; however, of the 57 confirmed cases, 39 (68%) had positive cultures, most commonly from blood, lymph nodes, or lesions, and 18 (32%) had a fourfold or greater difference in paired serum antibody titers. All probable cases were diagnosed based on a single elevated serum antibody titer to F. tularensis. Among the 39 culture-confirmed cases, 33 (85%) laboratory results were available before the health-care provider documented a suspicion of tularemia in the clinical record.
Among 78 cases for which exposure was known, tick bites were the most commonly noted exposures (72%) (Table), and 80% of tick bite exposures occurred during May--September. Cases associated with other exposures did not show a distinct seasonal trend (Figure 2). Animal and aerosol exposures accounted for 16% of cases, with aerosol exposures reported only for adults.
Reported by: G Turabelidze, MD, PhD, S Patrick, PhD, Missouri Dept of Health and Senior Svcs. PS Mead, MD, KS Griffith, MD, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; IB Weber, MBChB, MMed, EIS Officer, CDC.
Editorial Note:
With fewer than 200 incident cases reported annually in the United States, tularemia is an uncommon but serious human illness that is best prevented through the use of personal protective measures. The seasonal, age, and sex distributions of cases described in this report are consistent with national surveillance data (1). However, this report identifies age-specific differences in diagnosed clinical form that have not been documented previously, and suggests a higher proportion of tick-associated cases than earlier studies of tularemia in this region (5,6). The observed peaks in tick-associated cases in June and September coincide with periods of activity of questing nymphal ticks in spring and adults in late summer in Missouri. The findings in this report might not be representative of other areas of the United States because of differences in clinician or public awareness and exposure risk. Patients reporting fever and tick, animal, or aerosol (e.g., agricultural, lawnmowing, and laboratory aerosols) exposure should be evaluated promptly for infection with F. tularensis. Because F. tularensis takes several days to culture and seroconversion occurs 10--20 days after infection (4), the initiation of empiric antimicrobial therapy should not be delayed pending laboratory confirmation. Naturally occurring tularemia usually is sporadic, occurs in rural areas, and manifests as either ulceroglandular or glandular illness. An intentional aerosolized release might result in clusters of illness, occur in urban areas, and be characterized by a higher proportion of pneumonic disease (7). For this reason, cases of pneumonic tularemia should be reported urgently to local and state health departments and CDC.
F. tularensis is highly infectious when grown in culture (8); therefore, appropriate infection-control measures are needed to prevent laboratory-acquired infection. Although 85% of culture-confirmed cases described in this report were handled and processed before documented clinical concern for tularemia, no laboratory-acquired cases were identified. Diagnostic procedures with clinical materials can be performed in biosafety level 2 conditions; however, all work with suspect cultures of F. tularensis should be performed in a biosafety cabinet (9). Manipulation of cultures and other procedures that might produce aerosols or droplets (e.g., grinding, centrifuging, vigorous shaking, and animal studies) should be conducted under biosafety level 3 conditions (9). The state public health laboratory and public health department should be consulted immediately if tularemia is suspected (9). Moreover, laboratorians are encouraged to take appropriate precautions when processing culture specimens from endemic regions, even if suspicion of tularemia is not noted on the request form.
Currently, only aminoglycosides, tetracyclines, chloramphenicol, and rifampin are approved by the Food and Drug Administration for treatment of tularemia. Studies conducted in vitro and in animals suggest that fluoroquinolone antimicrobials are effective for treatment of F. tularensis infections (10), and drugs of this class have been included in the Strategic National Stockpile for potential use in the event of a bioterrorist attack (2). Although additional systematic information is needed regarding the efficacy of fluoroquinolones for treatment of tularemia, the 86% cure rate among patients receiving fluoroquinolone monotherapy described in this report is comparable with rates previously reported for gentamicin and doxycycline (10).
The findings in this report are subject to at least three limitations. First, although no differences were noted with respect to age, sex, year of diagnosis, or county of residence between patients for whom clinical records were and were not available, these groups might have differed with respect to other variables. Second, data on the full range of exposure and clinical variables were not available for all clinical charts. Finally, inter-laboratory thresholds for titer levels reported as positive might have led to variability in case detection across counties.
In 2003, MDHSS initiated a public awareness campaign on tick bite prevention. Outreach to hunters included billboard placement near state parks and an educational mailing to all hunting and fishing license registration sites. Tularemia experts participated in public media awareness events, and additional radio and print materials were made available to local public health agencies, a network of senior citizen sites, and the general public.
The prevention of tularemia requires educating those at greatest risk for exposure (e.g., hikers, campers, and hunters). The use of protective clothing, repellents containing DEET (N,N-dimethyl-meta-toluamide), and pesticides (e.g., permethrin) on clothing can help reduce the risk for exposure through tick and arthropod bites (3). Hunters and others who handle potentially infected animals should wear gloves to avoid introduction of F. tularensis through cuts or abrasions, and game meat should always be cooked thoroughly. To reduce the risk for aerosol exposures, grassy areas should be surveyed before mowing and any dead animals removed. Persons facing potential occupational risks such as agricultural and laboratory workers should follow safe practice guidelines.§
Acknowledgments
This report is based, in part, on contributions by D Pratt, F Fick, J Bos, P Franklin, A Grimm, C Butler, P Kishore Molakatalla, and A Turner of the Missouri Dept of Health and Senior Svcs; and K Kugeler and J Petersen, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, CDC.
References
- CDC. Tularemia---United States, 1990--2000. MMWR 2002;51:182--4.
- Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA 2001;285:2763--73.
- Hayes E. Tularemia. In: Goodman JL, Dennis DT, Sonenshine DE, eds. Tick-borne diseases of humans. Washington, DC: ASM Press; 2005:207--17.
- World Health Organization. WHO guidelines on tularemia. Geneva, Switzerland: World Health Organization; 2007. Available at http://www.cdc.gov/tularemia/resources/whotularemiamanual.pdf.
- Taylor JP, Istre GR, McChesney TC, Satalowich FT, Parker RL, McFarland LM. Epidemiologic characteristics of human tularemia in the southwest-central states, 1981--1987. Am J Epidemiol 1991;133:1032--8.
- Assal N, Blenden DC, Price ER. Epidemiologic study of human tularemia reported in Missouri, 1949--65. Public Health Rep 1967;82:627--32.
- CDC. Recognition of illness associated with the intentional release of a biologic agent. MMWR 2001;50:893--7.
- Overholt EL, Tigertt WD, Kadull PJ, et al. An analysis of forty-two cases of laboratory-acquired tularemia. Treatment with broad spectrum antibiotics. Am J Med 1961;30:785--806.
- CDC, American Society for Microbiology, Association of Public Health Laboratories. Basic protocols for level A laboratories for the presumptive identification of Francisella tularensis. Washington, DC: American Society for Microbiology; 2001. Available at http://www.asm.org/asm/files/leftmarginheaderlist/downloadfilename/0000000525/tularemiaprotocol%5b1%5d.pdf.
- Enderlin G, Morales L, Jacobs RF, Cross JT. Streptomycin and alternative agents for the treatment of tularemia: review of the literature. Clin Infect Dis 1994;19:42--7.
* CDC tularemia case report form available at http://www.cdc.gov/tularemia/tul_pubhealthofficials.html.
† A confirmed case was defined as clinically compatible illness with isolation of F. tularensis from a clinical specimen or a fourfold or greater change in paired serum antibody titers to F. tularensis antigen between acute and convalescent samples. A probable case was defined as clinically compatible illness with detection of F. tularensis in a clinical specimen by fluorescent assay or a single elevated serum antibody titer to F. tularensis antigen, as determined by individual laboratory cutoff values. Case definitions available at http://www.cdc.gov/ncphi/disss/nndss/casedef/tularemia_current.htm.
§ Additional information available at http://www.cdc.gov/niosh/topics/tick-borne.
FIGURE 1. Average annual incidence rate of tularemia, by age group and sex* --- Missouri, 2000--2007
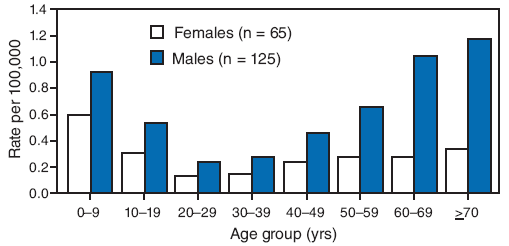
* Among 190 total cases. Reports were included in this analysis if the diagnosis of tularemia met the National Notifiable Disease Surveillance System case definition. A confirmed case was defined as clinically compatible illness with isolation of F. tularensis from a clinical specimen or a fourfold or greater change in paired serum antibody titers to F. tularensis antigen between acute and convalescent samples. A probable case was defined as clinically compatible illness with detection of F. tularensis in a clinical specimen by fluorescent assay or a single elevated serum antibody titer to F. tularensis antigen, as determined by individual laboratory cutoff values. Case definitions available at http://www.cdc.gov/ncphi/disss/nndss/casedef/tularemia_current.htm. Age-specific and sex-specific incidence calculated using 2000 census data.
Alternative Text: The figure above shows the average annual incidence rate of tularemia, by age group and sex in Missouri from 2000 through 2007. According to the figure, during 2000-2007, a total of 190 cases of tularemia (87 confirmed and 103 probable) were reported to MDHSS, yielding a statewide average annual incidence of 0.4 cases per 100,000 population. No increase or decrease was observed in annual trend (range: 13-32 cases per year). The majority of cases were reported from central and southwestern Missouri. The total number of cases by county for the 8-year period ranged from zero to 14, yielding average annual incidence rates that ranged from 0.03 to 5.25 cases per 100,000 population). Males accounted for 125 (66%) patients; median patient age was 37 years (range: 6 months-93 years), with a distinct bimodal distribution among males.
|
TABLE. Number and percentage of human tularemia cases among children (aged ≤18 years) and adults, by year of diagnosis, exposure source, primary clinical form, treatment prescribed, and outcome --- Missouri, 2000--2007* |
||||||
|---|---|---|---|---|---|---|
|
Children |
Adults |
Total |
||||
|
Characteristic |
No. |
(%) |
No. |
(%) |
No. |
(%) |
|
Year of diagnosis |
73 |
(100) |
117 |
(100) |
190 |
(100) |
|
2000 |
9 |
(12) |
14 |
(12) |
23 |
(12) |
|
2001 |
11 |
(15) |
14 |
(11) |
25 |
(13) |
|
2002 |
6 |
(8) |
10 |
(9) |
16 |
(9) |
|
2003 |
15 |
(21) |
15 |
(13) |
30 |
(16) |
|
2004 |
8 |
(11) |
18 |
(16) |
26 |
(14) |
|
2005 |
6 |
(8) |
19 |
(16) |
25 |
(13) |
|
2006 |
4 |
(6) |
9 |
(8) |
13 |
(7) |
|
2007 |
14 |
(19) |
18 |
(16) |
32 |
(17) |
|
Exposure source† |
34 |
(100) |
44 |
(100) |
78 |
(100) |
|
Tick bite |
26 |
(76) |
30 |
(68) |
56 |
(72) |
|
Animal/animal tissue contact |
2 |
(6) |
4 |
(9) |
6 |
(8) |
|
Agricultural or lawnmowing aerosols§ |
0 |
(0) |
6 |
(14) |
6 |
(8) |
|
Multiple exposure sources |
6 |
(18) |
4 |
(9) |
10 |
(13) |
|
Primary clinical form¶ |
45 |
(100)** |
62 |
(100) |
107 |
(100) |
|
Ulceroglandular |
19 |
(42) |
26 |
(42) |
45 |
(42) |
|
Glandular |
20 |
(44) |
10 |
(16) |
30 |
(28) |
|
Pneumonic |
2 |
(4) |
24 |
(39) |
26 |
(24) |
|
Oculoglandular |
3 |
(7) |
1 |
(2) |
4 |
(4) |
|
Oropharyngeal |
1 |
(2) |
1 |
(2) |
2 |
(2) |
|
Treatment prescribed†† |
47 |
(100) |
62 |
(100) |
109 |
(100) |
|
Tetracyclines |
8 |
(17) |
45 |
(71) |
53 |
(49) |
|
Aminoglycosides |
29 |
(62) |
22 |
(35) |
51 |
(47) |
|
Fluoroquinolones |
18 |
(38) |
27 |
(44) |
45 |
(41) |
|
Ineffective antibiotics§§ |
40 |
(82) |
42 |
(58) |
82 |
(75) |
|
Outcome |
49 |
(100) |
72 |
(100) |
121 |
(100) |
|
No complications |
35 |
(71) |
45 |
(63) |
80 |
(66) |
|
Required surgical intervention |
9 |
(18) |
8 |
(11) |
17 |
(14) |
|
Developed more severe secondary form of tularemia |
0 |
(0) |
7 |
(10) |
7 |
(6) |
|
Recurrence of disease¶¶ |
4 |
(8) |
3 |
(4) |
7 |
(6) |
|
Severe organ dysfunction |
0 |
(0) |
6 |
(8) |
6 |
(5) |
|
Multiple complications |
1 |
(2) |
2 |
(3) |
3 |
(2) |
|
Died |
0 |
(0) |
1 |
(1) |
1 |
(1) |
|
* Data on year of diagnosis are for 190 tularemia cases reported to the Missouri Department of Health and Senior Services during 2000--2007. Data on exposure source, primary clinical form, treatment prescribed, and outcome were abstracted from available clinical charts of 121 of these cases. Reports were included in this analysis if the diagnosis of tularemia met the National Notifiable Disease Surveillance System case definition. A confirmed case was defined as clinically compatible illness with isolation of F. tularensis from a clinical specimen or a fourfold or greater change in paired serum antibody titers to F. tularensis antigen between acute and convalescent samples. A probable case was defined as clinically compatible illness with detection of F. tularensis in a clinical specimen by fluorescent assay or a single elevated serum antibody titer to F. tularensis antigen, as determined by individual laboratory cutoff values. Case definitions available at http://www.cdc.gov/ncphi/disss/nndss/casedef/tularemia_current.htm. † Exposure source as documented by the health-care provider in the patient chart. § Lawnmowing aerosols generated by mowing over an animal carcass. ¶ Categorization of primary clinical form based on the recorded history, examination, and health-care provider assessment. ** Percentages do not sum to 100% because of rounding. †† Treatment by antimicrobial class; not mutually exclusive. §§ Beta-lactams, macrolides, and lincosamides are not considered effective for treatment of tularemia (4). ¶¶ Recurrence of disease after a course of an effective antimicrobial drug. |
||||||
FIGURE 2. Number of tularemia cases (N = 78), by month of onset and presumptive exposure source* --- Missouri, 2000--2007
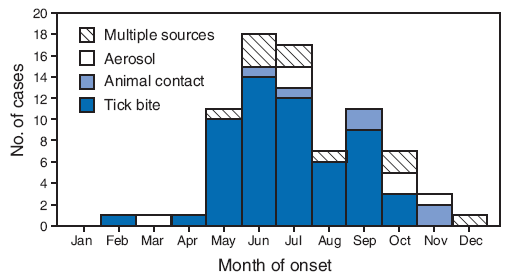
* Data on presumptive exposure source were abstracted as available from clinical charts of 121 cases reported in Missouri during 2000--2007. Reports were included in this analysis if the diagnosis of tularemia met the National Notifiable Disease Surveillance System case definition. A confirmed case was defined as clinically compatible illness with isolation of F. tularensis from a clinical specimen or a fourfold or greater change in paired serum antibody titers to F. tularensis antigen between acute and convalescent samples. A probable case was defined as clinically compatible illness with detection of F. tularensis in a clinical specimen by fluorescent assay or a single elevated serum antibody titer to F. tularensis antigen, as determined by individual laboratory cutoff values. Case definitions available at http://www.cdc.gov/ncphi/disss/nndss/casedef/tularemia_current.htm.
Alternative Text: The figure above show the number of tularemia cases (N = 78), by month of onset and presumptive exposure source from the state of Missouri for the years 200 through 2007. Among 78 cases for which exposure was known, tick bites were the most commonly noted exposures. Eighty percent of tick bite exposures occurred during May-September. Cases associated with other exposures did not show a distinct seasonal trend.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to [email protected].Date last reviewed: 7/16/2009


