 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Guidelines for National Human Immunodeficiency Virus Case Surveillance, Including Monitoring for Human Immunodeficiency Virus Infection and Acquired Immunodeficiency SyndromePlease note: An update has been published for this report. To view the update, please click here. The following CDC staff members prepared this report: Patricia L. Fleming, Ph.D., M.S. Ronald O. Valdiserri, M.D., M.P.H. in collaboration with Jeffrey L. Jones, M.D., M.P.H. Eva M. Seiler, M.P.A Harold W. Jaffe, M.D. Summary CDC recommends that all states and territories conduct case surveillance for human immunodeficiency virus (HIV) infection as an extension of current acquired immunodeficiency syndrome (AIDS) surveillance activities. The expansion of national surveillance to include both HIV infection and AIDS cases is a necessary response to the impact of advances in antiretroviral therapy, the implementation of new HIV treatment guidelines, and the increased need for epidemiologic data regarding persons at all stages of HIV disease. Expanded surveillance will provide additional data about HIV-infected populations to enhance local, state, and federal efforts to prevent HIV transmission, improve allocation of resources for treatment services, and assist in evaluating the impact of public health interventions. CDC will provide technical assistance to all state and territorial health departments to continue or establish HIV and AIDS case surveillance systems and to evaluate the performance of their surveillance programs. This report includes a revised case definition for HIV infection in adults and children, recommended program practices, and performance and security standards for conducting HIV/AIDS surveillance by local, state, and territorial health departments. The revised surveillance case definition and associated recommendations become effective January 1, 2000. INTRODUCTION AIDS surveillance has been the cornerstone of national efforts to monitor the spread of HIV infection in the United States and to target HIV-prevention programs and health-care services. Although AIDS is the end-stage of the natural history of HIV infection, in the past, monitoring AIDS-defining conditions provided population-based data that reflected changes in the incidence of HIV infection. However, recent advances in HIV treatment have slowed the progression of HIV disease for infected persons on treatment and contributed to a decline in AIDS incidence. These advances in treatment have diminished the ability of AIDS surveillance data to represent trends in the incidence of HIV infection or the impact of the epidemic on the health-care system. As a consequence, the capacity of local, state, and federal public health agencies to monitor the HIV epidemic has been compromised (1-3). In response to these changes and following consultations with multiple and diverse constituencies (including representatives of public health, government, and community organizations), CDC and the Council of State and Territorial Epidemiologists (CSTE) have recommended that all states and territories include surveillance for HIV infection as an extension of their AIDS surveillance activities (1,4). In this manner, the HIV/AIDS epidemic can be tracked more accurately and appropriate information about HIV infection and AIDS can be made available to policymakers. CDC continues to support a diverse set of epidemiologic methods to characterize persons affected by the epidemic in the United States (5-10). Although HIV/AIDS case surveillance represents only one component among multiple necessary surveillance strategies, this report focuses primarily on CDC's recommendation to implement HIV case reporting nationwide. This report provides a revised case definition for HIV infection in adults and children, recommended program practices, and performance and security standards for conducting HIV/AIDS surveillance by local, state, and territorial health departments. The case definition for HIV infection was revised in consultation with CSTE and includes the current AIDS surveillance criteria as a component (11). The recommended program practices and performance and security standards are based on a) the established practices of AIDS and other public health surveillance systems; b) reviews of state and local surveillance programs, confidentiality statutes, and security procedures; c) studies of the performance of surveillance systems; d) ongoing evaluations of determinants of test-seeking or test-avoidance in relation to state policies and practices on HIV testing and reporting; and e) discussions at a consultation held by CDC and CSTE in May 1997. A draft of this report was made available for public comment from December 10, 1998, to January 11, 1999, through a notice published in the Federal Register (12). BACKGROUND History of AIDS and HIV Case Surveillance Since the epidemic was first identified in the United States in 1981, population-based AIDS surveillance (i.e., reporting of AIDS cases and their characteristics to public health authorities for epidemiologic analysis) has been used to track the progression of the HIV epidemic from the initial case reports of opportunistic illnesses caused by a then unknown agent in a few large cities to the reporting of 711,344 AIDS cases nationwide through June 30, 1999 (5,13-15). The AIDS reporting criteria have been periodically revised to incorporate new understanding of HIV disease and changes in medical practice (16-19). In the absence of effective therapy for HIV infection, AIDS surveillance data have reliably detected changing patterns of HIV transmission and reflected the effect of HIV-prevention programs on the incidence of HIV infection and related illnesses in specific populations (20-25). Because of these attributes, AIDS surveillance data have been used as a basis for allocating many federal resources for HIV treatment and care services and as the epidemiologic basis for planning local HIV-prevention services. With the advent of more effective therapy that slows the progression of HIV disease, AIDS surveillance data no longer reliably reflect trends in HIV transmission and do not accurately represent the need for prevention and care services (26,27). In 1996, national AIDS incidence and AIDS deaths declined for the first time during the HIV epidemic (Figure 1). These declines have been primarily attributed to the early use of combination antiretroviral therapy, which delays the progression to AIDS and death for persons with HIV infection (1-3,9). Revised HIV treatment guidelines recommend antiretroviral therapy for many HIV-infected persons in whom AIDS-defining conditions have not yet developed (28-30). In addition, antiretroviral treatment of pregnant women and their newborns has reduced perinatal HIV transmission and resulted in dramatic declines in the incidence of perinatally acquired AIDS (31,32) (Figure 2). In response to these changes in HIV treatment practices and the information needs of public health and other policymakers, CDC and CSTE have recommended that all states and territories extend their AIDS case surveillance activities to include HIV case surveillance and the reporting of HIV-exposed infants (1,4,33). Since 1985, many states have implemented HIV case reporting as part of their comprehensive HIV/AIDS surveillance programs. As of November 1, 1999, a total of 34 states and the Virgin Islands (VI) had implemented HIV case surveillance using the same confidential system for name-based case reporting for both HIV infection and AIDS; two of these states conduct pediatric surveillance only (5) (Figure 3). Areas that conduct integrated HIV/AIDS surveillance for adults, adolescents, and children have reported 42% of cumulative U.S. AIDS cases. In addition, four states (Illinois, Maine, Maryland, and Massachusetts) and Puerto Rico, representing 11% of cumulative AIDS cases, are reporting cases of HIV infection using a coded identifier rather than patient name. Washington has implemented HIV reporting by patient name to enable public health follow-up; after services and referrals are offered, names are converted into codes. In most other states, HIV case reporting is under consideration or laws, rules, or regulations enabling HIV surveillance are expected to be implemented during 2000. In contrast to AIDS case surveillance, HIV case surveillance provides data to better characterize populations in which HIV infection has been newly diagnosed, including persons with evidence of recent HIV infection such as adolescents and young adults (13-24-year-olds) (34,35). Of the 52,690 HIV infections diagnosed from January 1994 through June 1997 in 25 states that conducted name-based HIV surveillance throughout this period, 14% of cases occurred in persons aged 13-24 years. In comparison, of the 20,215 persons in whom AIDS was diagnosed in these 25 states, only 3% of cases occurred in persons aged 13-24 years. Thus, AIDS case surveillance alone does not accurately reflect the extent of the HIV epidemic among adolescents and young adults. Compared with persons reported with AIDS, those reported with HIV infection in these 25 states were more likely to be women and from racial/ethnic minorities (36) (Table 1). These patterns reflect the characteristics of populations that were affected by the epidemic more recently, but they might also reflect changes in testing practices or behaviors (6,36,37). Compared with the diagnosis of AIDS, which can be delayed among HIV-infected persons receiving antiretroviral therapy, the first diagnosis of HIV infection is not delayed by treatment but is affected by testing behaviors and targeted testing programs. In addition, in these 25 states as of June 30, 1999, the total number of persons (159,083) who were reported as living with either a diagnosis of HIV infection (90,699) or AIDS (68,384) was 133% greater than that represented by the number living with AIDS alone (5). Therefore, these states have documented that the combined prevalence of those living with a diagnosis of HIV infection and those living with AIDS provides a more realistic and useful estimate of the resources needed for patient care and services than does AIDS prevalence alone. States with confidential name-based HIV case surveillance systems have used data on all perinatally exposed children to document the sharp decline in perinatally acquired HIV infection, the increase in the proportion of infected pregnant women who have been tested for HIV infection before delivery, and the high proportion of HIV-infected pregnant women who accept zidovudine therapy (31,38-44). These findings contribute to HIV-prevention policy development. CSTE and the American Academy of Pediatrics have recommended that all states and territories conduct pediatric HIV surveillance that includes all perinatally exposed infants to facilitate follow-up to assess infection status and access to care (11,31,33,40,45). Persons can choose to be tested for HIV in the following ways: a) anonymously -- whereby identifying information, including patient name and other locating information, are not linked to the HIV test result (e.g., at anonymous testing sites) and b) confidentially -- whereby the HIV test result is linked to identifying information such as patient and provider names (e.g., at medical clinics). In states that require HIV case reporting, providers in confidential medical or testing sites are required to report HIV-infected persons to public health authorities. Not all persons infected with HIV are tested, and of those who are, testing occurs at different stages of their infection. Therefore, HIV surveillance data provide a minimum estimate of the number of infected persons and are most representative of persons who have had HIV infection diagnosed in medical clinics and other confidential diagnostic settings. The data represent the characteristics of persons who recognize their risk and seek confidential testing, who are offered HIV testing (e.g., pregnant women and clients at sexually transmitted disease [STD] clinics), who are required to be tested (e.g., blood donors and military recruits), and who are tested because they present with symptoms of HIV-related illnesses. CDC estimated that, in 1996, approximately two thirds of all infected persons in the United States had HIV infection diagnosed in such settings (46). HIV surveillance data might not represent untested persons or those who seek testing at anonymous test sites or with home collection kits; such persons are not reported to confidential HIV/AIDS surveillance systems. However, the availability of anonymous testing is important in promoting knowledge of HIV status among at-risk populations and provides an opportunity for counseling to reduce high-risk behaviors and voluntary referrals to appropriate medical diagnosis and prevention services. Despite their current limitations, HIV and AIDS case surveillance data together can provide a clearer picture of the HIV epidemic than AIDS case surveillance data alone. Therefore, CDC and CSTE continue to recommend that all areas implement HIV case reporting as part of a comprehensive strategy to monitor HIV infection and HIV disease. The strategy should also include surveys of the incidence and prevalence of HIV infection; AIDS case surveillance; monitoring HIV-related mortality; supplemental research and evaluation studies, including behavioral surveillance; and statistical estimation of the incidence and prevalence of infection and disease. Considerations in Implementing Nationwide HIV Case Surveillance The nationwide implementation of the 1993 expanded AIDS surveillance case definition prompted renewed discussions of the rationale and need for data representing HIV-infected persons who did not meet the AIDS-defining criteria. Because many states were considering implementing HIV reporting, CDC held a consultation in 1993 with public health and community representatives to discuss relevant issues and concerns. Community representatives' main concerns were that the security and confidentiality standards of surveillance programs might not be sufficient to prevent disclosures of information and that many persons at risk for HIV infection might therefore delay seeking HIV counseling and testing because of these confidentiality concerns. The consensus of the consultants was that few published studies were of sufficient scientific quality to assess these concerns. Therefore, the consultants identified several areas that required additional research and policy development before CDC and CSTE should consider recommending further expansion of HIV surveillance efforts. These areas included a) the impact of reporting policies on testing behaviors and practices, including the decreased availability of anonymous testing in some states; b) the role of surveillance data in linking reported persons to prevention and care programs; c) the development of recommended standards for the security and confidentiality of publicly held HIV/AIDS surveillance data; and d) determining whether alternatives to reporting of patient names would reduce confidentiality risks while meeting the needs for high-quality surveillance data. In response to the consultants' recommendations, CDC initiated several research projects to a) assess the effect of confidential name-based HIV surveillance on persons' willingness to seek HIV testing and care; b) review program practices and legal requirements for the security and confidentiality of state and local HIV/AIDS surveillance data; and c) evaluate the performance of coded-identifier-based surveillance systems. Findings from these projects and expert advice from participants at numerous technical meetings and consultations held during the intervening period have guided formulation of the policies and practices recommended in this report. The findings from these projects are summarized in the following three subsections: HIV surveillance and testing behavior, HIV surveillance using non-name-based unique identifiers, and confidentiality of HIV surveillance data. HIV Surveillance and Testing Behavior Few studies have characterized test- or care-seeking behaviors in relation to state HIV reporting policies. A 1988 general population study of previous or planned use of HIV testing services did not identify an association of reporting policy with testing behavior (47). In contrast, interviews of persons seeking anonymous testing in 1989 documented that many would avoid testing if a positive test resulted in name reporting or partner notification (48). A review of the published literature on HIV testing behaviors highlighted several limitations and biases in previous studies (49), including small numbers, lack of geographic and risk-group representativeness, and analysis of intent to test rather than of actual testing behavior. An additional limitation of the available literature is that studies published 5-10 years ago might not reflect actual testing behaviors in the current treatment era. Literature that highlights potential misuse of public health surveillance data might have the unintended effect of increasing test avoidance among some at-risk persons (50). Examining knowledge of and perceptions about testing and reporting, as well as actual testing behavior, in the context of current treatment advances and evolving HIV reporting policies, can address some of the limitations of previous research. To determine the effect of changes in reporting policies on actual testing behaviors among persons seeking testing at publicly funded HIV counseling and testing sites, CDC and six state health departments reviewed data routinely collected from these sites to compare HIV testing patterns during the 12 months before and the 12 months after implementation of HIV case surveillance (51). In these areas, the number of HIV tests increased in four states and decreased in two states; the declines were not statistically significant. All the analysis periods (25-month periods during 1992-1996) antedated the widespread beneficial effects of highly active antiretroviral therapy. Slight variability in testing trends was observed among racial/ethnic subgroups and HIV-risk exposure categories; however, these data do not suggest that, in these states, the policy of implementing HIV case reporting adversely affected test-seeking behaviors overall (52). CDC also supported studies by researchers at the University of California at San Francisco and participating state health departments to identify the most important determinants of test seeking or test avoidance among high-risk populations and to assess the impact of changes in HIV testing and HIV reporting policies. Data from these surveys of high-risk persons in nine selected states about their perceptions and knowledge of HIV testing and HIV reporting practices documented that few respondents had knowledge of the HIV reporting policies in their respective states (53,54). In surveys conducted during 1995-1996, respondents reported high levels of testing, with approximately three fourths reporting that they had had an HIV test. The most commonly reported factors (by nearly half of respondents) that might have contributed to delays in seeking testing or not getting tested were fear of having HIV infection diagnosed or belief that they were not likely to be HIV infected. "Reporting to the government" was a concern that might have contributed to a delay in seeking HIV testing for 11% of heterosexuals, 18% of injecting-drug users, and 22% of men who have sex with men; less than 1%, 3%, and 2% of respondents in these risk groups, respectively, indicated that this was their main concern. Concern about name-based reporting of HIV infections to the government was a factor for not testing for HIV for 13% of heterosexuals, 18% of injecting-drug users, and 28% of men who have sex with men. As the main factor for not testing for HIV, concern about name-based reporting to the government was substantially lower in all risk groups (1% of heterosexuals, 1% of injecting-drug users, and 4% of men who have sex with men) (55). These findings suggest that name-based reporting policies might deter a small proportion of persons with high-risk sex or drug-using behaviors from seeking testing and, therefore, support the need for strict adherence to confidentiality safeguards of public health testing and surveillance data. In addition, the survey documented that the availability of an anonymous testing option is consistently associated with higher rates of intention to test in the future. In this survey, high levels of testing, together with high levels of test delay or avoidance associated with reasons other than concern about name reporting, suggest that addressing these other concerns may have a greater effect on testing behavior. For example, 59% of men who have sex with men reported being "afraid to find out" as a factor for not testing, and 27% reported it as the main factor for not testing. In addition, 52% of men who have sex with men reported "unlikely to have been exposed" as a factor for not testing, and 17% reported it as the main factor. In a companion survey of persons reported with AIDS in eight of these same states, participants who had recognized their HIV risk and sought testing at anonymous testing sites reported entering care at an earlier stage of HIV disease than persons who were first tested in a confidential testing setting (e.g., STD clinics, medical clinics, or hospitals), where persons are frequently first tested when they become ill (56). These data suggest that anonymous testing options are important in promoting timely knowledge of HIV status for some at-risk persons. HIV Surveillance Using Non-Name-Based Unique Identifiers To assess the feasibility of using alternatives to confidential name-based methods for HIV surveillance, several states implemented reporting of cases of HIV infection or CD4 (a marker of immunosuppression in HIV-infected persons) laboratory test results using various numeric or alphanumeric codes. Other states considered or tried to conduct case surveillance without name identifiers by using codes designed for nonsurveillance purposes (e.g., codes intended for use in tracking patients in case-management systems) (57). In May 1995, CDC convened a meeting at which these states identified operational, technical, and scientific challenges in conducting surveillance using coded identifiers rather than patient names. The states recommended that CDC evaluate additional coded identifiers and assist them in documenting and disseminating the results of their findings. In addition, CDC supported research to evaluate the performance of a coded unique identifier (UI) in two states that implemented a non-name-based HIV case-reporting system while maintaining name-based surveillance methods for AIDS (58). The study, conducted by Maryland and Texas during 1994-1996 in collaboration with CDC, documented nearly 50% incomplete reporting, in part because the social security number necessary to construct the identifier code was not uniformly available in medical or laboratory records. In Maryland, provider-maintained logs were needed to link the UI to name-based medical records to obtain follow-up data (e.g., on HIV risk/exposure). A more recent evaluation conducted by the Maryland Department of Health and Mental Hygiene (MDHMH) reported data from a publicly funded counseling and testing site and documented a higher level of completeness of HIV reporting (88%) than the 50% documented in the previous study (58,59). MDHMH reports that their code is unique to a given person and that assignment of two different codes to the same person is unlikely. That is, the probability that a given code can distinguish one person from any other is greater than 99% if all the elements of the code are complete and accurate. No published evaluations have assessed the probability of assigning the same code to different persons, which could occur if elements of the code were missing. In contrast to MDHMH's findings, analogous evaluations in Texas, as well as studies that used more diverse methods in Los Angeles and New Jersey, failed to identify a code that performs as well as name-based methods (58,60-67). On the basis of published evaluations (58), Texas recently switched to name-based HIV case surveillance. In addition to Maryland, three other states (Illinois, Maine, and Massachusetts) and Puerto Rico recently implemented HIV reporting using four different coded identifiers. CDC will assist these states in implementing their systems, establishing standardized criteria for assessing the overall performance of their systems, as well as assessing whether the required standards are achieved. Additional evaluations will be conducted by the respective state health departments, in collaboration with CDC, to determine a) the ability of coded identifiers to accurately track disease progression from HIV infection to AIDS to death, b) their utility for evaluating public health efforts to eliminate perinatal HIV transmission, c) their acceptability, and d) their usefulness in matching to other databases (e.g., tuberculosis). Confidentiality of HIV Surveillance Data A 1994 review of state confidentiality laws that protect HIV surveillance data documented that all states and many localities have legal safeguards for confidentiality of government-held health data (68). These laws provide greater protection than laws protecting the confidentiality of information in health records held by private health-care providers. Most states have specific statutory protections for public health data related to HIV infection and other STDs. However, state legal protections vary, and CDC supports additional efforts to strengthen privacy protections for public health data. On the basis of input from expert legal and public health consultants, the Model State Public Health Privacy Act (69) was developed by an independent contractor at the behest of CSTE. If enacted by states, the provisions of the Model Act would ensure the confidentiality of surveillance data, strengthen statutory protections against disclosure, and preclude the intended or unintended use of surveillance data for non-public health purposes. CDC has reviewed state and local security policies and procedures for HIV/AIDS surveillance data. Since 1981, states have conducted AIDS surveillance, and few breaches of security have resulted in the unauthorized release of data (70,71). Because survival has improved for HIV-infected persons, information about them might be maintained in public health surveillance databases for longer periods. This has resulted in increased concerns about confidentiality of surveillance data among public health and community groups (72). Therefore, CDC has issued technical guidance for security procedures that include enhanced confidentiality and security safeguards as evaluation criteria for federal funding of state HIV/AIDS surveillance activities (73). The receipt of federal surveillance funding depends on the recipient's ability to ensure the physical security and confidentiality of case reports. At the federal level, HIV/AIDS surveillance data are protected by several federal statutes, which ensure that CDC will not release HIV/AIDS surveillance data for non-public health purposes (e.g., for use in criminal, civil, or administrative proceedings). Privacy is also ensured by the removal of names and the encryption of data transmitted to CDC. On the basis of the importance of maintaining the confidentiality of persons in whom HIV infection has been diagnosed by public or private health-care providers, CDC has recommended additional standards to enhance the security and confidentiality of HIV and AIDS surveillance data (74,75). GUIDELINES FOR SURVEILLANCE OF HIV INFECTION AND AIDS HIV Surveillance Case Definition for Adults and Children CDC, in collaboration with CSTE, has established a new case definition for HIV infection in adults and children that includes revised surveillance criteria for HIV infection and incorporates the surveillance criteria for AIDS (17-19,76) (Appendix). HIV infection and AIDS case reports forwarded to CDC should be based on this definition. For adults and children aged greater than or equal to 18 months, the HIV surveillance case definition includes laboratory and clinical evidence specifically indicative of HIV infection and severe HIV disease (AIDS). For children aged less than 18 months (except for those who acquired HIV infection other than by perinatal transmission), the HIV surveillance case definition updates the definition in the 1994 revised classification system. In addition, the new case definition is based on recent data regarding the sensitivity and specificity of HIV diagnostic tests in infants and clinical guidelines for Pneumocystis carinii pneumonia (PCP) prophylaxis for children (19,77-88) and for use of antiretroviral agents for pediatric HIV infection (30). The revised surveillance case definitions for adults and children become effective January 1, 2000. HIV/AIDS Case Surveillance Practices and Standards CDC and CSTE recommend that all states require reporting to public health surveillance of all cases of perinatal HIV exposure in infants, the earliest diagnosis of HIV infection (exclusive of anonymous tests) and the earliest diagnosis of AIDS in persons of all ages, and deaths among these persons (4,33). Such reporting should constitute the core minimum performance standard for HIV/AIDS surveillance in all states and territories. CDC provides federal funds and technical assistance to states to establish and conduct active HIV/AIDS surveillance programs. On the basis of feasibility, needs, and resources, areas may be funded to implement additional surveillance activities (e.g., supplemental research and evaluation studies and serologic surveys), but these approaches might not be necessary in all areas. The following recommended practices update and revise the CDC Guidelines for HIV/AIDS Surveillance released in 1996 and updated in 1998 as a technical guide for state and local HIV/AIDS surveillance programs (34,73-75). Recommended practices represent CDC's guidance for best public health practice based on available scientific data. Programmatic standards set minimum requirements for states to receive support from CDC for HIV/AIDS surveillance activities. Recommended Surveillance Practices
Minimum Performance Standards
Recommended Security and Confidentiality Practices
Minimum Security and Confidentiality Standards The security and confidentiality policies and procedures of state and local surveillance programs should be consistent with CDC standards for the security of HIV/AIDS surveillance data (73,74). The minimum security criteria were established following reviews of all state and numerous local health department HIV/AIDS surveillance programs. In general, the reviews documented that health departments have achieved a high level of security and that most state health departments meet or exceed the minimum standards. Beginning in 2000, CDC will require that recipients of federal funds for HIV/AIDS surveillance establish the minimum security standards and include their security policy in applications for surveillance funds (73,74). Examples of these standards include the following:
Relation to HIV-Prevention and HIV-Care Programs: Recommended Practices At the federal level, the primary function of HIV/AIDS surveillance is collecting accurate and timely epidemiologic data for public health planning and policy. Consequently, CDC is authorized to provide federal funds to states through surveillance cooperative agreements, both to achieve the goals of the national surveillance program and to assist states in developing their surveillance programs in accordance with state and local laws and practices. Federal funds authorized for HIV/AIDS surveillance are not provided to states for developing or providing prevention or treatment case-management services; funds for such services are provided by CDC and other federal agencies under separate authorizations. Whether and how states establish a link between individual case-patients reported to their HIV/AIDS surveillance programs and other health department programs and services for HIV prevention and treatment is within the purview of the states. However, in considering or establishing such linkages, CDC recommends the following:
COMMENTARY Surveillance Case Definition for HIV Infection and AIDS The revised case definition for HIV infection in adults and children integrates reporting criteria for HIV infection and AIDS in a single case definition and incorporates new laboratory tests in the laboratory criteria for HIV case reporting. The 2000 case definition for HIV infection includes HIV nucleic acid (DNA or RNA) detection tests that were not commercially available when the AIDS case definition was revised in 1993. The revised case definition for HIV infection also permits states to report cases to CDC based on the result of any test licensed for diagnosing HIV infection in the United States. Although the reporting criteria generally reflect the recommendations for diagnosing HIV infection, the HIV reporting criteria are for public health surveillance and are not designed for making a diagnosis for an individual patient. The laboratory criteria include the serologic HIV tests described in the clinical standards for diagnosing HIV infection (92-95). The pediatric HIV reporting criteria include criteria for monitoring all children with perinatal exposures to HIV and reflect recent advances in diagnostic approaches that permit the diagnosis of HIV infection during the first months of life. With HIV nucleic acid detection tests, HIV infection can be detected in nearly all infants aged greater than or equal to 1 month. The timing of the HIV serologic and HIV nucleic acid detection tests and the number of HIV nucleic acid detection tests in the definitive and presumptive criteria for HIV infection are based on the recommended practices for diagnosing infection in children aged less than 18 months and on evaluations of the performance of these tests for children in this age group (30,77-88). The clinical criteria in the case definition for HIV infection are included to ensure the complete reporting of cases with documented evidence of HIV infection or conditions meeting the AIDS case definition. The AIDS-defining conditions are included as part of the single case definition for HIV infection. In adults and adolescents aged greater than or equal to 13 years, criteria for presumptive and definitive AIDS-defining conditions have not been revised since 1993 and continue to include the laboratory markers of severe HIV-related immunosuppression and the opportunistic illnesses indicative of severe HIV disease, which greatly increase mortality risks. Effect of National HIV Case Surveillance on Reporting Trends Changes in the HIV reporting criteria will have little effect on reporting trends in states already conducting HIV case surveillance. However, the number of cases of HIV infection reported nationally will increase primarily because of implementation of HIV surveillance by the remaining states and local areas. Many of the states that will implement HIV case surveillance in the future have high AIDS incidence rates. Similar to the effect on AIDS surveillance trends after the implementation of the revised reporting criteria in 1993, the initiation of HIV surveillance by additional states might result in a sudden and large increase in HIV case reports (96). On the basis of CDC's estimate that approximately 220,000 HIV-infected persons without AIDS-defining conditions had had HIV infection diagnosed in confidential testing settings and resided in states that were not conducting HIV case surveillance at the end of 1996 (46), the possibility exists that this number of persons could be reported with HIV infection from these states in 2000. However, reporting of prevalent HIV infections is more likely to be spread over several years, and the annual increases will most likely be more modest. Initially, most case reports will represent persons whose HIV infection was diagnosed before the implementation of HIV surveillance. As the reporting of prevalent cases of HIV infection reaches full implementation nationwide, the number of HIV case reports will decrease, and case reports will increasingly represent persons with recent diagnoses of HIV infection. To facilitate interpretation of HIV surveillance data and given that CDC strongly promotes continued availability of anonymous testing options, evaluations of HIV/AIDS surveillance systems will include assessments of the representativeness of HIV case surveillance data. These assessments will include special surveys to evaluate the delays between HIV testing and entry to care. In addition, these evaluations will be useful in determining the effectiveness of program efforts to refer persons into care services after the diagnosis of HIV infection in anonymous testing settings. AIDS cases have declined nationwide; however, because AIDS surveillance trends are affected by the incidence of HIV infection, as well as the effect of treatment on the progression of HIV disease, future AIDS trends cannot be predicted. AIDS surveillance will continue to be important in evaluating access to care for different populations and in identifying changes in trends that might signal a decrease in the effectiveness of treatment. The long-term benefits of antiretroviral therapy and antimicrobial prophylaxis for AIDS-related illnesses continue to be defined. In addition, various factors (e.g., access, adherence, treatment costs, and viral resistance) will influence the use and effectiveness of these therapies and their effects on AIDS incidence and mortality trends (97-99). Because trends in new diagnoses of HIV infection are affected by when in the course of disease a person seeks or is offered HIV testing, such trends do not reflect the incidence of HIV infection in the population. In addition, because all HIV-infected persons in the population might not have had the infection diagnosed, these data do not represent total HIV prevalence in the population. Currently, interpretation of these data is complicated by several factors. First, persons might have HIV infection diagnosed and later during the same calendar year have AIDS diagnosed, which can complicate presentation of the data. Second, delays in reporting cases of HIV infection tend to be shorter than for AIDS cases, necessitating development of stage-specific statistical adjustments. Third, methods of imputation of exposure risk data for AIDS cases have been developed based on historical patterns of reclassification after investigation, but comparable methods for cases of HIV infection are only recently available at the national level. Finally, whether a trend in the number of new HIV diagnoses is stable, increasing, or decreasing might reflect current or historical HIV transmission patterns, changes in testing behaviors, and/or stage of the epidemic in the local geographic area. Overall, in the United States, the incidence of HIV infection peaked approximately 15 years ago, and the annual number of HIV infections has been stable at approximately 40,000 since 1992, when CDC estimated the prevalence of HIV infection in the range of 650,000-900,000 infected persons (100,101). Based on HIV and AIDS case surveillance data, CDC estimates that the prevalence of HIV infection at the end of 1998 was in the range of 800,000-900,000 infected persons. Of these persons, approximately 625,000 (range: 575,000-675,000) had had HIV infection or AIDS diagnosed (CDC, unpublished data, 1999). Because the annual number of new infections in recent years is relatively lower than during the peak incidence years, over time the remaining untested or anonymously tested infected persons will have HIV infection diagnosed through test-seeking, targeted testing, entry to care, or progression of disease to AIDS. Ultimately, the number of new diagnoses of HIV infection will decrease each year as they increasingly represent the smaller pool of more recently infected persons. Thus, in states that have been conducting HIV case reporting for several years, the number of new diagnoses of HIV infection is expected to decrease, then stabilize at a lower rate if the number of new infections remains stable. For states that newly implement HIV reporting, a large bolus of reported prevalent infections is expected to occur, followed by a decline in the annual number of new cases until the number stabilizes at a lower level. Recently, since the impact of highly active antiretroviral therapy on survival, the estimated number of new infections each year probably exceeds the number of deaths, and the prevalence of HIV infection might be increasing by a small proportion of total prevalence. Thus, during the transition period to nationwide HIV-infection reporting, measures of the combined prevalence of HIV infection diagnoses and AIDS diagnoses will be most useful in projecting the need for resources for care and prevention. Trends in the numbers of new cases reported will not provide immediate insights into the dynamics of the epidemic because prevalent case reports represent a mixture of new and old HIV infections. Within the next several years, however, all states will be able to characterize new diagnoses of HIV infection or a representative sample by demographic and clinical characteristics that will provide meaningful insights into actual HIV transmission patterns and will have well-characterized the health and service needs of the population of prevalent HIV-infected persons. CDC will develop analysis profiles, statistical adjustments for reporting delays and imputation of risk data, and recommendations for data presentation to assist states in analyzing and interpreting their HIV/AIDS surveillance data during this transition period. HIV/AIDS Surveillance Practices Laboratories will be an increasingly important source of information from which to initiate reporting. HIV infection is frequently diagnosed in the outpatient clinical setting, and laboratory-initiated reporting will be particularly useful in identifying outpatient sources of HIV testing (89) although contact with individual providers is necessary to complete the reporting process. The routine collection of HIV and CD4 test data from laboratories and managed-care organizations promotes completeness of reporting and may increase the simplicity and efficiency of initial case-finding activities by local surveillance programs. Nonetheless, repeated testing of the same persons results in multiple reports and necessitates labor-intensive follow-up to eliminate duplicates. CDC is increasing its efforts to promote standards in laboratory reporting and to facilitate the transfer of data from public health and commercial laboratories to health departments. Performance criteria for HIV and AIDS surveillance are necessary to ensure that surveillance data are of sufficient quality to target prevention and care resources and to detect emerging trends in the HIV epidemic. Evaluations of HIV and AIDS surveillance programs have documented that areas should be able to meet these performance criteria (5,36,61-67,89,90). According to these evaluations of name-based surveillance systems, the completeness of HIV surveillance (from 79% to approximately 95%) and AIDS surveillance (from 85% to approximately 95%) is high, and reporting is timely with nearly one half of AIDS cases and three quarters of cases of HIV infection reported to the national HIV/AIDS reporting system within 3 months of diagnosis (5). CDC estimates that the duplication rate of cases of HIV infection reported from different states to the national surveillance database was approximately 2%; for AIDS cases, the rate was approximately 3% (5,36). The performance criteria also reflect the need for public health surveillance systems to identify and follow-up on cases of public health importance. On the basis of current evaluation studies of non-name-based case identifiers and the current infrastructure of state and local health departments, name-based methods for collecting and reporting public health data provide the most feasible, simple, and reliable means for ensuring timely, accurate, and complete reporting of persons in whom HIV infection or AIDS has been diagnosed. Confidential name-based reporting also facilitates follow-up of perinatally exposed infants to determine their infection status and of persons reported with HIV infection to determine progression to AIDS and vital status (36,42). A name-based patient identifier allows providers to report cases directly from their name-based medical records, facilitates elimination of duplicate case reports, enables cross-matching of HIV and AIDS data with other name-based public health data (e.g., tuberculosis surveillance), permits follow-up with providers to collect information regarding risk for HIV infection and other data of public health importance. Through follow-up with providers, the HIV/AIDS surveillance system has provided an effective means to identify rare or unusual modes of HIV transmission and infection with rare strains of HIV and to improve prevention of HIV-related opportunistic illnesses (102-106). CDC will assist states in monitoring the impact of changing medical interventions, epidemiology, and HIV case surveillance policies on test- and care-seeking behaviors. Security and Confidentiality of HIV and AIDS Surveillance The revision of the case definition for HIV infection provides an opportunity to review and strengthen state and local confidentiality laws and regulations. Although state HIV/AIDS surveillance confidentiality laws and regulations adequately protect privacy compared with the statutory protections of other health-care data, state statutes differ in the degree of privacy protections afforded health information and the criteria for permissible disclosures of personal information. Most state statutes describe some permissible disclosures of public health information. To help ensure uniform confidentiality protections, the Georgetown University Law Center developed the Model State Public Health Privacy Act (69). Public health, legislative, legal, and community advocacy representatives provided expert consultation. The model legislative language protects confidential, identifiable information held by state and local public health departments against unauthorized and inappropriate non-public health uses but still allows public health officials to use surveillance information to accomplish the public health objectives defined by the law (69). CDC recommends that states planning to implement HIV case surveillance should consider adopting the model legislation, if necessary, to strengthen the current level of protection of public health data. Although HIV/AIDS surveillance systems have exemplary records of security and confidentiality, it is essential for all programs to identify ways to strengthen data protection because of a perceived greater sensitivity of HIV case surveillance compared with that of AIDS case surveillance alone (71). Providing accurate public education and factual media messages to inform vulnerable populations, as well as promoting testing programs that facilitate referrals into treatment and prevention services, will be important to ensure that test seeking and acceptance are not adversely affected as additional states implement HIV case reporting. The revised security standards (74) promote enhancements to further reduce any potential for disclosure of sensitive surveillance data. CDC continues to conduct evaluations of methods to further enhance data security, including the use of coding and encryption of data collected in the HIV/AIDS reporting system. HIV Prevention and Care CDC has published guidelines concerning the provision and targeting of HIV counseling and testing services (29,41,107-111) and provides support for most public sources of HIV testing. The availability of anonymous HIV testing services might be particularly important for persons who delay seeking testing because of a concern that others might learn of their serologic status (55). Studies have documented that the availability of anonymous HIV testing is associated with increased numbers of persons seeking testing services (112-115). Anonymous HIV testing services are a required element of federally supported prevention programs unless prohibited by state law or regulation. Currently, 39 states, Puerto Rico, and the District of Columbia provide anonymous HIV testing services. CDC advises that the decision to refer persons reported to the surveillance system to prevention and care services (e.g., partner counseling and referral services [PCRS]) be made at the local level. PCRS programs provide HIV counseling and testing to persons who might be unaware of HIV risk exposures, and these services are a required component of federally sponsored HIV-prevention programs (116,117). The provision of such services to persons in whom HIV infection or AIDS has been diagnosed, especially those who receive services in publicly funded testing and clinic settings, is conducted successfully by states regardless of whether they have implemented HIV reporting (118). Referrals from surveillance to other health department services, when they occur, should be established in a manner that ensures both the quality of the surveillance data and the security of the surveillance system, as well as the quality, confidentiality, and voluntary nature of HIV-prevention services (119). At the federal level, the primary function of HIV/AIDS surveillance remains the provision of accurate epidemiologic data for public health information, planning, and evaluation. Persons in whom HIV infection has been diagnosed at either confidential or anonymous test sites should be promptly referred to facilities that provide confidential HIV care. Recent studies have documented disparities in ensuring timely testing and access to care by demographic, socioeconomic, and other factors (120,121). Although not directly responsible for the delivery of medical care, CDC provides federal direction for state and local programs that facilitate referral of HIV-infected persons from counseling and testing centers and health education/risk-reduction programs to HIV care facilities. CDC has developed guidelines to strengthen the system of referrals between HIV testing sites and care programs, in part by increasing coordination with the Health Resources and Services Administration and the Ryan White CARE Act grantees (122). To provide further guidance, CDC has participated in developing model contract language for Medicaid programs that serve persons with HIV infection to ensure cooperation with public health authorities in case reporting and follow-up. A well-developed and well-implemented HIV and AIDS case surveillance system is integral to public health efforts to identify disparities, target programs and resources to vulnerable populations, and assess the impact of these programs in reducing infection, disease, and premature death. CDC is undertaking a national effort to further reduce perinatal HIV transmission in the United States. This effort will incorporate HIV counseling and voluntary testing, treatment, and outreach to pregnant women, especially those who are racial/ethnic minorities and substance abusers, and will integrate prevention and treatment services for women and children. Surveillance for perinatally HIV-exposed and HIV- infected children will remain a critical measure of the effectiveness of this campaign (32,40,41,123,124). CONCLUSION The implementation of a national surveillance network to include both HIV and AIDS case reporting is a necessary response to epidemiologic trends and new standards for HIV care (125-127). Integrated HIV/AIDS surveillance programs will provide data to characterize persons in whom HIV infection has been newly diagnosed, including those with evidence of recent infection, persons with severe HIV disease (AIDS), and those dying of HIV disease or AIDS. The revised HIV surveillance case definition and the establishment of minimum performance standards will promote uniform case ascertainment and will ensure that the surveillance data are of sufficient quality for effective planning and allocation of resources for prevention and care programs. References
Table 1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. TABLE 1. Characteristics of persons aged >=13 years with HIV, by disease status at initial diagnosis* -- 25 states†, January 1994-June 1997
* For persons who had not had an HIV diagnosis before being diagnosed with AIDS, their AIDS diagnosis date is considered their earliest HIV diagnosis date; for persons initially reported with HIV who subsequently had AIDS diagnosed and reported, they are presented by the earliest diagnosis date, which is their HIV diagnosis. Return to top. Figure 1 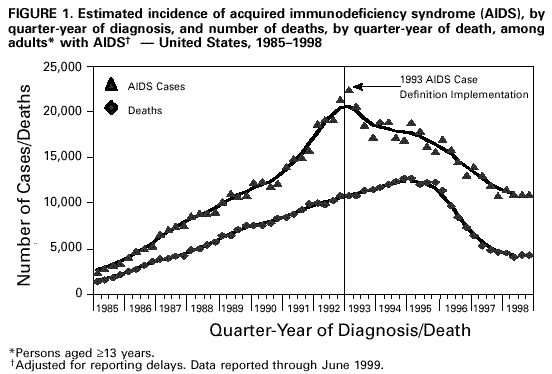 Return to top. Figure 2 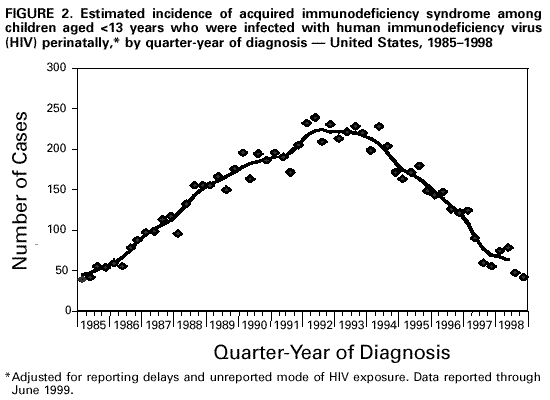 Return to top. Figure 3 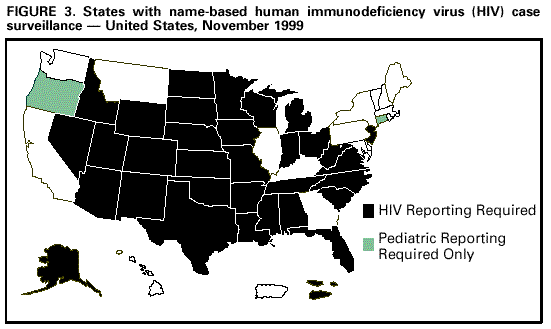 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 12/8/1999 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This page last reviewed 5/2/01
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||