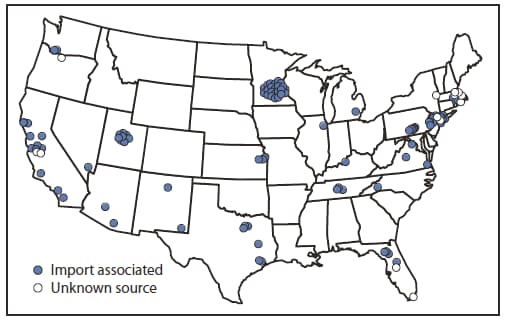
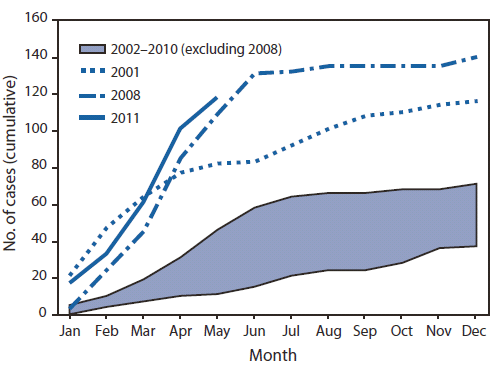
* Additional information is available at http://www.cdc.gov/osels/ph_surveillance/nndss/casedef/measles_2010.htm.
† Documented receipt of 2 doses of live measles virus-containing vaccine, laboratory evidence of immunity, documentation of physician-diagnosed measles, or birth before 1957.
 ShareCompartir
ShareCompartir