Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Introduction and Transmission of 2009 Pandemic Influenza A (H1N1) Virus --- Kenya, June--July 2009
In April 2009, in the United States, the first cases of 2009 pandemic influenza A (H1N1) virus infection were reported (1). On June 11, the World Health Organization (WHO) declared an influenza pandemic because of widespread transmission worldwide (2). As of September 13, all six WHO regions had reported approximately 296,471 cases of pandemic H1N1, including 3,486 deaths (3). On June 29, the first case of pandemic H1N1 was confirmed in Kenya. At that time, only four other countries in sub-Saharan Africa had reported cases (3), and secondary transmission had not been documented in the region. Surveillance activities in Kenya detected four separate introductions of the virus into the country. The introductions provided an opportunity to study transmission of the virus (including calculation of secondary household attack rates) in a virus-naive population that had not yet initiated the use of antiviral drugs. This report describes the four introductions and the accompanying analysis. The overall rate of secondary household transmission of laboratory-confirmed pandemic H1N1 was 26% (range: 7%--33%), which is comparable to secondary household attack rates reported for laboratory-confirmed seasonal influenza virus infection (4,5). However, additional and more rigorous studies are needed to better understand the secondary attack rates associated with the current pandemic.
Since 2006, the Kenya Ministry of Public Health and Sanitation (MoPHS) has maintained 26 influenza sentinel surveillance hospitals and clinics that identify patients with severe acute respiratory illness and influenza-like illness (ILI). Nasopharyngeal and oropharyngeal specimens are collected from severe acute respiratory illness and ILI patients and tested for influenza at two laboratories located in Nairobi: the National Influenza Center (NIC) and the CDC-Kenya International Emerging Infections Program. From the beginning of May through June 28, a total of 28 samples from suspected pandemic H1N1 cases were tested, all of which were negative for pandemic H1N1.
After the first case of pandemic H1N1 was identified in Kenya on June 29, MoPHS conducted contact tracing to identify additional laboratory-confirmed cases. A laboratory-confirmed case was defined by a positive test result for 2009 pandemic influenza A (H1N1) virus by real-time reverse transcription--polymerase chain reaction (rRT-PCR) at the NIC and CDC-Kenya laboratories. A household contact was defined as a person who stayed in the same dwelling (i.e., house or hotel floor) as a patient with a laboratory-confirmed case and had close contact (within 2 meters) beginning 1 day before to 7 days after symptom onset in the patient with laboratory-confirmed infection. As part of contact tracing, all symptomatic close contacts (defined as those having fever or any upper respiratory symptom) had combined nasopharyngeal and oropharyngeal swabs taken, which were tested for pandemic H1N1 using rRT-PCR. Secondary attack rates were computed by dividing the number of contacts who acquired laboratory-confirmed infection and had an epidemiologic link to an index patient by the overall number of household contacts. Prophylactic antivirals were not used in Kenya during the period before and during the investigations described in this report, and none of the travelers to Kenya reported having access to antivirals.
Transmission Groups
Group 1. On June 21, 2009, a group of 34 medical students from Nottingham, United Kingdom (UK), flew from London to Nairobi. During the 9-hour flight, a male student aged 22 years developed headache and chills. The next day, the group took a 5-hour bus trip to Kisumu, a city in western Kenya, where an educational service program had been arranged. The mildly ill student participated in group activities for 3 days, including working at a school and at a center for homeless children. The students from Nottingham, including the ill student, stayed in shared rooms on one floor of a local hotel and had their meals together in the same hotel dining area. On June 24, the index patient developed a cough, sore throat, and subjective fever. On June 25, he heard from a friend in the UK with whom he had close contact before departure that she had become ill with suspected pandemic H1N1. He notified the group leader and voluntarily stayed in isolation in the hotel. Public health authorities were contacted, and nasopharyngeal and oropharyngeal swabs were taken from the index patient on June 27. Infection with pandemic H1N1 was confirmed by rRT-PCR on June 29.
Of the 33 other students and student leaders in the group, 23 (70%) developed subjective fever or upper respiratory symptoms during June 24--30, from 3 to 6 days after symptom onset (June 21) in the index patient (Figure). Specimens were collected from all 23 students; infection with pandemic H1N1 was confirmed by rRT-PCR in 11 (48%) of the symptomatic students (Figure). Influenza B virus was detected by rRT-PCR in one of the 23 students. None of 11 patients with secondary transmission of pandemic H1N1 reported contact with persons having confirmed or suspected pandemic H1N1 cases in the month before their arrival in Kenya, or with any persons in Kenya with ILI. The calculated household secondary attack rate for confirmed pandemic H1N1 in this group was 33% (11 of 33).
Group 2. On June 26, a group of four public health students from London flew to Nairobi and then traveled to Kisumu to work on malaria-related projects. The next day, one student, a woman aged 22 years, developed subjective fever and malaise, followed by a cough 2 days later. On June 29, two of the other students, who reported no contact with anyone with ILI during the preceding month in the UK or while in Kenya (other than the ill student), developed sore throat. On June 30, public health officials in Kenya were notified, and all four students agreed to remain in isolation together in a private house. An rRT-PCR test for pandemic H1N1 was positive in specimens taken from the index patient on July 1. One of the other two ill students tested positive on July 3. For group 2, the secondary attack rate for confirmed pandemic H1N1 among household contacts was 33% (one of three).
Group 3. On June 28, a boy aged 5 years traveled by plane, accompanied by two members of his family, from London to Nairobi on vacation. On June 29, he developed a subjective fever, cough, and vomiting and sought treatment at a Nairobi clinic. Pandemic H1N1 virus was detected by rRT-PCR in specimens taken on June 30. By the time the results became available, the patient had traveled with two family members by road in a private vehicle to Northeastern Province, nearly 500 km away. Contact tracing was conducted, and nine family members in Nairobi and six family members in Northeastern Province (including the patient and the two family members who had traveled with him from Nairobi) were put under voluntary quarantine. On July 3, all 14 quarantined family members were interviewed; none were symptomatic. On July 4, the index patient's brother aged 8 years, who also had traveled from the UK on June 28, developed a runny nose; a specimen was taken, which tested positive for pandemic H1N1 on July 5. The secondary attack rate for confirmed pandemic H1N1 among household contacts was 7% (one of 14).
Group 4. On July 2, a Kenyan man aged 21 years who was studying in the UK traveled by plane to Nairobi on vacation. Upon arrival in Nairobi the same day, he developed sore throat, cough, and headache, but no fever. He reported contact with a person with confirmed pandemic H1N1 while he was in the UK during the week before his departure. On July 4, he sought care for his illness at a Nairobi hospital and had a respiratory specimen taken, which was positive for pandemic H1N1 by rRT-PCR. He remained in isolation at a house in Nairobi for 7 days. Of his four household contacts, one, a woman aged 51 years with hypothyroidism, tested positive for pandemic H1N1; the others had negative test results. The secondary attack rate for confirmed pandemic H1N1 among household contacts was 25% (one of four).
Reported by: C Tabu, Kenya Field Epidemiology and Laboratory Training Program and Kenya Ministry of Public Health and Sanitation. S Sharif, P Okoth, J Kioko, C Nzioka, P Muthoka, M Ope, S Makama, R Kalani, Kenya Ministry of Public Health and Sanitation; W Ochieng, J Simwa, Kenya Medical Research Institute; D Schnabel, W Bulimo, R Achilla, US Army Medical Research Unit (Kenya); J Onsongo, Kenya Country Office, World Health Organization; K Njenga, R Breiman, A Kearney, A Sick, R Harris, E Lebo, P Munyua, L Wakhule, L Waiboci-Muhia, S Gikundi, S Gikunju, V Omballa, L Nderitu, L Mayieka, W Kabura, S Omulo, D Odhiambo, C Wachira, G Kikwai, D Feikin, M Katz, Influenza Program and International Emerging Infections Program, Global Disease Detection Center, CDC-Kenya.
Editorial Note:
This report documents household transmission from the first four laboratory-confirmed cases of pandemic H1N1 in Kenya. The overall 26% secondary attack rate (range: 7%--33%) for laboratory-confirmed pandemic H1N1 is similar to the recently reported 30% secondary attack rate for laboratory-confirmed pandemic H1N1 in a tourist group in China (6). However, among the two student groups (groups 1 and 2), the 33% secondary household attack rate was slightly higher than the 21%--26% usually reported for laboratory-confirmed seasonal influenza (4,5).
The student groups were defined as household contacts because they lived together, ate together, and spent much of their time together, like members of typical households. However, unlike most households, the students were healthy young adults, and the nature of the students' interactions might have differed from typical household interactions. The fact that the students were together throughout the day (unlike usual households, where persons might go to work and to school) might have created an environment to facilitate transmission of the pandemic H1N1.
The findings in this report are subject to at least four limitations. First, extensive contact tracing was conducted only for these four groups at the beginning of the outbreak in Kenya. Therefore, the secondary attack rates reported are based on small numbers and are not generalizable. Second, for contacts who developed infection shortly after the index patient (e.g., the student contact in group 2), whether these patients acquired infection from a common exposure (e.g., an ill contact of the index patient) rather than via secondary transmission cannot be determined. Third, if infected contacts had not been shedding virus at the time of specimen collection, because they were early or very late in the course of their illness, the specimen would have tested negative. Testing of acute and convalescent sera or sampling symptomatic contacts on multiple days might have identified such cases. Therefore, the secondary attack rates in this report might be an underestimation of the actual rates. Finally, because contacts might not have remembered being exposed to persons with ILI, the findings might be subject to recall bias.
Since the introduction of the four cases, pandemic H1N1 has spread throughout all of Kenya's six provinces. As of October 9, Kenya had identified 359 cases of pandemic H1N1; 28 (7.8%) had been in patients who subsequently were hospitalized, and none had died. Despite the mild nature of the initial infections in Kenya, the H1N1 pandemic ultimately might have severe outcomes in Africa. In Africa, relatively high rates of malnutrition and human immunodeficiency virus, malaria, and other infectious diseases, combined with limited health-care infrastructure and reduced health-care utilization, create the possibility that the H1N1 pandemic might cause more severe disease and deaths in Africa (7,8).
The results of this investigation should be interpreted with caution. Although the overall 26% secondary attack rate of laboratory-confirmed 2009 pandemic influenza A (H1N1) virus infection appears comparable to that for laboratory-confirmed seasonal influenza, more studies are needed to better understand the secondary attack rates in both traditional and nontraditional household settings.
References
- World Health Organization. Influenza-like illness in the United States and Mexico. Available at http://www.who.int/csr/don/2009_04_24/en/index.html. Accessed October 14, 2009.
- World Health Organization. New influenza A (H1N1) virus: global epidemiologic situation, June 2009. Wkly Epidemiol Rec 2009;84:249--57.
- World Health Organization. Pandemic (H1N1) 2009. Available at http://www.who.int/csr/disease/swineflu/en/index.html. Accessed October 14, 2009.
- Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA 2001;285:748--54.
- Hayden FG, Belshe R, Villanueva C, et al., Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis 2004;189:440--9.
- Han K, Zhu X, He F, et al. Lack of airborne transmission during outbreak of pandemic (H1N1) 2009 among tour group members, China, June 2009. Emerg Infect Dis 2009;15(10). Available at http://www.cdc.gov/eid/content/15/10/1578.htm. Accessed October 14, 2009.
- Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918--20 pandemic: a quantitative analysis. Lancet 2006;368:2211--8.
- Breiman RF, Nasidi A, Katz MA, Kariuki Njenga M, Vertefeuille J. Preparedness for highly pathogenic avian influenza pandemic in Africa. Emerg Infect Dis 2007;13:1453--8.
|
What is already known on this topic? One small, retrospective study reported a 30% secondary attack rate for laboratory-confirmed 2009 pandemic influenza A (H1N1) virus infection among tourists in China. What is added by this report? Investigations into the initial cases of pandemic H1N1 in Kenya during June--July 2009 showed an overall laboratory-confirmed secondary attack rate of 26% in households, which is similar to laboratory-confirmed secondary attack rates reported for seasonal influenza. What are the implications for public health practice? Additional studies are needed to better understand the secondary attack rates for laboratory-confirmed pandemic H1N1 in both traditional and nontraditional household settings. |
FIGURE. Number of laboratory-confirmed cases of 2009 pandemic influenza A (H1N1) virus infection,* by transmission group and date of symptom onset† --- Kenya, June--July 2009
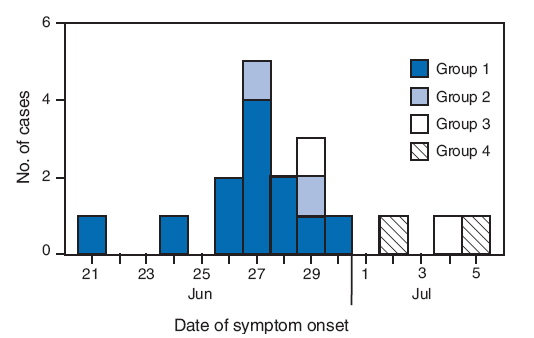
* N = 18. A laboratory-confirmed case was defined by a positive test result for 2009 pandemic influenza A (H1N1) virus by real-time reverse transcription--polymerase chain reaction at two laboratories located in Nairobi, Kenya: the National Influenza Center and the CDC-Kenya International Emerging Infections Program.
† Onset of fever or any upper respiratory symptom.
Alternative Text: The figure above shows the number of laboratory-confirmed cases of 2009 pandemic influenza A (H1N1) virus infection, by transmission group and date of symptom onset in Kenya from June through July 2009. On June 21, 2009, a group of 34 medical students from Nottingham, United Kingdom, flew from London to Nairobi. During the 9-hour flight, a male student aged 22 years developed headache and chills. Of the 33 other students and student leaders in the group, 23 (70%) developed subjective fever or upper respiratory symptoms during June 24-30, from 3 to 6 days after symptom onset (June 21) in the index patient.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to [email protected].Date last reviewed: 10/21/2009


