Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail.
West Nile Virus Transmission via Organ Transplantation and Blood Transfusion --- Louisiana, 2008
Three years after the introduction and spread of West Nile virus (WNV) in the United States, transmission through blood transfusion and solid organ transplantation was documented in 2002 (1--3). Within a year, these findings led to nationwide screening of blood donors for WNV. Although screening is extremely sensitive, current methods still do not detect all WNV-infected blood donations, and organ donors are not screened routinely. In October 2008, the Louisiana Department of Health (LDH) was notified of a heart transplant recipient with suspected West Nile neuroinvasive disease (WNND). LDH launched an investigation to confirm the diagnosis and determine whether the organ recipient's infection was derived from the organ donor or blood products the donor received before organ donation. The investigation concluded that two cases of probable transfusion-transmitted WNV resulted from a common blood donor; one infection resulted in WNND via an organ donor, and the other resulted in asymptomatic WNV infection via blood transfusion directly. This investigation also found that criteria used by the blood-screening laboratory to screen the implicated blood donation for WNV were less stringent than criteria used by other blood collection centers in the area. Use of the more stringent screening criteria might have detected the WNV and prevented the blood donation from being used. To increase the likelihood of detecting WNV-positive donations, blood centers should use the most sensitive screening criteria feasible and communicate frequently with nearby blood centers on screening results during times of high WNV activity in their geographic area. In addition, health-care providers should consider WNND as a possible cause of neurologic complications in patients after blood transfusion or organ transplantation.
Organ Transplant
On October 23, 2008, LDH was notified of a transplant recipient with suspected WNND (Table). The patient, a man aged 62 years, had undergone a heart transplant on September 23, 2008, because of hypertrophic cardiomyopathy and congestive heart failure. The patient had an uneventful postoperative course until the eighth day after his transplantation, when he had tonic-clonic seizures requiring intubation and transfer to the intensive-care unit (ICU). In the ICU, the patient was febrile (101°F [38°C]) with a normal peripheral white blood cell (WBC) count (8.33 × 109/L) and blood chemistries. He was treated empirically with a combination of piperacillin and tazobactam for possible bacterial infection. He was extubated the next day, but his mental status continued to deteriorate. Because neurologic complications occurring in patients after transfusion or organ transplantation can result from donor-related WNV transmission,* specimens from the patient were tested for WNV infection.† Serum and cerebrospinal fluid (CSF) obtained 22 days (October 15) and 31 days (October 24) posttransplantation, respectively, were positive for WNV immunoglobulin M (IgM) antibodies by enzyme-linked immunosorbent assay (ELISA) performed at a commercial laboratory. Testing at CDC on a serum specimen obtained 43 days posttransplantation showed WNV-specific neutralizing antibodies with a titer of 20,480 (positive titer ≥10), confirming the WNND diagnosis.
A pretransplant serum specimen tested negative for WNV RNA by reverse transcription--polymerase chain reaction and negative for WNV IgM and immunoglobulin (IgG) antibodies, suggesting the patient's infection occurred around the time of the transplantation. At discharge on December 17, 2008, he was unable to walk without assistance and was transferred to a skilled nursing facility for rehabilitation; he was discharged from that facility on January 9, 2009, to continue physical therapy as an outpatient.
The recipient reported that, before his heart transplantation, he went outside his home infrequently and applied mosquito repellent when going outside. He did not recall any mosquito bites before his hospitalization. Perioperatively, he received 10 blood products from 16 donors while on cardiopulmonary bypass pump. Blood from these donations was not available for testing. Eight of the 16 donors were contacted successfully and voluntarily agreed to testing; all eight were WNV IgM negative by ELISA.
The heart donor, a man aged 18 years, was admitted to the hospital with a gunshot wound to the head on September 21, 2008. He received 10 blood products before being declared brain dead on September 23. His heart was the only organ or tissue procured. After potential donor-related WNV transmission was recognized on October 15, donor serum collected at the time of heart donation and after receipt of blood products was tested by both a commercial laboratory and CDC, and was negative for WNV RNA and WNV IgM and IgG antibodies. All blood donations received by the heart donor were tested by the blood screening laboratory used by blood center A and were negative by WNV minipool nucleic acid-amplification testing (MP-NAT) on pools of 16 samples. The blood screening laboratory used by blood center A at the time of these donations had not met its criteria for switching from MP-NAT to individual donation NAT (ID-NAT)§ (Figure). All blood donors were contacted and returned for WNV IgM screening; nine tested negative for WNV IgM and IgG at a reference laboratory. One blood donor's serum collected 8 weeks after donation tested positive at CDC for WNV IgM antibodies and WNV-specific neutralization antibodies with a titer of 1:640, indicating recent WNV infection.
Blood Transfusion
The blood donation from the WNV-positive donor occurred on September 15, 2008. The blood donor lived in a parish with little WNV activity but had spent time outdoors near the time of donation. The donor recalled having a self-limited illness characterized by weakness, body aches, and fever on September 24. Fresh frozen plasma and packed red blood cells were derived from this donation. The fresh frozen plasma was given to the heart donor and the packed red blood cells to a woman nursing home resident aged 76 years. The woman was admitted to a local hospital for atrial fibrillation with rapid ventricular response on September 19 and received the unit of packed red blood cells for anemia. Although the woman reportedly never developed a febrile illness, serum obtained as part of the investigation on December 1, 2008, tested positive for WNV-specific IgM and neutralizing antibodies at a titer of 1:1280.
Reported by: E Stanley, MPH, R Ratard, MD, Louisiana Dept of Health. JE Staples, MD, R Royce, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; WA Bower, MD, KD Ellingson, PhD, MJ Kuehnert, MD, Div of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, CDC.
Editorial Note:
This report describes two cases of probable transfusion-transmitted WNV from a common blood donor. One infection resulted in WNND via an organ donor, and the other resulted in asymptomatic WNV infection via blood transfusion directly. The source of infection cannot be proved definitively because blood samples or other components from the implicated donation were unavailable for testing. However, evidence of WNV infection in the two patients linked to blood products from a common donor, along with serologic evidence of recent infection and a febrile illness in the donor shortly after blood donation, make these probable cases of transfusion-transmitted WNV (4).
After heart transplantation, the organ recipient described in this report was immunosuppressed when he had onset of WNND. This patient likely was infected with WNV after receipt of the transplant heart from a donor who received multiple blood transfusions, at least one of which was suspected to contain WNV. If exposed to WNV, transplant recipients are at high risk for WNND; however, organ donors are not routinely screened for WNV. Both organ donors and transplant recipients often receive multiple transfusions. Early recognition and notification of a potential donor-related transmitted disease could result in earlier WNV infection diagnosis and initiation of supportive care in organ transplant recipients who received organs from the same donor. Health-care providers should consider WNND when neurologic complications occur in patients after transfusion or organ transplantation. In addition, timely recognition of potential contamination with WNV or other transfusion-transmitted pathogens could lead to removal of potentially infectious blood products from the blood supply.
Screening blood or organ and tissue donors based on clinical symptoms is ineffective at preventing donor-related WNV infections (5). The Food and Drug Administration (FDA) provides guidance to blood centers for donor screening to reduce the risk for transfusion-transmitted disease. FDA recommends screening year-round for WNV using a licensed NAT (MP-NAT or ID-NAT) on donor samples of whole blood and blood components (6). Because of the dilution effect inherent in screening by MP-NAT, this method is less sensitive than ID-NAT. FDA further recommends that blood centers using MP-NAT screening establish criteria to switch to ID-NAT during periods of high WNV activity in their geographic area of collection. No cases of transfusion-related transmission have been reported when using ID-NAT to screen for WNV. However, routine ID-NAT screening is not feasible for many blood centers because of the resulting logistic and financial burdens. Therefore, most blood centers use WNV MP-NAT screening until a trigger threshold of one or more positive MP-NAT results is reached over a specific period and then switch to ID-NAT. Each blood center has its own triggering threshold, developed within the constraints of FDA guidance and standards of AABB (formerly known as the American Association of Blood Banks) (7).¶
The WNV screening policy at the laboratory used by blood center A, where the presumed WNV-contaminated donation was collected, set the trigger to switch from MP-NAT to ID-NAT as identification of two MP-NAT positive donations from the same postal code area within a 7-day period. Blood center A had collected an MP-NAT--positive donation on September 11, and collected the implicated blood donation on September 15. Blood center B, another blood center collecting in the same region, had a policy of transitioning from MP-NAT to ID-NAT after identification of one MP-NAT positive donation within its blood collection area, including those identified by other blood centers in the region. Screening data from September indicated that blood center B transitioned to ID-NAT during the period the implicated donation was collected by blood center A, based on the positive MP-NAT collected on September 11 by another blood center, which collects blood in a region that overlaps that of blood center A. Use of triggering criteria, at a minimum, as sensitive as that used by blood center B could have resulted in the implicated donation being tested by ID-NAT, found reactive, and removed from the blood supply.
Adoption of a single standard for all triggering criteria would be desirable; however, differences exist between blood centers because of geographic variability of WNV activity, amplifying logistical concerns. Although universal ID-NAT screening is the more conservative option, MP-NAT can be a highly effective screening strategy if coupled with an appropriate strategy for triggering ID-NAT testing. Recent modeling has suggested that initiation of ID-NAT in a previously defined geographic region or zone should be based on one MP-NAT--reactive donation (8). A second model examining 27 triggering strategies suggested that effectiveness increased when triggering was based on one positive MP-NAT rather than two during a 7-day period (9). Further simulations based on data from a 2006 transfusion-transmitted WNV investigation, in which the positive donation went undetected by minipool testing (10), suggest that triggering based on one MP-NAT might have resulted in detection of the WNV positive unit (CDC, unpublished data, 2008). Similarly, the triggering data described in this report suggest that the WNV-contaminated donation might have been detected before use by triggering to ID-NAT on one MP-NAT positive donation. In regions served by more than one blood center, close communication between blood centers locally is critical. Blood centers can increase the likelihood of detecting WNV in donated blood by using screening strategies that trigger the most timely use of ID-NAT, selection of geographic areas larger than a single postal code area, and ongoing communication of screening results between all facilities that collect blood in a geographic area.
Acknowledgments
The findings in this report are based, in part, on contributions from JB Garcia-Diaz, MD, FR Rodwig Jr, MD, Ochsner Medical Center, New Orleans; and C Moore, Our Lady of the Lake Regional Medical Center, Baton Rouge, Louisiana.
References
- CDC. Outbreak of West Nile-like viral encephalitis---New York, 1999. MMWR 1999;48:845--9.
- Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med 2003;348:2196--203.
- Pealer LN, Marfin AA, Petersen LR, et al. Transmission of West Nile virus through blood transfusion---United States, 2002. N Engl J Med 2003;349:1236--45.
- Montgomery SP, Brown JA, Kuehnert M, et al. Transfusion-associated transmission of West Nile virus, United States 2003 through 2005. Transfusion 2006;46:2038--46.
- Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion 2006;46:272--7.
- Food and Drug Administration. Guidance for industry: use of nucleic acid tests to reduce the risk of transmission of West Nile virus from donors of whole blood and blood components intended for transfusion. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration; 2008.
- Custer B, Busch MP, Marfin AA, Petersen LR. The cost-effectiveness of screening the U.S. blood supply for West Nile virus. Ann Intern Med 2005;143:486--92.
- Kleinman SH, Williams JD, Robertson G, et al. West Nile virus testing experience in 2007: evaluation of different criteria for triggering individual-donation nucleic acid testing. Transfusion 2009;49:1160--70.
- Biggerstaff BJ, Petersen LR. A modeling framework for evaluation and comparison of trigger strategies for switching from minipool to individual-donation testing for West Nile Virus. Transfusion 2009;49:1151--9.
- CDC. West Nile virus transmission through blood transfusion---South Dakota, 2006. MMWR 2007;56:76--9.
* Although most WNV infections are asymptomatic, approximately 20% of infected persons develop a self-limited febrile illness after an incubation of 3--6 days. Incubation periods up to 1 month have been documented after blood transfusion and organ transplantation (2,3). Less than 1% of infected immunocompetent persons will develop more severe neuroinvasive disease (e.g., encephalitis, meningoencephalitis, meningitis, or poliomyelitis-like acute flaccid paralysis). However, as many as 40%--60% of immunocompromised persons infected as a result of receiving a WNV-infected organ develop neuroinvasive disease.
† WNV infections typically are diagnosed based on serologic testing and detection of WNV immunoglobulin M (IgM) and neutralizing antibodies in serum or cerebrospinal fluid. WNV IgM antibodies are normally detected within 8 days of illness onset in immunocompetent persons. Nucleic acid-amplification testing (NAT) detects the presence of WNV before development of WNV IgM antibodies and is used by blood centers to screen for WNV in donated blood. WNV screening by blood centers is performed on pooled samples (i.e., minipool NAT [MP-NAT]). Individual blood centers have differing criteria for switching to individual sample testing (ID-NAT) when a positive MP-NAT is detected.
§ Two MP-NAT positive donations from the same postal code area within a 7-day period.
¶ After reviewing comments and announcing the availability of guidance on use of NATs for screening for blood WNV transmission, FDA noted, "At this time, there is insufficient data to recommend uniform threshold criteria for switching from MP-NAT screening to ID-NAT screening. Until we have sufficient data to support the development of suitable uniform threshold criteria, we consider it appropriate for each blood establishment to define its own threshold criteria for switching from MP-NAT to ID-NAT screening and for reverting to MP-NAT screening." Federal Register 2009;74:57685--6.
|
What is already known on this topic? Despite sensitive screening assays for West Nile virus (WNV) in blood donations, risks remain for WNV transmission through blood transfusion and organ transplant. What is added by this report? This report found that blood screening using a less sensitive protocol failed to detect WNV in donated blood, leading to probable transmission to two persons, one directly through blood transfusion and the other by organ transplant through a transfused organ donor. What are the implications for public health practice? To further decrease the incidence of WNV transfusion- and transplant-related disease transmission, blood centers should adopt the most sensitive triggering strategies feasible during periods of high WNV activity, by optimizing the transition from pooled blood sample testing to individual sample screening through standard protocols and communication. |
FIGURE. Strategies used by laboratories to screen donated blood for West Nile virus (WNV) --- Louisiana, 2008
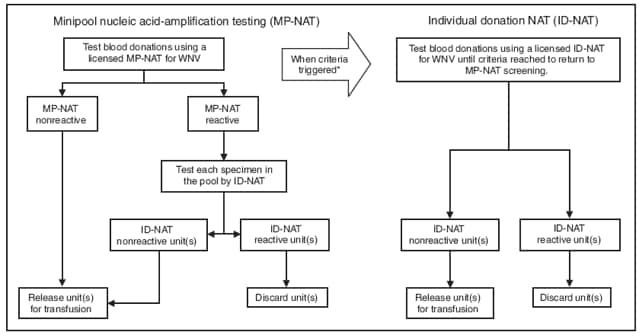
* Blood centers generally pool blood samples for screening until WNV is identified in one or more samples. Established criteria for switching from MP-NAT to ID-NAT can vary greatly between blood centers. Some centers transition from MP-NAT to ID-NAT after identification of two MP-NAT positive donations from donors living in the same postal code area within a 7-day period. Other centers transition to ID-NAT if one donation is identified as positive by MP-NAT at any blood center within their collection area. When the established criteria are met, all donations then are screened by ID-NAT until no positive donations are detected for a predetrimined period (usually 7 days); then they transition back to MP-NAT.
Alternate Text: The figure shows strategies used by blood screening laboratories to screen donated blood for West Nile virus (WNV) in Louisiana in 2008. Laboratories use nucleic acid-amplification testing to detect WNV antibodies in blood samples. Several samples may be pooled and tested as one to save time and money. The figure shows the decision trees laboratories typically follow for testing pooled samples and deciding when to test individual samples.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to [email protected].Date last reviewed: 11/19/2009


