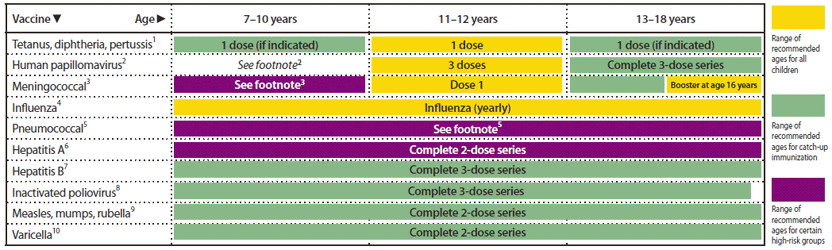
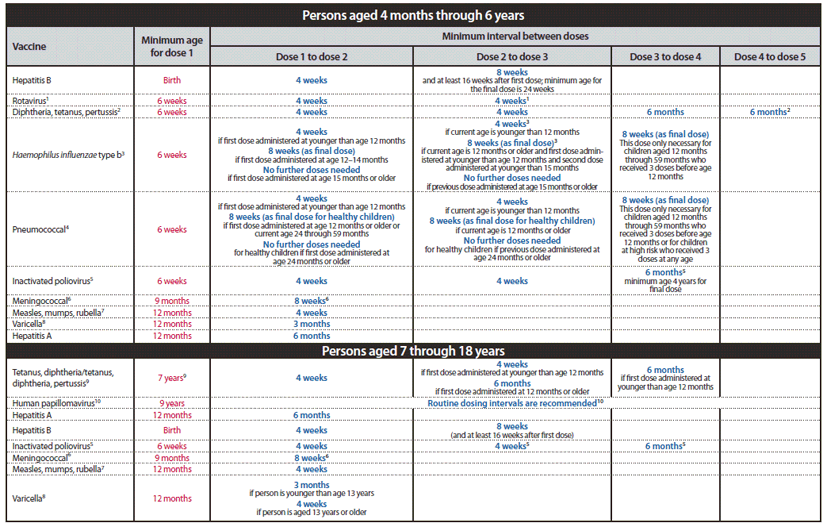
FIGURE 1. Recommended immunization schedule for persons aged 0 through 6 years — United States, 2012 (for those who fall behind or start late, see the catch-up schedule [Figure 3])
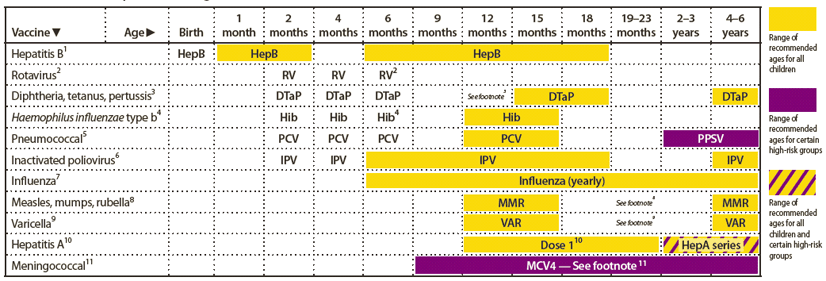
Alternate Text: The figure above shows the recommended immunization schedule for persons aged 0 through 6 years in the United States for the year 2012. For persons who fall behind or start late, this schedule and the catch-up schedule (Figure 3) should be consulted.
 ShareCompartir
ShareCompartir