FIGURE. Estimated influenza A (H1N1) 2009 monovalent vaccination coverage among children and adults,* by age group and state --- United States, Behavioral Risk Factor Surveillance System and National 2009 H1N1 Flu Survey, end of January 2010
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Interim Results: State-Specific Influenza A (H1N1) 2009 Monovalent Vaccination Coverage --- United States, October 2009--January 2010
In July 2009, the Advisory Committee on Immunization Practices (ACIP) issued recommendations for the use of influenza A (H1N1) 2009 monovalent vaccine (1). Distribution of 2009 H1N1 vaccine in the United States began on October 5, using a system that allocated available vaccine to states proportional to their populations. By the end of 2009, approximately 61 million persons had been vaccinated (2). By January 29, 2010, approximately 124 million doses had been distributed. To provide preliminary state-specific estimates of 2009 H1N1 vaccination coverage as of the end of January, CDC analyzed results from the Behavioral Risk Factor Surveillance System (BRFSS) and the National 2009 H1N1 Flu Survey (NHFS), using data collected during November 2009--February 2010. This report summarizes the results of that analysis, which found that, by state, estimated 2009 H1N1 vaccination coverage as of the end of January among persons aged ≥6 months ranged from 12.9% to 38.8% (median: 23.9%). Median coverage was 36.8% for children aged 6 months--17 years, 20.1% for adults aged ≥18 years, and 33.2% for persons in the ACIP initial target group.* The wide variation in 2009 H1N1 vaccination rates among states suggests opportunities for improvement in future seasons, such as maintaining and increasing the reach of networks of private providers as vaccinators and distributing more vaccine through public venues (e.g., schools).
To provide state-specific estimates for selected groups (1), CDC combined data collected during November 2009--February 2010 from two separate surveys, BRFSS and NHFS (3). BRFSS is a state-based telephone survey collecting information from approximately 400,000 randomly selected persons aged ≥18 years among the noninstitutionalized, U.S. civilian population.† To determine 2009 H1N1 vaccination coverage, starting in October 2009, BRFSS respondents in 49 states, the District of Columbia, and two territories were asked if they (or their children, in 46 states and the District of Columbia) had been vaccinated for the "H1N1 flu" since September, and if so, in which month.§ NHFS is a new telephone survey operating from October 2009 through June 2010 to track 2009 H1N1 and seasonal vaccination coverage (2). To determine influenza vaccination status, NHFS respondents aged ≥18 years were asked whether they (or their children) had received "an H1N1 flu vaccination" since September, and if so, in which month.¶
To improve the precision of state-level estimates from each survey, CDC combined data collected during November 2009--February 2010 and estimated the cumulative proportion of persons vaccinated with at least 1 dose during October--January using the Kaplan-Meier survival analysis procedure. If a child aged 6 months through 9 years received 2 doses, the month of the first dose was used in the analysis.
To further improve precision for subgroups within states, particularly for children, CDC averaged the BRFSS and NHFS estimates.** Overall sample sizes for children were 52,322 from NHFS and 21,542 from BRFSS, and for adults, 24,924 from NHFS and 115,528 from BRFSS. For both NHFS and BRFSS, respondents with missing 2009 H1N1 vaccination information were excluded; persons with reported vaccination in September were included as unvaccinated.
Results from both surveys were weighted and analyzed with statistical software that accounts for complex survey design. For NHFS, the Council of American Survey and Research Organizations (CASRO) response rate for the weekly sample groups released through January were 35% for landline telephones and 27% for cellular telephones; cooperation rates were 45% and 57% for landline and cellular telephones, respectively. For BRFSS, the median state CASRO response and cooperation rates for data collected during October--February were 54% (range: 47%--57%) and 76% (range: 72%--78%), respectively.
Among persons aged ≥6 months, 2009 H1N1 vaccination rates by end of January ranged from 12.9% in Mississippi to 38.8% in Rhode Island (U.S. median: 23.9%). Coverage among children aged 6 months through 17 years ranged from 21.3% in Georgia to 84.7% in Rhode Island (U.S. median: 36.8%), and was higher than the rate among adults for all states (U.S. median: 20.1%) (Figure and Table). Child and adult coverage were highly correlated (r = 0.84).
For persons in the ACIP initial target groups, 2009 H1N1 vaccination coverage ranged from 19.4% in Mississippi to 57.5% in Rhode Island (U.S. median: 33.2%). Median vaccination coverage for the subset of adults aged 25--64 years with high-risk conditions included in the initial target group was 25.2% (range: 10.4%--47.2%). Among adults not in the initial target group, coverage was higher in most states for adults aged ≥65 years (median: 22.0%) compared with adults aged 25--64 years (median: 14.4%).
Reported by
PJ Lu, MD, PhD, H Ding, MD, GL Euler, DrPH, C Furlow, PhD, LN Bryan, MS, B Bardenheier, MA, MPH, D Yankey, MS, E Monsell, AG Gonzalez-Feliciano, MPH, C LeBaron, MD, JA Singleton, MS, Immunization Svcs Div, National Center for Immunization and Respiratory Diseases; M Town, MS, L Balluz, ScD, Div of Adult and Community Health, National Center for Chronic Disease Prevention and Health Promotion, CDC.
Editorial Note
The majority of states achieved 2009 H1N1 vaccination rates among children that were higher than previous pediatric seasonal influenza vaccination rates (4,5) and the 30% measured for the 2008--09 season (CDC, unpublished data, 2009). Four states in the New England region had estimated 2009 H1N1 vaccination coverage ≥60%, and 10 states had coverage <30%. Of the four New England states achieving high coverage in children, three had conducted statewide school vaccination campaigns that coincided with a period of high demand for vaccine. The relatively high 2009 H1N1 vaccination coverage among children is a reflection of the focus in many states on childhood vaccination, the ability to use the infrastructure of the childhood vaccination program, the use of school-located vaccination clinics in some states, recognition by providers and parents of the risk for severe outcomes among children (particularly those with certain medical conditions), and other factors.
The 2009 H1N1 vaccination coverage rate among adults at high risk aged 25--64 years was lower (median: 25%) than the rate among children. Reasons for this might include a lesser emphasis on vaccination of this population compared with children, lack of preexisting relationship of state immunization programs with providers who serve adults at high risk, difficulty in implementing a risk-condition--based recommendation for persons in this age group (resulting in vaccination program implementation challenges), and historically low seasonal influenza vaccination rates in this population (4,5).
2009 H1N1 vaccine was funded by the federal government and distributed free of charge in partnership with state and local health departments. Available vaccine supplies were allocated to states proportional to their total populations, and shipped to public and private provider vaccination sites determined by the states. States developed implementation strategies in accordance with ACIP recommendations for the initial target groups (1), specifying vaccine distribution by provider type in consideration of local supply and demand or other factors. By late December, vaccine supply was sufficient for most states to broaden their vaccination recommendations to include the entire population, and vaccine was widely available in community sites such as pharmacies. States used multiple strategies, with varying emphasis on school-located vaccination or other mass vaccination strategies and reliance on vaccination in provider offices.
The state-level estimates of 2009 H1N1 coverage in this report measure coverage as of the end of January 2010. To assess the extent of continued H1N1 vaccination through February nationally, CDC analyzed interview data through March 14 for BRFSS and March 20 for NHFS. As of the end of February, based on data from BRFSS and NHFS combined, the estimated coverage rate was 24.0% (95% confidence interval [CI] = 23.4%--24.6%), representing 72 million persons (CI = 70--74 million persons) vaccinated. Using NHFS data alone, the rate was 27.1% (CI = 25.9%--28.3%), representing 81 million persons (CI = 77--85 million persons) vaccinated. Counting all doses reported, an estimated 81--91 million doses were administered.
Combining BRFSS and NHFS estimates increased the size of the sample to approximately 200,000 persons and increased precision of estimates but might also constitute a limitation in interpretation of the results. Although BRFSS and NHFS are measuring the same population, differences in survey methods might lead to different levels of bias (e.g., inclusion of a cellular telephone sample in NHFS, variations in survey questions, the context of a general health survey [BRFSS] versus a influenza-specific focus [NHFS], survey operations and weighting, and response rates) (3). These differences might contribute to different levels of bias that are averaged over in the combined estimates in this report. For example, estimated 2009 H1N1 vaccination coverage by end of January was higher when based on NHFS alone compared with combined estimates (1.0, 3.6, and 2.8 percentage points higher for children, adults, and all ages, respectively). In the future, comparisons with the National Health Interview Survey might help determine the potential bias of the combined estimates.
The findings in this report are subject to at least three other limitations. First, BRFSS is a landline, telephone-based survey and thus excludes persons in households without landline telephones; NHFS includes households with landlines as well as those with only cellular telephone service; and both BRFSS and NHFS exclude households with no telephone service. Second, response rates for both surveys were low, and nonresponse bias might remain after weighting adjustments. Finally, self-reported 2009 H1N1 vaccination status is subject to respondents' recall and was not validated with medical records; persons also might have confused receipt of 2009 H1N1 vaccination with seasonal influenza vaccination.
CDC is collecting additional information on state vaccination programs to understand reasons for variations in state-level coverage and identify program factors associated with high vaccination coverage. CDC will continue to analyze BRFSS and NHFS data to provide updated estimates of vaccine utilization and identify specific aspects of the 2009 H1N1 vaccination program that were successful and could be integrated to improve future season vaccination rates.
Acknowledgments
The findings in this report are based, in part, on contributions by K Copeland, N Ganesh, M Montgomery, M Stanislawski, K Wolter, and others at the National Opinion Research Center, Chicago, Illinois; state BRFSS coordinators; members of the CDC H1N1 Vaccine Coverage Monitoring Team; and members of the CDC Behavioral Surveillance Branch.
References
- CDC. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-10).
- CDC. Interim results: influenza A (H1N1) 2009 monovalent vaccination coverage---United States, October--December 2009. MMWR 2010;59:44--8.
- Schenker N, Raghunathan TE. Combining information from multiple surveys to enhance estimation of measures of health. Stat Med 2007;26:1802--11.
- CDC. Influenza vaccination coverage among children and adults---United States, 2008--09 season. MMWR 2009;58:1091--5.
- CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-8):26.
* Initial target groups identified by ACIP include pregnant women, health-care and emergency medical services personnel, children and young adults aged 6 months through 24 years, persons aged 25--64 years who have medical conditions that put them at higher risk for influenza-related complications, and persons who live with or provide care for infants aged <6 months. For this report, the latter group was not included in the analysis because contact with infants aged <6 months was not identified by BRFSS. Questions regarding health-care personnel used in both BRFSS and NHFS included: "Do you currently volunteer or work in a hospital, medical clinic, doctor's office, dentist's office, nursing home, or some other health-care facility? This includes part-time and unpaid work in a health-care facility as well as professional nursing care provided in the home." and "Do you provide direct patient care as part of your routine work? By direct patient care we mean physical or hands-on contact with patients." Influenza-related high-risk conditions included in both surveys were diabetes, asthma, myocardial infarction, coronary heart disease, lung problems other than asthma, kidney problems, anemia (including sickle cell), and a weakened immune system caused by illness or medicines.
† Additional information is available at http://www.cdc.gov/brfss.
§ The wording of the questions regarding 2009 H1N1 vaccination included the following: "There are two ways to get the H1N1 flu vaccination. One is a shot in the arm and the other is a spray, mist, or drop in the nose. Since September 2009, have you been vaccinated either way for the H1N1 flu?"
¶ Respondents were asked, "Since September 2009, have you had an H1N1 flu vaccination?" The landline telephone sample was augmented with a sample of children aged <18 years identified during screening for the National Immunization Survey. Additional information is available at http://www.cdc.gov/nis/h1n1_introduction.htm and http://www.cdc.gov/nis/data/h1n1_flu_survey.pdf.
** Combined estimates were weighted averages of the BRFSS and NHFS estimates, with weights being determined by the effective sample sizes. The effective sample sizes take into account the design of each survey and are determined as the unweighted sample size divided by the design effect. The design effect is the ratio of the variance of a survey estimate to the variance had the survey been a simple random sample; surveys with large design effects are less efficient. CDC estimated state and age group specific design effects based on estimated proportions vaccinated each month, using data from each survey from November 2009 through February 2010. Among states, the median design effects for children were 2.2 for NHFS and 1.3 for BRFSS, with the BRFSS estimate receiving a median of 50% of the weight in the combined average estimate. For adults, median design effects were 1.2 for NHFS and 1.7 for BRFSS, with BRFSS estimates receiving a median of 80% of the weight. The NHFS estimate was used alone when no data were available from BRFSS.
What is already known on this topic?
Distribution of 2009 H1N1 vaccine in the United States began on October 5, 2009, using a system that allocated available vaccine to states proportional to their populations; by January 29, 2010, approximately 124 million doses of H1N1 influenza vaccine had been distributed.
What is added by this report?
Estimated 2009 H1N1 vaccination coverage as of the end of January among persons aged ≥6 months ranged among states from 12.9% to 38.8% (U.S. median: 23.9%) and 33.2% (range: 19.4%--57.5%) for persons in the Advisory Committee on Immunization Practices (ACIP) initial target group.
What are the implications for public health practice?
The wide variation in H1N1 vaccination rates among states suggests opportunities for improvement in future seasons, such as increasing the reach of networks of private providers as vaccinators, and distributing more vaccine through public venues (e.g., schools).
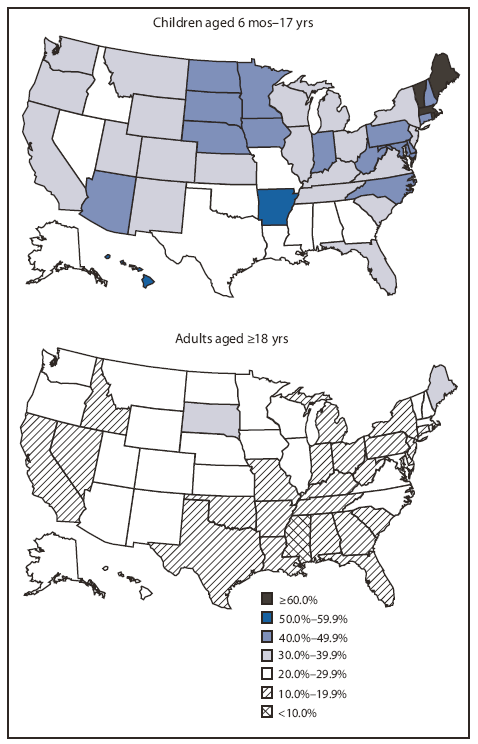
* Coverage estimates are for persons with reported vaccination during October 2009--January 2010 who were interviewed during November 2009--February 2010.
Alternate Text: The figure above shows estimated influenza A (H1N1) 2009 monovalent vaccination coverage among children and adults, by age group and state, based on results from the United States, Behavioral Risk Factor Surveillance System and National 2009 H1N1 Flu Survey, through the end of January 2010. Coverage among children aged 6 months through 17 years ranged from 21.3% in Georgia to 84.7% in Rhode Island (U.S. median: 36.8%), and was higher than the rate among adults for all state (U.S. median: 20.1%).
|
TABLE. (Continued) Estimated influenza A (H1N1) 2009 monovalent vaccination coverage among children and adults,* by U.S. Department of Health and Human Services (DHHS) region, state, and selected age and priority subgroups --- United States, Behavioral Risk Factor Surveillance System (BRFSS) and National 2009 H1N1 Flu Survey (NHFS), end of January 2010† |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
DHHS region and state |
Unweighted sample size |
Children aged 6 mos to 17 yrs |
Persons aged ≥18 yrs |
Persons in the initial target groups¶ |
Persons aged 25--64 yrs at high risk** |
Persons aged 25--64 yrs not in the initial target groups |
Persons aged ≥65 yrs |
Persons aged ≥6 mos |
|||||||
|
% |
(95% CI§) |
% |
(95% CI) |
% |
(95% CI) |
% |
(95% CI) |
% |
(95% CI) |
% |
(95% CI) |
% |
(95% CI) |
||
|
Region 10 |
17,208 |
34.6 |
(±3.9) |
22.0 |
(±2.0) |
34.6 |
(±3.4) |
33.3 |
(±6.7) |
14.7 |
(±2.2) |
25.9 |
(±3.9) |
25.0 |
(±1.8) |
|
Alaska |
2,066 |
26.6 |
(±5.6) |
24.5 |
(±8.8) |
29.4 |
(±5.8) |
26.5 |
(±11.7)†† |
---¶¶ |
--- |
---¶¶ |
--- |
25.0 |
(±6.7) |
|
Idaho |
3,303 |
29.5 |
(±5.6) |
17.8 |
(±3.2) |
27.8 |
(±4.5) |
24.4 |
(±9.0) |
14.4 |
(±3.9) |
12.4 |
(±4.6) |
21.2 |
(±2.8) |
|
Oregon |
3,390 |
35.3 |
(±6.3) |
20.9 |
(±3.3) |
31.8 |
(±5.0) |
30.1 |
(±9.0) |
13.7 |
(±3.9) |
25.4 |
(±8.5) |
23.9 |
(±2.9) |
|
Washington |
8,449 |
36.6 |
(±6.5) |
23.4 |
(±3.1) |
37.5 |
(±5.3) |
37.7 |
(±11.4)†† |
14.5 |
(±3.0) |
28.8 |
(±5.1) |
26.4 |
(±2.8) |
|
Median |
36.8 |
20.1 |
33.2 |
25.2 |
14.4 |
22.0 |
23.9 |
||||||||
|
Range |
21.3--84.7 |
8.7--34.4 |
19.4--57.5 |
10.4--47.2 |
6.1--24.6 |
8.9--43.8 |
12.9--38.8 |
||||||||
|
U.S. territories*** |
2,374 |
26.9 |
(±9.8) |
12.9 |
(±4.1) |
18.6 |
(±6.7) |
---¶¶ |
--- |
14.8 |
(±7.4) |
13.3 |
(±5.7) |
14.8 |
(±4.1) |
|
Puerto Rico |
1,491 |
26.9 |
(±9.8) |
13.0 |
(±4.1) |
18.6 |
(±6.9) |
---¶¶ |
--- |
15.1 |
(±7.6) |
13.2 |
(±5.9) |
14.9 |
(±4.3) |
|
U.S. Virgin Islands††† |
883 |
--- |
--- |
9.3 |
(±2.9) |
--- |
--- |
---¶¶ |
--- |
7.0 |
(±3.7) |
---¶¶ |
--- |
--- |
--- |
|
* Coverage estimates are for persons with reported vaccination during October 2009--January 2010 who were interviewed during November 2009--February 2010. † Percentages are weighted. § Confidence interval. ¶ Pregnant women, health-care and emergency medical services personnel, children and young adults aged 6 months through 24 years, and persons aged 25--64 years who have medical conditions that put them at higher risk for influenza-related complications. ** Persons aged 25--64 years who have medical conditions that put them at higher risk for influenza-related complications. †† Estimate might be unreliable because confidence interval half-width is >10. §§ Estimates are based on NHFS only. ¶¶ Estimates are not reliable because relative standard error is >0.3. *** Estimates are based on BRFSS only because NHFS did not collect data for U.S. territories. ††† Estimates are limited to persons aged ≥18 years. |
|||||||||||||||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
[email protected].


