 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommendations for Using Fluoride to Prevent and Control Dental Caries in the United StatesFluoride Recommendations Work Group Steven M. Adair, D.D.S., M.S. School of Dentistry Medical College of Georgia Augusta, Georgia William H. Bowen, Ph.D. Caries Research Center University of Rochester Rochester, New York Brian A. Burt, B.D.S., M.P.H., Ph.D. School of Public Health University of Michigan Ann Arbor, Michigan Jayanth V. Kumar, D.D.S., M.P.H. New York Department of Health Albany, New York Steven M. Levy, D.D.S., M.P.H. College of Dentistry University of Iowa Iowa City, Iowa David G. Pendrys, D.D.S., Ph.D. School of Dental Medicine University of Connecticut Farmington, Connecticut R. Gary Rozier, D.D.S., M.P.H. School of Public Health University of North Carolina Chapel Hill, North Carolina Robert H. Selwitz, D.D.S., M.P.H. National Institute of Dental and Craniofacial Research Bethesda, Maryland John W. Stamm, D.D.S., D.D.P.H. School of Dentistry University of North Carolina Chapel Hill, North Carolina George K. Stookey, Ph.D., D.D.S. School of Dentistry Indiana University Indianapolis, Indiana Gary M. Whitford, Ph.D., D.M.D. School of Dentistry Medical College of Georgia Augusta, Georgia Fluoride Recommendations Reviewers Myron Allukian, Jr., D.D.S., M.P.H. Director of Oral Health Boston Public Health Commission Boston, Massachusetts John P. Brown, B.D.S., Ph.D. Department of Community Dentistry University of Texas Health Science Center San Antonio, Texas Joseph A. Ciardi, Ph.D. National Institute of Dental and Craniofacial Research Bethesda, Maryland D. Christopher Clark, D.D.S., M.P.H. Faculty of Dentistry University of British Columbia North Vancouver, Canada Stephen B. Corbin, D.D.S., M.P.H. Oral Health America Brookeville, Maryland Michael W. Easley, D.D.S., M.P.H. School of Dental Medicine State University of New York Buffalo, New York Caswell A. Evans, D.D.S., M.P.H. County Dental Director Los Angeles, California Lawrence J. Furman, D.D.S., M.P.H. National Institute of Dental and Craniofacial Research Bethesda, Maryland Stanley B. Heifetz, D.D.S., M.P.H. Department of Dental Medicine and Public Health School of Dentistry University of Southern California Los Angeles, California Keith E. Heller, D.D.S., Dr.P.H. School of Public Health University of Michigan Ann Arbor, Michigan Amid I. Ismail, D.D.S., Dr.P.H. School of Dentistry University of Michigan Ann Arbor, Michigan David W. Johnston, B.D.S., M.P.H. School of Dentistry University of Western Ontario London, Canada John V. Kelsey, D.D.S., M.B.A. US Food and Drug Administration Rockville, Maryland James A. Lalumandier, D.D.S., M.P.H. School of Dentistry Case Western Reserve University Hudson, Ohio Stephen J. Moss, D.D.S., M.S. College of Dentistry New York University New York, New York Ernest Newbrun, D.M.D., Ph.D. School of Dentistry University of California, San Francisco San Francisco, California Kathy R. Phipps, Dr.P.H. School of Dentistry Oregon Health Sciences University Portland, Oregon Mel L. Ringelberg, D.D.S., Dr.P.H. State Dental Director State of Florida Department of Health Tallahassee, Florida Jay D. Shulman, D.M.D., M.S.P.H. Baylor College of Dentistry Dallas, Texas Phillip A. Swango, D.D.S., M.P.H. Private dental consultant Albuquerque, New Mexico Gerald R. Vogel, Ph.D. ADA Health Foundation Paffenbarger Research Center Gaithersburg, Maryland James S. Wefel, Ph.D. College of Dentistry University of Iowa Iowa City, Iowa B. Alex White, D.D.S., Dr.P.H. Kaiser-Permanente, Inc. Portland, Oregon Summary Widespread use of fluoride has been a major factor in the decline in the prevalence and severity of dental caries (i.e., tooth decay) in the United States and other economically developed countries. When used appropriately, fluoride is both safe and effective in preventing and controlling dental caries. All U.S. residents are likely exposed to some degree to fluoride, which is available from multiple sources. Both health-care professionals and the public have sought guidance on selecting the best way to provide and receive fluoride. During the late 1990s, CDC convened a work group to develop recommendations for using fluoride to prevent and control dental caries in the United States. This report includes these recommendations, as well as a) critical analysis of the scientific evidence regarding the efficacy and effectiveness of fluoride modalities in preventing and controlling dental caries, b) ordinal grading of the quality of the evidence, and c) assessment of the strength of each recommendation. Because frequent exposure to small amounts of fluoride each day will best reduce the risk for dental caries in all age groups, the work group recommends that all persons drink water with an optimal fluoride concentration and brush their teeth twice daily with fluoride toothpaste. For persons at high risk for dental caries, additional fluoride measures might be needed. Measured use of fluoride modalities is particularly appropriate during the time of anterior tooth enamel development (i.e., age <6 years). The recommendations in this report guide dental and other health-care providers, public health officials, policy makers, and the public in the use of fluoride to achieve maximum protection against dental caries while using resources efficiently and reducing the likelihood of enamel fluorosis. The recommendations address public health and professional practice, self-care, consumer product industries and health agencies, and further research. Adoption of these recommendations could further reduce dental caries in the United States and save public and private resources. INTRODUCTIONDental caries (i.e., tooth decay) is an infectious, multifactorial disease afflicting most persons in industrialized countries and some developing countries (1). Fluoride reduces the incidence of dental caries and slows or reverses the progression of existing lesions (i.e., prevents cavities). Although pit and fissure sealants, meticulous oral hygiene, and appropriate dietary practices contribute to caries prevention and control, the most effective and widely used approaches have included fluoride use. Today, all U.S. residents are exposed to fluoride to some degree, and widespread use of fluoride has been a major factor in the decline in the prevalence and severity of dental caries in the United States and other economically developed countries (1). Although this decline is a major public health achievement, the burden of disease is still considerable in all age groups. Because many fluoride modalities are effective, inexpensive, readily available, and can be used in both private and public health settings, their use is likely to continue. Fluoride is the ionic form of the element fluorine, the 13th most abundant element in the earth's crust. Fluoride is negatively charged and combines with positive ions (e.g., calcium or sodium) to form stable compounds (e.g., calcium fluoride or sodium fluoride). Such fluorides are released into the environment naturally in both water and air. Fluoride compounds also are produced by some industrial processes that use the mineral apatite, a mixture of calcium phosphate compounds. In humans, fluoride is mainly associated with calcified tissues (i.e., bones and teeth) because of its high affinity for calcium. Fluoride's ability to inhibit or even reverse the initiation and progression of dental caries is well documented. The first use of adjusted fluoride in water for caries control began in 1945 and 1946 in the United States and Canada, when the fluoride concentration was adjusted in the drinking water supplying four communities (2--5). The U.S. Public Health Service (PHS) developed recommendations in the 1940s and 1950s regarding fluoride concentrations in public water supplies. At that time, public health officials assumed that drinking water would be the major source of fluoride for most U.S. residents. The success of water fluoridation in preventing and controlling dental caries led to the development of fluoride-containing products, including toothpaste (i.e., dentifrice), mouthrinse, dietary supplements, and professionally applied or prescribed gel, foam, or varnish. In addition, processed beverages, which constitute an increasing proportion of the diets of many U.S. residents (6,7), and food can contain small amounts of fluoride, especially if they are processed with fluoridated water. Thus, U.S. residents have more sources of fluoride available now than 50 years ago. Much of the research on the efficacy and effectiveness of individual fluoride modalities in preventing and controlling dental caries was conducted before 1980, when dental caries was more common and more severe. Modalities were usually tested separately and with the assumption that the method would provide the main source of fluoride. Thus, various modes of fluoride use have evolved, each with its own recommended concentration, frequency of use, and dosage schedule. Health-care professionals and the public have sought guidance regarding selection of preventive modalities from among the available options. The United States does not have comprehensive recommendations for caries prevention and control through various combinations of fluoride modalities. Adoption of such recommendations could further reduce dental caries while saving public and private resources and reducing the prevalence of enamel fluorosis, a generally cosmetic developmental condition of tooth enamel. This report presents comprehensive recommendations on the use of fluoride to prevent and control dental caries in the United States. These recommendations were developed by a work group of 11 specialists in fluoride research or policy convened by CDC during the late 1990s and reviewed by an additional 23 specialists. Although the recommendations were developed specifically for the United States, aspects of this report could be relevant to other countries. The recommendations guide health-care providers and the public on efficient and appropriate use of fluoride modalities, direct attention to fluoride intake among children aged <6 years to decrease the risk for enamel fluorosis, and suggest areas for further research. This report focuses on critical analysis of the scientific evidence regarding the efficacy and effectiveness of each fluoride modality in preventing and controlling dental caries and on the use of multiple sources of fluoride. The safety of fluoride, which has been documented comprehensively by other scientific and public health organizations (e.g., PHS [8], National Research Council [9], World Health Organization [10], and Institute of Medicine [11]) is not addressed. HOW FLUORIDE PREVENTS AND CONTROLS DENTAL CARIESDental caries is an infectious, transmissible disease in which bacterial by-products (i.e., acids) dissolve the hard surfaces of teeth. Unchecked, the bacteria can penetrate the dissolved surface, attack the underlying dentin, and reach the soft pulp tissue. Dental caries can result in loss of tooth structure, pain, and tooth loss and can progress to acute systemic infection. Cariogenic bacteria (i.e., bacteria that cause dental caries) reside in dental plaque, a sticky organic matrix of bacteria, food debris, dead mucosal cells, and salivary components that adheres to tooth enamel. Plaque also contains minerals, primarily calcium and phosphorus, as well as proteins, polysaccharides, carbohydrates, and lipids. Cariogenic bacteria colonize on tooth surfaces and produce polysaccharides that enhance adherence of the plaque to enamel. Left undisturbed, plaque will grow and harbor increasing numbers of cariogenic bacteria. An initial step in the formation of a carious lesion takes place when cariogenic bacteria in dental plaque metabolize a substrate from the diet (e.g., sugars and other fermentable carbohydrates) and the acid produced as a metabolic by-product demineralizes (i.e., begins to dissolve) the adjacent enamel crystal surface (Figure 1). Demineralization involves the loss of calcium, phosphate, and carbonate. These minerals can be captured by surrounding plaque and be available for reuptake by the enamel surface. Fluoride, when present in the mouth, is also retained and concentrated in plaque. Fluoride works to control early dental caries in several ways. Fluoride concentrated in plaque and saliva inhibits the demineralization of sound enamel and enhances the remineralization (i.e., recovery) of demineralized enamel (12,13). As cariogenic bacteria metabolize carbohydrates and produce acid, fluoride is released from dental plaque in response to lowered pH at the tooth-plaque interface (14). The released fluoride and the fluoride present in saliva are then taken up, along with calcium and phosphate, by de-mineralized enamel to establish an improved enamel crystal structure. This improved structure is more acid resistant and contains more fluoride and less carbonate (12,15--19) (Figure 1). Fluoride is more readily taken up by demineralized enamel than by sound enamel (20). Cycles of demineralization and remineralization continue throughout the lifetime of the tooth. Fluoride also inhibits dental caries by affecting the activity of cariogenic bacteria. As fluoride concentrates in dental plaque, it inhibits the process by which cariogenic bacteria metabolize carbohydrates to produce acid and affects bacterial production of adhesive polysaccharides (21). In laboratory studies, when a low concentration of fluoride is constantly present, one type of cariogenic bacteria, Streptococcus mutans, produces less acid (22--25). Whether this reduced acid production reduces the cariogenicity of these bacteria in humans is unclear (26). Saliva is a major carrier of topical fluoride. The concentration of fluoride in ductal saliva, as it is secreted from salivary glands, is low --- approximately 0.016 parts per million (ppm) in areas where drinking water is fluoridated and 0.006 ppm in nonfluoridated areas (27). This concentration of fluoride is not likely to affect cariogenic activity. However, drinking fluoridated water, brushing with fluoride toothpaste, or using other fluoride dental products can raise the concentration of fluoride in saliva present in the mouth 100- to 1,000-fold. The concentration returns to previous levels within 1--2 hours but, during this time, saliva serves as an important source of fluoride for concentration in plaque and for tooth remineralization (28). Applying fluoride gel or other products containing a high concentration of fluoride to the teeth leaves a temporary layer of calcium fluoride-like material on the enamel surface. The fluoride in this material is released when the pH drops in the mouth in response to acid production and is available to remineralize enamel (29). In the earliest days of fluoride research, investigators hypothesized that fluoride affects enamel and inhibits dental caries only when incorporated into developing dental enamel (i.e., preeruptively, before the tooth erupts into the mouth) (30,31). Evidence supports this hypothesis (32--34), but distinguishing a true preeruptive effect after teeth erupt into a mouth where topical fluoride exposure occurs regularly is difficult. However, a high fluoride concentration in sound enamel cannot alone explain the marked reduction in dental caries that fluoride produces (35,36). The prevalence of dental caries in a population is not inversely related to the concentration of fluoride in enamel (37), and a higher concentration of enamel fluoride is not necessarily more efficacious in preventing dental caries (38). The laboratory and epidemiologic research that has led to the better understanding of how fluoride prevents dental caries indicates that fluoride's predominant effect is posteruptive and topical and that the effect depends on fluoride being in the right amount in the right place at the right time. Fluoride works primarily after teeth have erupted, especially when small amounts are maintained constantly in the mouth, specifically in dental plaque and saliva (37). Thus, adults also benefit from fluoride, rather than only children, as was previously assumed. RISK FOR DENTAL CARIESThe prevalence and severity of dental caries in the United States have decreased substantially during the preceding 3 decades (39). National surveys have reported that the prevalence of any dental caries among children aged 12--17 years declined from 90.4% in 1971--1974 to 67% in 1988--1991; severity (measured as the mean number of decayed, missing, or filled teeth) declined from 6.2 to 2.8 during this period (40--43). These decreases in caries prevalence and severity have been uneven across the general population; the burden of disease now is concentrated among certain groups and persons. For example, 80% of the dental caries in permanent teeth of U.S. children aged 5--17 years occurs among 25% of those children (43). To develop and apply appropriate and effective caries prevention and control strategies, identification and assessment of groups and persons at high risk for developing new carious lesions is essential (44). Caries risk assessment is difficult because it attempts to account for the complex interaction of multiple factors. Although various methods for assessing risk exist, no single model predominates in this emerging science. Models that take multiple factors into account predict the risk more accurately, especially for groups rather than persons. However, for persons in a clinical setting, models do not improve on a dentist's perception of risk after examining a patient and considering the personal circumstances (45). Populations believed to be at increased risk for dental caries are those with low socioeconomic status (SES) or low levels of parental education, those who do not seek regular dental care, and those without dental insurance or access to dental services (45--47). Persons can be at high risk for dental caries even if they do not have these recognized factors. Individual factors that possibly increase risk include active dental caries; a history of high caries in older siblings or caregivers; root surfaces exposed by gingival recession; high levels of infection with cariogenic bacteria; impaired ability to maintain oral hygiene; malformed enamel or dentin; reduced salivary flow because of medications, radiation treatment, or disease; low salivary buffering capacity (i.e., decreased ability of saliva to neutralize acids); and the wearing of space maintainers, orthodontic appliances, or dental prostheses. Risk can increase if any of these factors are combined with dietary practices conducive to dental caries (i.e., frequent consumption of refined carbohydrates). Risk decreases with adequate exposure to fluoride (44,45). Risk for dental caries and caries experience* exists on a continuum, with each person at risk to some extent; 85% of U.S. adults have experienced tooth decay (48). Caries risk can vary over time --- perhaps numerous times during a person's lifetime --- as risk factors change. Because caries prediction is an inexact, developing science, risk is dichotomized as low and high in this report. If these two categories of risk were applied to the U.S. population, most persons would be classified as low risk at any given time. Children and adults who are at low risk for dental caries can maintain that status through frequent exposure to small amounts of fluoride (e.g., drinking fluoridated water and using fluoride toothpaste). Children and adults at high risk for dental caries might benefit from additional exposure to fluoride (e.g., mouthrinse, dietary supplements, and professionally applied products). All available information on risk factors should be considered before a group or person is identified as being at low or high risk for dental caries. However, when classification is uncertain, treating a person as high risk is prudent until further information or experience allows a more accurate assessment. This assumption increases the immediate cost of caries prevention or treatment and might increase the risk for enamel fluorosis for children aged <6 years, but reduces the risk for dental caries for groups or persons misclassified as low risk. RISK FOR ENAMEL FLUOROSISThe proper amount of fluoride helps prevent and control dental caries. Fluoride ingested during tooth development can also result in a range of visually detectable changes in enamel opacity (i.e., light refraction at or below the surface) because of hypomineralization. These changes have been broadly termed enamel fluorosis, certain extremes of which are cosmetically objectionable (49). (Many other developmental changes that affect the appearance of enamel are not related to fluoride [50].) Severe forms of this condition can occur only when young children ingest excess fluoride, from any source, during critical periods of tooth development. The occurrence of enamel fluorosis is reported to be most strongly associated with cumulative fluoride intake during enamel development, but the severity of the condition depends on the dose, duration, and timing of fluoride intake. The transition and early maturation stages of enamel development appear to be most susceptible to the effects of fluoride (51); these stages occur at varying times for different tooth types. For central incisors of the upper jaw, for example, the most sensitive period is estimated at age 15--24 months for boys and age 21--30 months for girls (51,52). Concerns regarding the risk for enamel fluorosis are limited to children aged <8 years; enamel is no longer susceptible once its preeruptive maturation is complete (11). Fluoride sources for children aged <8 years are drinking water, processed beverages and food, toothpaste, dietary supplements that include fluoride (tablets or drops), and other dental products. This report discusses the risk for enamel fluorosis among children aged <6 years. Children aged >6 years are considered past the age that fluoride ingestion can cause cosmetically objectionable fluorosis because only certain posterior teeth are still at a susceptible stage of enamel development, and these will not be readily visible. In addition, the swallowing reflex has developed sufficiently by age 6 years for most children to be able to control inadvertent swallowing of fluoride toothpaste and mouthrinse. The very mild and mild forms of enamel fluorosis appear as chalklike, lacy markings across a tooth's enamel surface that are not readily apparent to the affected person or casual observer (53). In the moderate form, >50% of the enamel surface is opaque white. The rare, severe form manifests as pitted and brittle enamel. After eruption, teeth with moderate or severe fluorosis might develop areas of brown stain (54). In the severe form, the compromised enamel might break away, resulting in excessive wear of the teeth. Even in its severe form, enamel fluorosis is considered a cosmetic effect, not an adverse functional effect (8,11,55,56). Some persons choose to modify this condition with elective cosmetic treatment. The benefits of reduced dental caries and the risk for enamel fluorosis are linked. Early studies that examined the cause of "mottled enamel" (now called moderate to severe enamel fluorosis) led to the unexpected discovery that fluoride in community drinking water inhibits dental caries (57). Historically, a low prevalence of the milder forms of enamel fluorosis has been accepted as a reasonable and minor consequence balanced against the substantial protection from dental caries from drinking water containing an optimal concentration of fluoride, either naturally occurring or through adjustment (11,53). When enamel fluorosis was first systematically investigated during the 1930s and 1940s, its prevalence was 12%--15% for very mild and mild forms and zero for moderate and severe forms among children who lived in communities with drinking water that naturally contained 0.9--1.2 ppm fluoride (53). Although the prevalence of this condition in the United States has since increased (8,58,59), most fluorosis today is of the mildest form, which affects neither cosmetic appearance nor dental function. The increased prevalence in areas both with and without fluoridated community drinking water (8) indicates that, during the first 8 years of life (i.e., the window of time when this condition can develop), the total intake of fluoride from all sources has increased for some children. The 1986--1987 National Survey of Dental Caries in U.S. School Children (the most recent national estimates of enamel fluorosis prevalence) indicated that the prevalence of any enamel fluorosis among children was 22%--23% (range: 26% of children aged 9 years to 19% of those aged 17 years) (60,61). Almost all cases reported in the survey were of the very mild or mild form, but some cases of the moderate (1.1%) and severe (0.3%) forms were observed. Cases of moderate and severe forms occurred even among children living in areas with low fluoride concentrations in the drinking water (61). Although this level of enamel fluorosis is not considered a public health problem (53), prudent public health practice should seek to minimize this condition, especially moderate to severe forms. In addition, changes in public perceptions of what is cosmetically acceptable could influence support for effective caries-prevention measures. Research into the causes of enamel fluorosis has focused on identifying risk factors (62--65). Adherence to the recommendations in this report regarding appropriate use of fluoride for children aged <6 years will reduce the prevalence and severity of enamel fluorosis. NATIONAL GUIDELINES FOR FLUORIDE USEPHS recommendations for fluoride use include an optimally adjusted concentration of fluoride in community drinking water to maximize caries prevention and limit enamel fluorosis. This concentration ranges from 0.7 ppm to 1.2 ppm depending on the average maximum daily air temperature of the area (66--68). In 1991, PHS also issued policy and research recommendations for fluoride use (8). The U.S. Environmental Protection Agency (EPA), which is responsible for the safety and quality of drinking water in the United States, sets a maximum allowable limit for fluoride in community drinking water at 4 ppm and a secondary limit (i.e., nonenforceable guideline) at 2 ppm (69,70). The U.S. Food and Drug Administration (FDA) is responsible for approving prescription and over-the-counter fluoride products marketed in the United States and for setting standards for labeling bottled water (71) and over-the-counter fluoride products (e.g., toothpaste and mouthrinse) (72). Nonfederal agencies also have published guidelines on fluoride use. The American Dental Association (ADA) reviews fluoride products for caries prevention through its voluntary Seal of Acceptance program; accepted products are listed in the ADA Guide to Dental Therapeutics (73). A dosage schedule for fluoride supplements for infants and children aged <16 years, which is scaled to the fluoride concentration in the community drinking water, has been jointly recommended by ADA, the American Academy of Pediatric Dentistry (AAPD), and the American Academy of Pediatrics (AAP) (Table 1) (44,74,75). In 1997, the Institute of Medicine published age-specific recommendations for total dietary intake of fluoride (Table 2). These recommendations list adequate intake to prevent dental caries and tolerable upper intake, defined as a level unlikely to pose risk for adverse effects in almost all persons. FLUORIDE SOURCES AND THEIR EFFECTSFluoridated community drinking water and fluoride toothpaste are the most common sources of fluoride in the United States and are largely responsible for the low risk for dental caries for most persons in this country. Persons at high risk for dental caries might require more frequent or more concentrated exposure to fluoride and might benefit from use of other fluoride modalities (e.g., mouthrinse, dietary supplements, and topical gel, foam, or varnish). The effects of each of these fluoride sources on dental caries and enamel fluorosis are described. Fluoridated Drinking Water and Processed Beverages and Food Fluoridated drinking water contains a fluoride concentration effective for preventing dental caries; this concentration can occur naturally or be reached through water fluoridation, which is the controlled addition of fluoride to a public water supply. When fluoridated water is the main source of drinking water, a low concentration of fluoride is routinely introduced into the mouth. Some of this fluoride is taken up by dental plaque; some is transiently present in saliva, which serves as a reservoir for plaque fluoride; and some is loosely held on the enamel surfaces (76). Frequent consumption of fluoridated drinking water and beverages and food processed in fluoridated areas maintains the concentration of fluoride in the mouth. Estimates of fluoride intake among U.S. and Canadian adults have ranged from <1.0 mg fluoride per day in nonfluoridated areas to 1--3 mg fluoride per day in fluoridated areas (77--80). The average daily dietary fluoride intake for both children and adults in fluoridated areas has remained relatively constant for several years (11). For children who live in optimally fluoridated areas, this average is approximately 0.05 mg/kg/day (range: 0.02--0.10); for children who live in nonfluoridated areas, the average is approximately half (11). In a survey of four U.S. cities with different fluoride concentrations in the drinking water (range: 0.37--1.04 ppm), children aged 2 years ingested 0.41--0.61 mg fluoride per day and infants aged 6 months ingested 0.21--0.54 mg fluoride per day (81,82). In the United States, water and processed beverages (e.g., soft drinks and fruit juices) can provide approximately 75% of a person's fluoride intake (83). Many processed beverages are prepared in locations where the drinking water is fluoridated. Foods and ingredients used in food processing vary in their fluoride content (11). As consumption of processed beverages by children increases, fluoride intake in communities without fluoridated water will increase whenever the water source for the processed beverage is fluoridated (84). In fluoridated areas, dietary fluoride intake has been stable because processed beverages have been substituted for tap water and for beverages prepared in the home using tap water (11). A study of Iowa infants estimated that the mean fluoride intake from water during different periods during the first 9 months of life, either consumed directly or added to infant formula or juice, was 0.29--0.38 mg per day, although estimated intake for some infants was as high as 1.73 mg per day (85). As foods are added to an infant's diet, replacing some of the formula prepared with fluoridated water, the amount of fluoride the infant receives typically decreases (86). The Iowa study also reported that infant formula and processed baby food contained variable amounts of fluoride. Since 1979, U.S. manufacturers of infant formula have voluntarily lowered the fluoride concentration of their products, both ready-to-feed and concentrates, to <0.3 ppm fluoride (87). Drinking Water Community Water. During the 1940s, researchers determined that 1 ppm fluoride was the optimal concentration in community drinking water for climates similar to the Chicago area (88,89). This concentration would substantially reduce the prevalence of dental caries, while allowing an acceptably low prevalence (i.e., 10%--12%) of very mild and mild enamel fluorosis and no moderate or severe enamel fluorosis. Water fluoridation for caries control began in 1945 and 1946, when the fluoride concentration was adjusted in the drinking water supplying four communities in the United States and Canada (2--5). This public health approach followed a long period of epidemiologic research into the effects of naturally occurring fluoride in drinking water (53,57,88,89). Current federal fluoridation guidelines, maintained by the PHS since 1962, state that community drinking water should contain 0.7--1.2 ppm fluoride, depending on the average maximum daily air temperature of the area. These temperature-related guidelines are based on epidemiologic studies conducted during the 1950s that led to the development of an algebraic formula for determining optimal fluoride concentrations (67,90--92). This formula determined that a lower fluoride concentration was appropriate for communities in warmer climates because persons living in warmer climates drank more tap water. However, social and environmental changes since 1962 (e.g., increased use of air conditioning and more sedentary lifestyles) have reduced the likelihood that persons in warmer regions drink more tap water than persons in cooler regions (7). By 1992, fluoridated water was reaching 144 million persons in the United States (56% of the total population and 62% of those receiving municipal water supplies) (93). Approximately 10 million of these persons were receiving water containing naturally occurring fluoride at a concentration of >0.7 ppm. In 11 states and the District of Columbia, >90% of the population had such access, whereas <5% received this benefit in two states. In 2000, a total of 38 states and the District of Columbia provided access to fluoridated public water supplies to >50% of their population (CDC, unpublished data, 2000) (Figure 2). Initial studies of community water fluoridation demonstrated that reductions in childhood dental caries attributable to fluoridation were approximately 50%--60% (94--97). More recent estimates are lower --- 18%--40% (98,99). This decrease in attributable benefit is likely caused by the increasing use of fluoride from other sources, with the widespread use of fluoride toothpaste probably the most important. The diffusion or "halo" effect of beverages and food processed in fluoridated areas but consumed in nonfluoridated areas also indirectly spreads some benefit of fluoridated water to nonfluoridated communities. This effect lessens the differences in caries experience among communities (100). Quantifying the benefits of water fluoridation among adults is more complicated because adults are rarely surveyed, their fluoride histories are potentially more varied, and their tooth loss or restorations might be caused by dental problems other than caries (e.g., trauma or periodontal diseases). Nevertheless, adults are reported to receive caries-preventive benefits from community water fluoridation (99,101--103). These benefits might be particularly advantageous for adults aged >50 years, many of whom are at increased risk for dental caries. Besides coronal caries, older adults typically experience gingival recession, which results in teeth with exposed root surfaces. Unlike the crowns of teeth, these root surfaces are not covered by enamel and are more susceptible to caries. Because tooth retention among older age groups has increased in recent decades in the United States (39), these groups' risk for caries will increase as the country's population ages. Older adults also frequently require multiple medications for chronic conditions, and many of these medications can reduce salivary output (104). Drinking water containing an optimal concentration of fluoride can mitigate the risk factors for caries among older adults. Studies have reported that the prevalence of root caries among adults is inversely related to fluoride concentration in the community drinking water (105--107). Water fluoridation also reduces the disparities in caries experience among poor and nonpoor children (108--111). Caries experience is considerably higher among persons in low SES strata than among those in high SES strata (39,46,112). The reasons for this discrepancy are not well understood; perhaps persons in low SES strata have less knowledge of oral diseases, have less access to dental care, are less likely to follow recommended self-care practices, or are harder to reach through traditional approaches, including public health programs and private dental care (48). Thus, these persons might receive more benefit from fluoridated community water than persons from high SES strata. Regardless of SES, water fluoridation is the most effective and efficient strategy to reduce dental caries (112). Enamel fluorosis occurs among some persons in all communities, even in communities with a low natural concentration of fluoride. During 1930--1960, U.S. studies documented that, in areas with a natural or adjusted concentration of fluoride of approximately 1.0 ppm in the community drinking water, the permanent teeth of 7%-- 16% of children with lifetime residence in those areas exhibited very mild or mild forms of enamel fluorosis (53,113,114). Before 1945, when naturally fluoridated drinking water was virtually the only source of fluoride, the moderate and severe forms of this condition were not observed unless the natural fluoride concentration was >2 ppm (53). The likelihood of a child developing the mild forms of enamel fluorosis might be higher in a fluoridated area than in a nonfluoridated area, but prevalence might not change in every community (115,116). The most recent national study of this condition indicated that its prevalence had increased in both fluoridated and nonfluoridated areas since the 1940s, with the relative increase higher in nonfluoridated areas. In communities with drinking water containing 0.7--1.2 ppm fluoride, the prevalence was 1.3% for the moderate form of enamel fluorosis and zero for the severe form; thus, few cases of enamel fluorosis were likely to be of cosmetic consequence (8,61). Because combined fluoride intake from drinking water and processed beverages and food by children in fluoridated areas has reportedly remained stable since the 1940s, the increase in fluoride intake resulting in increased enamel fluorosis almost certainly stems from use of fluoride-containing dental products by children aged <6 years (11). Two studies reported that extended consumption of infant formula beyond age 10--12 months was a risk factor for enamel fluorosis, especially when formula concentrate was mixed with fluoridated water (62,63). These studies examined children who used pre-1979 formula (with higher fluoride concentrations). Whether fluoride intake from formula that exceeds the recommended amount during only the first 10--12 months of life contributes to the prevalence or severity of enamel fluorosis is unknown. Fluoride concentrations in drinking water should be maintained at optimal levels, both to achieve effective caries prevention and because changes in fluoride concentration as low as 0.2 ppm can result in a measurable change in the prevalence and severity of enamel fluorosis (52,117). Since the late 1970s, CDC has provided guidelines and recommendations for managers of fluoridated water supply systems at state and local levels to help them establish and maintain appropriate fluoride concentrations. CDC periodically updates these guidelines; the most recent revision was published in 1995 (68). School Water Systems. In some areas of the United States where fluoridating a community's drinking water was not feasible (e.g., rural areas), the alternative of fluoridating a school's public water supply system was promoted for many years. This method was used when a school had its own source of water and was not connected to a community water supply system (i.e., stand-alone systems). Because children are at school only part of each weekday, a fluoride concentration of 4.5 times the optimal concentration for a community in the same geographic area was recommended (118) to compensate for the more limited consumption of fluoridated water. At the peak of this practice in the early 1980s, a total of 13 states had initiated school water fluoridation in 470 schools serving 170,000 children (39). Since then, school water fluoridation has been phased out in several states; the current extent of this practice is not known. Studies of the effects of school water fluoridation in the United States reported that this practice reduced caries among schoolchildren by approximately 40% (118--122). A more recent study indicated that this effect might no longer be as pronounced (123). Several concerns regarding school water fluoridation exist. Operating and maintaining small fluoridation systems (i.e., those serving <500 persons) create practical and logistical difficulties (68). These difficulties have occasionally caused higher than recommended fluoride concentrations in the school drinking water, but no lasting effects among children have been observed (124--126). In schools that enroll preschoolers in day care programs, children aged <6 years might receive more than adequate fluoride. Bottled Water. Many persons drink bottled water, replacing tap water partially or completely as a source of drinking water. Water is classified as "bottled water" if it meets all applicable federal and state standards, is sealed in a sanitary container, and is sold for human consumption. Although some bottled waters marketed in the United States contain an optimal concentration of fluoride (approximately 1.0 ppm), most contain <0.3 ppm fluoride (127--129). Thus, a person substituting bottled water with a low fluoride concentration for fluoridated community water might not receive the full benefits of community water fluoridation (130). For water bottled in the United States, current FDA regulations require that fluoride be listed on the label only if the bottler adds fluoride during processing; the concentration of fluoride is regulated but does not have to be stated on the label (Table 3). Few bottled water brands have labels listing the fluoride concentration. Determining Fluoride Concentration. Uneven geographic coverage of community water fluoridation throughout the United States, wide variations in natural fluoride concentrations found in drinking water, and almost nonexistent labeling of fluoride concentration in bottled water make knowing the concentration of fluoride in drinking water difficult for many persons. Persons in nonfluoridated areas can mistakenly believe their water contains an optimal concentration of fluoride. To obtain the fluoride concentration of community drinking water, a resident can contact the water supplier or a local public health authority, dentist, dental hygienist, physician, or other knowledgeable source. EPA requires that all community water supply systems provide each customer an annual report on the quality of water, including the fluoride concentration (131). Testing for private wells is available through local and state public health departments as well as some private laboratories. If the fluoride concentration is not listed on the label of bottled water, the bottler can be contacted directly to obtain this information. Fluoride Toothpaste Fluoride is the only nonprescription toothpaste additive proven to prevent dental caries. When introduced into the mouth, fluoride in toothpaste is taken up directly by dental plaque (132--134) and demineralized enamel (135,136). Brushing with fluoride toothpaste also increases the fluoride concentration in saliva 100- to 1,000-fold; this concentration returns to baseline levels within 1--2 hours (137). Some of this salivary fluoride is taken up by dental plaque. The ambient fluoride concentration in saliva and plaque can increase during regular use of fluoride toothpaste (132,133). By the 1990s, fluoride toothpaste accounted for >90% of the toothpaste market in the United States, Canada, and other developed countries (138). Because water fluoridation is not available in many countries, toothpaste might be the most important source of fluoride globally (1). Studies of 2--3 years duration have reported that fluoride toothpaste reduces caries experience among children by a median of 15%--30% (139--148). This reduction is modest compared with the effect of water fluoridation, but water fluoridation studies usually measured lifetime --- rather than a few years' --- exposure. Regular lifetime use of fluoride toothpaste likely provides ongoing benefits that might approach those of fluoridated water. Combined use of fluoride toothpaste and fluoridated water offers protection above either used alone (99,149,150). Few studies evaluating the effectiveness of fluoride toothpaste, gel, rinse, and varnish among adult populations are available. Child populations have typically been used for studies on caries prevention because of perceived increased caries susceptibility and logistical reasons. However, teeth generally remain susceptible to caries throughout life, and topically applied fluorides could be effective in preventing caries in susceptible patients of any age (151,152). Most persons report brushing their teeth at least once per day (153,154), but more frequent use can offer additional protection (139,141,155--158). Brushing twice a day is a reasonable social norm that is both effective and convenient for most persons' daily routines, and this practice has become a basic recommendation for caries prevention. Whether increasing the number of daily brushings from two to three times a day results in lower dental caries experience is unclear. Because the amount and vigor of rinsing after toothbrushing affects fluoride concentration in the mouth and reportedly affects caries experience (157--160), persons aged >6 years can retain more fluoride in the mouth by either rinsing briefly with a small amount of water or not at all. In the United States, the standard concentration of fluoride in fluoride toothpaste is 1,000--1,100 ppm. Toothpaste containing 1,500 ppm fluoride has been reported to be slightly more efficacious in reducing dental caries in U.S. and European studies (161--164). Products with this fluoride concentration have been marketed in the United States, but are not available in all areas. These products might benefit persons aged >6 years at high risk for dental caries. Children who begin using fluoride toothpaste at age <2 years are at higher risk for enamel fluorosis than children who begin later or who do not use fluoride toothpaste at all (62,63,165--170). Because studies have not used the same criteria for age of initiation, amount of toothpaste used, or frequency of toothpaste use, the specific contribution of each factor to enamel fluorosis among this age group has not been established. Fluoride toothpaste contributes to the risk for enamel fluorosis because the swallowing reflex of children aged <6 years is not always well controlled, particularly among children aged <3 years (171,172). Children are also known to swallow toothpaste deliberately when they like its taste. A child-sized toothbrush covered with a full strip of toothpaste holds approximately 0.75--1.0 g of toothpaste, and each gram of fluoride toothpaste, as formulated in the United States, contains approximately 1.0 mg of fluoride. Children aged <6 years swallow a mean of 0.3 g of toothpaste per brushing (11) and can inadvertently swallow as much as 0.8 g (138,173--176). As a result, multiple brushings with fluoride toothpaste each day can result in ingestion of excess fluoride (177). For this reason, high-fluoride toothpaste (i.e., containing 1,500 ppm fluoride) is generally contraindicated for children aged <6 years. Use of a pea-sized amount (approximately 0.25 g) of fluoride toothpaste <2 times per day by children aged <6 years is reported to sharply reduce the importance of fluoride toothpaste as a risk factor for enamel fluorosis (65). Since 1991, manufacturers of fluoride toothpaste marketed in the United States have, as a requirement for obtaining the ADA Seal of Acceptance, placed instructions on the package label stating that children aged <6 years should use only this amount of toothpaste. Toothpaste labeling requirements mandated by FDA in 1996 (72) also direct parents of children aged <2 years to seek advice from a dentist or physician before introducing their child to fluoride toothpaste. The propensity of young children to swallow toothpaste has led to development of "child-strength" toothpaste with lower fluoride concentrations (176). Such a product would be a desirable alternative to currently available products for many young children. Clinical trials outside the United States have reported that toothpaste containing 250 ppm fluoride is less effective than toothpaste containing 1,000 ppm fluoride in preventing dental caries (178,179). However, toothpaste containing 500--550 ppm fluoride might be almost as efficacious as that containing 1,000 ppm fluoride (180). A British study reported that the prevalence of diffuse enamel opacities (an indicator of mild enamel fluorosis) in the upper anterior incisors was substantially lower among children who used toothpaste containing 550 ppm fluoride than among those who used toothpaste containing 1,050 ppm fluoride (181). Toothpaste containing 400 ppm fluoride has been available in Australia and New Zealand for approximately 20 years, but has not been tested in clinical trials, and no data are available to assess whether toothpaste at this concentration has reduced the prevalence of enamel fluorosis in those countries. A U.S. clinical trial of the efficacy of toothpaste with lower fluoride concentrations, required by FDA before approval for marketing and distribution, has not been conducted (182). Fluoride Mouthrinse Fluoride mouthrinse is a concentrated solution intended for daily or weekly use. The fluoride from mouthrinse, like that from toothpaste, is retained in dental plaque and saliva to help prevent dental caries (183). The most common fluoride compound used in mouthrinse is sodium fluoride. Over-the-counter solutions of 0.05% sodium fluoride (230 ppm fluoride) for daily rinsing are available for use by persons aged >6 years. Solutions of 0.20% sodium fluoride (920 ppm fluoride) are used in supervised, school-based weekly rinsing programs. Throughout the 1980s, approximately 3 million children in the United States participated in school-based fluoride mouthrinsing programs (39). The current extent of such programs is not known. Studies indicating that fluoride mouthrinse reduces caries experience among schoolchildren date mostly from the 1970s and early 1980s (184--191). In one review, the average caries reduction in nonfluoridated communities attributable to fluoride mouthrinse was 31% (191). Two studies reported benefits of fluoride mouthrinse approximately 2.5 and 7 years after completion of school-based mouthrinsing programs (192,193), but a more recent study did not find such benefits 4 years after completion of a mouthrinsing program (194). The National Preventive Dentistry Demonstration Program (NPDDP), a large project conducted in 10 U.S. cities during 1976--1981 to compare the cost and effectiveness of combinations of caries-prevention procedures, reported that fluoride mouthrinse had little effect among schoolchildren, either among first-grade students with high and low caries experience (195) or among all second- and fifth-grade students (196). NPDDP documented only a limited reduction in dental caries attributable to fluoride mouthrinse, especially when children were also exposed to fluoridated water. Although no studies of enamel fluorosis associated with use of fluoride mouthrinse have been conducted, studies of the amount of fluoride swallowed by children aged 3--5 years using such rinses indicated that some young children might swallow substantial amounts (191). Use of fluoride mouthrinse by children aged >6 years does not place them at risk for cosmetically objectionable enamel fluorosis because they are generally past the age that fluoride ingestion might affect their teeth. Dietary Fluoride Supplements Dietary fluoride supplements in the form of tablets, lozenges, or liquids (including fluoride-vitamin preparations) have been used throughout the world since the 1940s. Most supplements contain sodium fluoride as the active ingredient. Tablets and lozenges are manufactured with 1.0, 0.5, or 0.25 mg fluoride. To maximize the topical effect of fluoride, tablets and lozenges are intended to be chewed or sucked for 1--2 minutes before being swallowed. For infants, supplements are available as a liquid and used with a dropper. In 1986, an estimated 16% of U.S. children aged <2 years used fluoride supplements (197). All fluoride supplements must be prescribed by a dentist or physician. The prescription should be consistent with the 1994 dosage schedule developed by ADA, AAPD, and AAP (Table 1). Because fluoride supplements are intended to compensate for fluoride-deficient drinking water, the dosage schedule requires knowledge of the fluoride content of the child's primary drinking water; consideration should also be given to other sources of water (e.g., home, child care settings, school, or bottled water) and to other sources of fluoride (e.g., toothpaste or mouthrinse), which can complicate the prescribing decision. The evidence for using fluoride supplements to mitigate dental caries is mixed. Use of fluoride supplements by pregnant women does not benefit their offspring (198). Several studies have reported that fluoride supplements taken by infants and children before their teeth erupt reduce the prevalence and severity of caries in teeth (98,199--207), but several other studies have not (19,208--212). Among children aged 6--16 years, fluoride supplements taken after teeth erupt reduce caries experience (213--215). Fluoride supplements might be beneficial among adults who have limitations with toothbrushing, but this use requires further study. A few studies have reported no association between supplement use by children aged <6 years and enamel fluorosis (208,216), but most have reported a clear association (19,62,64,165,170,199--201,209,210,212,217--222 ). In one study, the risk for this condition was high when supplements were used in fluoridated areas (odds ratio = 23.74; 95% confidence interval = 3.43--164.30) (62), a use inconsistent with the supplement schedule. Reports of the frequency of supplement use in fluoridated areas have ranged from 7% to 35% (223--228). In response to the accumulated data on fluoride intake and the prevalence of enamel fluorosis, the supplement dosage schedule for children aged <6 years was markedly reduced in 1994 when ADA, AAPD, and AAP jointly established the current schedule (Table 1) (73). The risk for enamel fluorosis among children this age attributable to fluoride supplements could be lower, but not enough information is available yet to evaluate the effects of this change. When prescribing any pharmaceutical agent, dentists and physicians should attempt to maximize benefit and minimize harm (229). For infants and children aged <6 years, both a benefit of dental caries prevention and a risk for enamel fluorosis are possible. Although the primary (i.e., "baby") teeth of children aged 1--6 years would benefit from fluoride's posteruptive action, and some preeruptive benefit for developing permanent teeth could exist, fluoride supplements also could increase the risk for enamel fluorosis at this age (138,223). Professionally Applied Fluoride Compounds In the United States, dentists and dental hygienists have been applying high-concentration fluoride compounds directly to patients' teeth for approximately 50 years. Application procedures were developed on the assumption that the fluoride would be incorporated into the crystalline structure of the dental enamel and develop a more acid-resistant enamel. To maximize this reaction, a professional tooth cleaning was considered mandatory before the application. However, subsequent research has demonstrated that high-concentration fluoride compounds (e.g., those in gel or varnish) do not directly enter the enamel's crystalline structure (230). The compound forms a calcium fluoride-like material on the enamel's surface that releases fluoride for remineralization when the pH in the mouth drops. Thus, professional tooth cleaning solely to prepare the teeth for application of a fluoride compound is unnecessary; toothbrushing and flossing appear equally effective in improving the efficacy of high-concentration fluoride compounds (231). Fluoride Gel and Foam Because an early study reported that fluoride uptake by dental enamel increased in an acidic environment (232), fluoride gel is often formulated to be highly acidic (pH of approximately 3.0). Products available in the United States include gel of acidulated phosphate fluoride (1.23% [12,300 ppm] fluoride), gel or foam of sodium fluoride (0.9% [9,040 ppm] fluoride), and self-applied (i.e., home use) gel of sodium fluoride (0.5% [5,000 ppm] fluoride) or stannous fluoride (0.15% [1,000 ppm] fluoride) (73). Clinical trials conducted during 1940--1970 demonstrated that professionally applied fluorides effectively reduce caries experience in children (233). In more recent studies, semiannual treatments reportedly caused an average decrease of 26% in caries experience in the permanent teeth of children residing in nonfluoridated areas (191,234--236). The application time for the treatments was 4 minutes. In clinical practice, applying fluoride gel for 1 minute rather than 4 minutes is common, but the efficacy of this shorter application time has not been tested in human clinical trials. In addition, the optimal schedule for repeated application of fluoride gel has not been adequately studied to support definitive guidelines, and studies that have examined the efficacy of various gel application schedules in preventing and controlling dental caries have reported mixed results. On the basis of the available evidence, the usual recommended frequency is semiannual (151,237,238). Because these applications are relatively infrequent, generally at 3- to 12-month intervals, fluoride gel poses little risk for enamel fluorosis, even among patients aged <6 years. Proper application technique reduces the possibility that a patient will swallow the gel during application. Fluoride Varnish High-concentration fluoride varnish is painted directly onto the teeth. Fluoride varnish is not intended to adhere permanently; this method holds a high concentration of fluoride in a small amount of material in close contact with the teeth for many hours. Fluoride varnish has practical advantages (e.g., ease of application, a nonoffensive taste, and use of smaller amounts of fluoride than required for gel applications). Such varnishes are available as sodium fluoride (2.26% [2,600 ppm] fluoride) or difluorsilane (0.1% [1,000 ppm] fluoride) preparations. Fluoride varnish has been widely used in Canada and Europe since the 1970s to prevent dental caries (152,239). FDA's Center for Devices and Radiological Health has cleared fluoride varnish as a medical device to be used as a cavity liner (i.e., to provide fluoride at the junction of filling material and tooth) and root desensitizer (i.e., to reduce sensitivity to temperature and touch that sometimes occurs on root surfaces exposed by receding gingiva) (240); FDA has not yet approved this product as an anticaries agent. Caries prevention is regarded as a drug claim, and companies would be required to submit appropriate clinical trial evidence for review before this product could be marketed as an anticaries agent. However, a prescribing practitioner can use fluoride varnish for caries prevention as an "off-label" use, based on professional judgement (241). Studies conducted in Canada (242) and Europe (243--246) have reported that fluoride varnish is efficacious in preventing dental caries in children. Applied semiannually, this modality is as effective as professionally applied fluoride gel (247). Some researchers advocate application of fluoride varnish as many as four times per year to achieve maximum effect, but the evidence of benefits from more than two applications per year remains inconclusive (240,246,248). Other studies have reported that three applications in 1 week, once per year, might be more effective than the more conventional semiannual regimen (249,250). European studies have reported that fluoride varnish prevents decalcification (i.e., an early stage of dental caries) beneath orthodontic bands (251) and slows the progression of existing enamel lesions (252). Studies examining the effectiveness of varnish in controlling early childhood caries are being conducted in the United States. Research on fluoride varnish (e.g., optimal fluoride concentration, the most effective application protocols, and its efficacy relative to other fluoride modalities) is likely to continue in both Europe and North America. No published evidence indicates that professionally applied fluoride varnish is a risk factor for enamel fluorosis, even among children aged <6 years. Proper application technique reduces the possibility that a patient will swallow varnish during its application and limits the total amount of fluoride swallowed as the varnish wears off the teeth over several hours. Fluoride Paste Fluoride-containing paste is routinely used during dental prophylaxis (i.e., cleaning). The abrasive paste, which contains 4,000--20,000 ppm fluoride, might restore the concentration of fluoride in the surface layer of enamel removed by polishing, but it is not an adequate substitute for fluoride gel or varnish in treating persons at high risk for dental caries (151). Fluoride paste is not accepted by FDA or ADA as an efficacious way to prevent dental caries. Combinations of Fluoride Modalities Studies comparing various combinations of fluoride modalities have generally reported that their effectiveness in preventing dental caries is partially additive. That is, the percent reduction in the prevalence or severity of dental caries from a combination of modalities is higher than the percent reduction from each modality, but less than the sum of the percent reduction of the modalities combined. Attempts to use a formula to apply sequentially the percent reduction of an additional modality to the estimated remaining caries increment have overestimated the effect (151,253). For example, if the first modality reduces caries by 40% and the second modality reduces caries by 30%, then the calculation that caries will be reduced by a total of 58% (i.e., 40% plus 18% [30% of the 60% decay remaining after the first modality]) will likely be an overestimate. QUALITY OF EVIDENCE FOR DENTAL CARIES PREVENTION AND CONTROLMembers of the work group convened by CDC identified the published research in their areas of expertise and evaluated the quality of scientific evidence for each fluoride modality in preventing and controlling dental caries. Evidence was drawn from the most relevant English-language, peer-reviewed scientific publications regarding the current effectiveness of fluoride modalities. Additional references were suggested by reviewers. Members used their own methods for critically analyzing articles. A formal protocol for duplicate review was not followed, but members collectively agreed on the grade reflecting the quality of evidence regarding each fluoride modality. Criteria used to grade the quality of scientific evidence (i.e., ordinal grading) was adapted from the U.S. Preventive Services Task Force (Box 1) (254). Grades range from I to III. Community Water Fluoridation Studies on the effectiveness of adjusting fluoride in community water to the optimal concentration cannot be designed as randomized clinical trials. Random allocation of study subjects is not possible when a community begins to fluoridate the water because all residents in a community have access to and are exposed to this source of fluoride. In addition, clinical studies cannot be conducted double-blind because both study subjects and researchers usually know whether a community's water has been fluoridated. Efforts to blind the examiners by moving study subjects to a neutral third site for clinical examinations, using radiographs of teeth without revealing where the subjects live, or including transient residents as study subjects have not fully resolved these inherent limitations. Early studies that led to the unexpected discovery that dental caries was less prevalent and severe among persons with mottled enamel (subsequently identified as a form of enamel fluorosis) were conducted before the caries-preventive effects of fluoride were known (255). In those studies, researchers did not have an a priori reason to suspect they would find either reduced or higher levels of dental caries experience in communities with low levels of mottled enamel. Researchers also had no reason to believe that patients selected where they lived according to their risk for dental caries. In that regard, these studies were randomized, and examiners were blinded. Despite the strengths of early studies of the efficacy of naturally occurring fluoride in community drinking water, the limitations of these studies make summarizing the quality of evidence on community water fluoridation as Grade I inappropriate (Table 1). The quality of evidence from studies on the effectiveness of adjusting fluoride concentration in community water to optimal levels is Grade II-1. Research limitations are counterbalanced by broadly similar results from numerous well-conducted field studies by other investigators that included thousands of persons throughout the world (256,257). School Water Fluoridation Field trials on the effect of school water fluoridation were not blindly conducted and had no concurrent controls (118). Thus, the quality of evidence for this modality is Grade II-3. Fluoride Toothpaste Studies that have demonstrated the efficacy of fluoride toothpaste in preventing and controlling dental caries include all of the essential features of well-conducted clinical trials. These include randomized groups, double-blind designs, placebo controls, and meticulous procedural protocols. Taken together, the trials on fluoride toothpaste provide solid evidence that fluoride is efficacious in controlling caries (144). The quality of evidence for toothpaste is Grade I. Fluoride Mouthrinse Early studies of the efficacy of fluoride mouthrinse in reducing dental caries experience were randomized clinical trials (184,185) or studies that used historical control groups rather than concurrent control groups (186--189). The quality of evidence for fluoride mouthrinse is Grade I. Dietary Fluoride Supplements The only randomized controlled trial to assess fluoride supplements taken by pregnant women provides Grade I evidence of no benefit for their children. Many studies of the effectiveness of fluoride supplements in preventing dental caries among children aged <6 years have been flawed in design and conduct. Problems included self-selection into test and control groups, absence of concurrent controls, high attrition rates, and nonblinded examiners. Because of these flaws, the quality of evidence to support use of fluoride supplements by children aged <6 years is Grade II-3. The well-conducted randomized clinical trials on the effects of fluoride supplements on dental caries among children aged 6--16 years in programs conducted in schools provide Grade I evidence. Fluoride Gel The quality of evidence for using fluoride gel to prevent and control dental caries in children is Grade I. However, data were gathered when dental caries was more prevalent and severe than today. Subjects in earlier studies were probably more representative of persons who now would be characterized as being at high risk for caries. Fluoride Varnish The quality of evidence for the efficacy of high-concentration fluoride varnish in preventing and controlling dental caries in children is Grade I. Although the randomized controlled clinical studies that established Grade I evidence were conducted in Europe, U.S. results should be the same. COST-EFFECTIVENESS OF FLUORIDE MODALITIESDocumented effectiveness is the most basic requirement for providing a health-care service and an important prerequisite for preventive services (e.g., caries-preventive modalities). However, effectiveness alone is not a sufficient reason to initiate a service. Other factors, including cost, must be considered (254). A modality is more cost-effective when deemed a less expensive way, from among competing alternatives, of meeting a stated objective (258). In public health planning, determination of the most cost-effective alternative for prevention is essential to using scarce resources efficiently. Dental-insurance carriers are also interested in cost-effectiveness so they can help purchasers use funds efficiently. Because half of dental expenditures are out of pocket (259), this topic interests patients and their dentists as well. Potential improvement to quality of life is also a consideration. The contribution of a healthy dentition to quality of life at any age has not been quantified, but is probably valued by most persons. Although solid data on the cost-effectiveness of fluoride modalities alone and in combination are needed, this information is scarce. In 1989, the Cost Effectiveness of Caries Prevention in Dental Public Health workshop, which was attended by health economists, epidemiologists, and dental public health professionals, attempted to assess the cost-effectiveness of caries-preventive approaches available in the United States (260). All other things being equal, fluoride modalities are most cost-effective for persons at high risk for dental caries. Because persons at low risk develop little dental caries, limited benefit is gained by adding caries-preventive modalities to water fluoridation and fluoride toothpaste, even those demonstrated to be effective among populations at high risk. Members of the CDC work group reached consensus regarding the populations for which each modality would be expected to have the necessary level of cost-effectiveness to warrant its use. Community Water Fluoridation Health economists at the 1989 workshop on cost-effectiveness of caries prevention calculated that the average annual cost of water fluoridation in the United States was $0.51 per person (range: $0.12--$5.41) (260). In 1999 dollars,** this cost would be $0.72 per person (range: $0.17--$7.62). Factors reported to influence the per capita cost included
When the effects of caries are repaired, the price of the restoration is based on the number of tooth surfaces affected. A tooth can have caries at >1 location (i.e., surface), so the number of surfaces saved is a more appropriate measure in calculating cost-effectiveness than the number of teeth with caries. The 1989 workshop participants concluded that water fluoridation is one of the few public health measures that results in true cost savings (i.e., the measure saves more money than it costs to operate); in the United States, water fluoridation cost an estimated average of $3.35 per carious surface saved ($4.71 in 1999 dollars**) (260). Even under the least favorable assumptions in 1989 (i.e., cities with populations <10,000, higher operating costs, and effectiveness projected at the low end of the range), the cost of a carious surface saved because of community water fluoridation ranged from $8 to $12 ($11--$17 in 1999 dollars**) (260), which is still lower than the fee for a one-surface restoration ($54 in 1995 or $65 in 1999 dollars***) (261). A Scottish study conducted in 1980 reported that community water fluoridation resulted in a 49% saving in dental treatment costs for children aged 4--5 years and a 54% saving for children aged 11--12 years (262). These savings were maintained even after the secular decline in the prevalence of dental caries was recognized (263). The effect of community water fluoridation on the costs of dental care for adults is less clear. This topic cannot be fully explored until the generations who grew up drinking optimally fluoridated water are older. School Water Fluoridation Costs for school water fluoridation are similar to those of any public water supply system serving a small population (i.e., <1,000 persons). In 1988, the average annual cost of school water fluoridation was $4.52 per student per year (range: $0.81--$9.72) (264). In 1999 dollars,**** this cost would be $6.37 per person (range: $1.14--$13.69). Use of this modality must be carefully weighed in the current environment of low caries prevalence, widespread use of fluoride toothpaste, and availability of other fluoride modalities that can be delivered in the school setting. Fluoride Toothpaste Fluoride toothpaste is widely available, no more expensive than nonfluoride toothpaste, and periodically improved. Use of a pea-sized amount (0.25 g) twice per day requires approximately two tubes of toothpaste per year, for an estimated annual cost of $6--$12, depending on brand, tube size, and retail source (265). Persons who brush and use toothpaste regularly to maintain periodontal health and prevent stained teeth and halitosis (i.e., bad breath) incur no additional cost for the caries-preventive benefit of fluoride in toothpaste. Because of its multiple benefits, most persons consider fluoride toothpaste a highly cost-effective caries-preventive modality. Fluoride Mouthrinse Public health programs of fluoride mouthrinsing have long been presumed to be cost-effective, especially when teachers can supervise weekly rinsing in classrooms at no direct cost to the program. In other programs, volunteers or hourly workers provide supervision. Under these circumstances, administrators of fluoride mouthrinsing programs have claimed annual program costs of approximately $1 per child ($1.41 in 1999 dollars****) (264). This figure likely is an underestimate because indirect costs are not included (196,266). Fluoride mouthrinsing is a reasonable procedure for groups and persons at high risk for dental caries, but its cost-effectiveness as a universal, population-wide strategy in the modern era of widespread fluoride exposure is questionable (267). Dietary Fluoride Supplements Dietary fluoride supplements prescribed to persons cost an estimated $37 per year. Fluoride supplements in school programs have direct costs of approximately $2.50 per child ($3.52 in 1999 dollars****) for the tablet or lozenge (264); program administrative costs and considerations are similar to those in school mouthrinsing programs. Professionally Applied Fluoride Compounds High-concentration fluoride gel and varnish are effective in preventing dental caries, but because application requires professional expertise, they are inherently more expensive than self-applied methods (e.g., drinking fluoridated water or brushing with fluoride toothpaste). For groups and persons at low risk for dental caries, professionally applied methods are unlikely to be cost-effective (268,269). In the NPDDP study, prophylactic cleaning and gel application costs were $23 per year ($66 in 1999 dollars*****) for semiannual applications, which prevented 0.03--0.26 decayed surfaces per year (196). A Swedish study claimed that fluoride varnish was cost-effective, but few supporting data were presented (270). Varnish might be cost-effective in Scandinavian school dental services, in which dental professionals regularly examine and treat each student, but the cost-effectiveness of fluoride varnish in public health programs in the United States remains undocumented. Whether fluoride varnish or gel would be most efficiently used in clinical programs targeting groups at high risk for dental caries or should be reserved for individual patients at high risk is unclear. Combinations of Fluoride Modalities Because the caries-preventive effects of a combination of fluoride modalities are only partially additive, estimates of the cost-effectiveness when adding a modality (e.g., fluoride mouthrinse for a group already drinking fluoridated water and using fluoride toothpaste) should take into account these smaller, incremental reductions in caries. This consideration is particularly relevant for groups and persons at low risk for caries (253). The scarcity of research on the costeffectiveness of combinations limits the ability to draw more detailed conclusions. RECOMMENDATIONSIn developing the recommendations for specific fluoride modalities that address public health and clinical practice and self-care, the CDC work group considered the quality of evidence of each modality's effect on dental caries, its association with enamel fluorosis, and its cost-effectiveness. The strength of the recommendation for each fluoride modality was determined by the work group, which adapted a coding system used by the U.S. Preventive Services Task Force (Box 2). The work group considered these factors when determining the population for which each recommendation applies (Table 4). The work group recognized that some recommendations can only be addressed by health-care industries or agencies and that additional research is required to resolve some questions regarding fluoride modalities. Before promoting a fluoride modality or combination of modalities, the dental-care or other health-care provider must consider a person's or group's risk for dental caries, current use of other fluoride sources, and potential for enamel fluorosis. Although these recommendations are based on assessments of caries risk as low or high, the health-care provider might also differentiate among patients at high risk and provide more intensive interventions as needed. Also, a risk category can change over time; the type and frequency of preventive interventions should be adjusted accordingly. Public Health and Clinical Practice Continue and Extend Fluoridation of Community Drinking Water Community water fluoridation is a safe, effective, and inexpensive way to prevent dental caries. This modality benefits persons in all age groups and of all SES, including those difficult to reach through other public health programs and private dental care. Community water fluoridation also is the most cost-effective way to prevent tooth decay among populations living in areas with adequate community water supply systems. Continuation of community water fluoridation for these populations and its adoption in additional U.S. communities are the foundation for sound caries-prevention programs. In contrast, the appropriateness of fluoridating stand-alone water systems that supply individual schools is limited. Widespread use of fluoride toothpaste, availability of other fluoride modalities that can be delivered in the school setting, and the current environment of low caries prevalence limit the appropriateness of fluoridating school drinking water at 4.5 times the optimal concentration for community drinking water. Decisions to initiate or continue school fluoridation programs should be based on an assessment of present caries risk in the target school(s), alternative preventive modalities that might be available, and periodic evaluation of program effectiveness. Counsel Parents and Caregivers Regarding Use of Fluoride Toothpaste by Young Children, Especially Those Aged <2 Years Fluoride toothpaste is a cost-effective way to reduce the prevalence of dental caries. However, for children aged <6 years, especially those aged <2 years, an increased risk for enamel fluorosis exists because of inadequately developed control of the swallowing reflex. Parents or caregivers should be counseled regarding selfcare recommendations for toothpaste use for young children (i.e., limit the child's toothbrushing to <2 times a day, apply a peasized amount to the toothbrush, supervise toothbrushing, and encourage the child to spit out excess toothpaste). For children aged <2 years, the dentist or other healthcare provider should consider the fluoride level in the community drinking water, other sources of fluoride, and factors likely to affect susceptibility to dental caries when weighing the risk and benefits of using fluoride toothpaste. Target Mouthrinsing to Persons at High Risk Because fluoride mouthrinse has resulted in only limited reductions in caries experience among schoolchildren, especially as their exposure to other sources of fluoride has increased, its use should be targeted to groups and persons at high risk for caries (see Risk for Dental Caries). Children aged <6 years should not use fluoride mouthrinse without consultation with a dentist or other health-care provider because enamel fluorosis could occur if such mouthrinses are repeatedly swallowed. Judiciously Prescribe Fluoride Supplements Fluoride supplements can be prescribed for children at high risk for dental caries and whose primary drinking water has a low fluoride concentration. For children aged <6 years, the dentist, physician, or other health-care provider should weigh the risk for caries without fluoride supplements, the caries prevention offered by supplements, and the potential for enamel fluorosis. Consideration of the child's other sources of fluoride, especially drinking water, is essential in determining this balance. Parents and caregivers should be informed of both the benefit of protection against dental caries and the possibility of enamel fluorosis. The prescription dosage of fluoride supplements should be consistent with the schedule established by ADA, AAPD, and AAP. Supplements can be prescribed for persons as appropriate or used in school-based programs. When practical, supplements should be prescribed as chewable tablets or lozenges to maximize the topical effects of fluoride. Apply High-Concentration Fluoride Products to Persons at High Risk for Dental Caries High-concentration fluoride products can play an important role in preventing and controlling dental caries among groups and persons at high risk. Dentists and other health-care providers must consider the risk status and age of the patient to determine the appropriate intensity of treatment. Routine use of professionally applied fluoride gel or foam likely provides little benefit to persons not at high risk for dental caries, especially those who drink fluoridated water and brush daily with fluoride toothpaste. If FDA approves use of fluoride varnish to prevent and control dental caries, its indications for use will be similar to those of fluoride gel. Such varnishes have practical advantages for children aged <6 years at high risk. Self-Care Know the Fluoride Concentration in the Primary Source of Drinking Water All persons should know whether the fluoride concentration in their primary source of drinking water is below optimal, optimal, or above optimal. This knowledge is the basis for all individual and professional decisions regarding use of other fluoride modalities (e.g., mouthrinse or supplements). Parents and caregivers of children, especially children aged <6 years, must know the fluoride concentration in their child's drinking water when considering whether to alter the child's fluoride intake. For example, in nonfluoridated areas where the natural fluoride concentration is below optimal, fluoride supplements might be considered, whereas in areas where the natural fluoride concentration is >2 ppm, children should use alternative sources of drinking water. Knowledge of the water's fluoride concentration is also key in public policy discussions regarding community water fluoridation. Frequently Use Small Amounts of Fluoride All persons should receive frequent exposure to small amounts of fluoride, which minimizes dental caries by inhibiting demineralization of tooth enamel and facilitating tooth remineralization. This exposure can be readily accomplished by drinking water with an optimal fluoride concentration and brushing with a fluoride toothpaste twice daily. Supervise Use of Fluoride Toothpaste Among Children Aged <6 Years Children's teeth should be cleaned daily from the time the teeth erupt in the mouth. Parents and caregivers should consult a dentist or other health-care provider before introducing a child aged <2 years to fluoride toothpaste. Parents and caregivers of children aged <6 years who use fluoride toothpaste should follow the directions on the label, place no more than a pea-sized amount (0.25 g) of toothpaste on the toothbrush, brush the child's teeth (recommended particularly for preschool-aged children) or supervise the toothbrushing, and encourage the child to spit excess toothpaste into the sink to minimize the amount swallowed. Indiscriminate use can result in inadvertent swallowing of more fluoride than is recommended. Consider Additional Measures for Persons at High Risk for Dental Caries Persons at high risk for dental caries might require additional fluoride or other preventive measures to reduce development of caries. This additional fluoride can come from daily use of another fluoride product at home or from professionally applied, topical fluoride products. Other preventive measures might include dental sealants and targeted antimicrobial therapies. Parents and caregivers should not provide additional fluoride to children aged <6 years without consulting a dentist or other health-care provider regarding the associated benefits and potential for enamel fluorosis. Persons should seek professional advice regarding their risk status or that of their children. Use an Alternative Source of Water for Children Aged <8 Years Whose Primary Drinking Water Contains >2 ppm Fluoride In some regions in the United States, community water supply systems and home wells contain a natural concentration of fluoride >2 ppm. At this concentration, children aged <8 years are at increased risk for developing enamel fluorosis, including the moderate and severe forms, and should have an alternative source of drinking water, preferably one containing fluoride at an optimal concentration. In areas where community water supply systems contain >2 ppm but <4 ppm fluoride, EPA requires that each household be notified annually of the desirability of using an alternative source of water for children aged <8 years. For families receiving water from home wells, testing is necessary to determine the natural fluoride concentration. Consumer Product Industries and Health Agencies Label the Fluoride Concentration of Bottled Water Producers of bottled water should label the fluoride concentration of their products. Such labeling will allow consumers to make informed decisions and dentists, dental hygienists, and other health-care professionals to appropriately advise patients regarding fluoride intake and use of fluoride products. Promote Use of Small Amounts of Fluoride Toothpaste Among Children Aged <6 Years Labels and advertisements for fluoride toothpaste should promote use of a pea-sized amount (0.25 g) of toothpaste on a child-sized toothbrush for children aged <6 years. Efforts to educate parents and caregivers and to encourage supervised use of fluoride toothpaste among young children can reduce inadvertent swallowing of excess toothpaste. Develop a Low-Fluoride Toothpaste for Children Aged <6 Years Manufacturers are encouraged to develop a dentifrice for children aged <6 years that is effective in preventing dental caries but alleviates the risk for enamel fluorosis. A "child-strength" toothpaste with a fluoride concentration lower than current products could reduce the risk for cosmetic concerns associated with inadvertent swallowing of toothpaste. Collaborate to Educate Health-Care Professionals and the Public Professional health-care organizations, public health agencies, and suppliers of oral-care products should collaborate to educate health-care professionals and trainees and the public regarding the recommendations in this report. Broad collaborative efforts to educate health-care professionals and the public and to encourage behavior change can promote improved, coordinated use of fluoride modalities. Further Research Continue Metabolic Studies of Fluoride Metabolic studies with animals and humans to determine the influence of environmental, physiological, and pathological conditions on the pharmacokinetics and effects of fluoride should continue. Research in these areas will enhance the knowledge base concerning fluoride use, thereby resulting in more effective and efficient use of fluoride. Identify Biomarkers of Fluoride As an alternative to direct fluoride intake measurement, biomarkers (i.e., distinct biological indicators) should be identified to estimate a person's fluoride intake and the amount of fluoride in the body. Identification of such biomarkers could allow more efficient research. Reevaluate the Method of Determining Optimal Fluoride Concentration of Community Drinking Water The current method of determining the optimal concentration of fluoride in community drinking water, which depends on the average maximum annual ambient air temperature, should be reevaluated because of the social and environmental changes that have occurred since it was adopted in 1962. Research into current consumption patterns of water, processed beverages, and processed foods is also needed. Such research will either validate the current method for determining optimal fluoride concentration in community drinking water or indicate improved methods. Evaluate the Effect of Fluoride Mouthrinse, Fluoride Supplements, and Other Fluoride Modalities on Dental Caries Additional clinical trials are needed to evaluate the current effect of fluoride mouthrinse, supplements, and other modalities on dental caries both individually and in combination. Cohorts of particular interest are groups and persons at high risk for dental caries, including older adults (i.e., those aged >50 years). Such research, as well as studies to determine the effects of new fluoride modalities and various combinations among groups and persons at high risk, could lead to more effective and efficient use of these interventions. Study the Current Cost-Effectiveness of Fluoride Modalities The increasing availability of multiple fluoride modalities and the lower caries prevalence in the United States indicate a need for current cost-effectiveness studies of fluoride modalities, especially logical combinations of regimens in populations with different caries risks. Such research will allow both more efficient use of resources and a better understanding of the additive effects of combined modalities. Conduct Descriptive and Analytic Epidemiologic Studies Descriptive and analytic epidemiologic studies should be conducted to determine the association between dental caries and fluoride exposure from several sources, as well as the current role of community water fluoridation in preventing coronal and root caries among adults. Studies should assess the effect of interruption or discontinuation of water fluoridation; the prevalence of fluorosis associated with different patterns of fluoride use and intake among various populations; and the relationship between objectively measured fluorosis and the aesthetic perceptions of persons, parents, and dentists and other health-care professionals. Studies are needed to refine methods of caries risk assessment. As appropriate, studies should use national, state, and local data. Research addressing these questions will improve understanding of the relationships between fluoride modalities and the benefits and unintended effects of their use. Identify Effective Strategies to Promote Adoption of Recommendations for Using Fluoride Effective strategies should be identified to promote adherence by parents, caregivers, children, adults, and health-care providers to recommendations regarding fluoride use. Such research could result in more effective behavior change, more efficient use of resources, improved caries prevention, and less enamel fluorosis. CONCLUSIONWhen used appropriately, fluoride is a safe and effective agent that can be used to prevent and control dental caries. Fluoride has contributed profoundly to the improved dental health of persons in the United States and other countries. Fluoride is needed regularly throughout life to protect teeth against tooth decay. To ensure additional gains in oral health, water fluoridation should be extended to additional communities, and fluoride toothpaste should be used widely. Adoption of these and other recommendations in this report could lead to considerable savings in public and private resources without compromising fluoride's substantial benefit of improved dental health. References
*For this report, the term "caries experience" is used to mean the sum of filled and unfilled cavities, along with any missing teeth resulting from tooth decay. **US$ 1988 converted to US$ 1999 using the Consumer Price Index for All Urban Customers (CPI-Urban) (all items). More information is available at the U.S. Department of Labor, Bureau of Labor Statistics website at <http://stats.bls.gov/cpihome.htm>. Accessed June 25, 2001. ***US$ 1995 converted to US$ 1999 using CPI-Urban (dental services). More information is available at the U.S. Department of Labor, Bureau of Labor Statistics website at <http://stats.bls.gov/cpihome.htm>. Accessed June 25, 2001. ****US$ 1988 converted to US$ 1999 using CPI-Urban (all items). More information is available at the U.S. Department of Labor, Bureau of Labor Statistics website at <http://stats.bls.gov/cpihome.htm>. Accessed June 25, 2001. *****US$ 1981 converted to US$ 1999 using CPI-Urban (dental services). More information is available at the U.S. Department of Labor, Bureau of Labor Statistics website at <http://stats.bls.gov/cpihome.htm>. Accessed June 25, 2001. Table 1 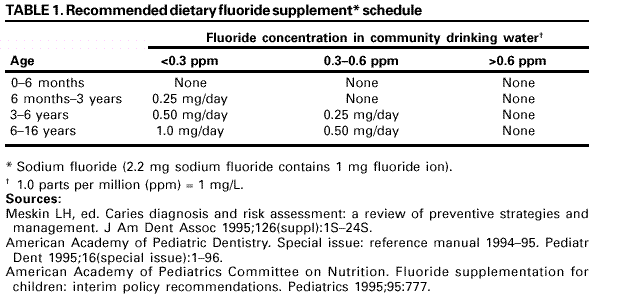 Return to top. Figure 1 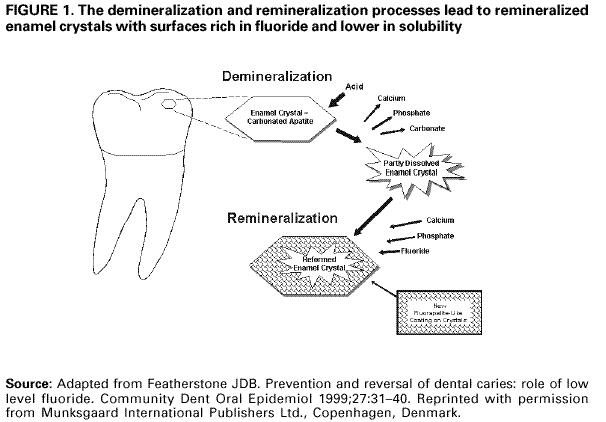 Return to top. Table 2 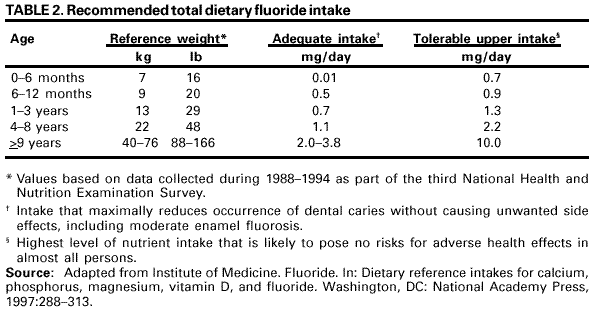 Return to top. Figure 2 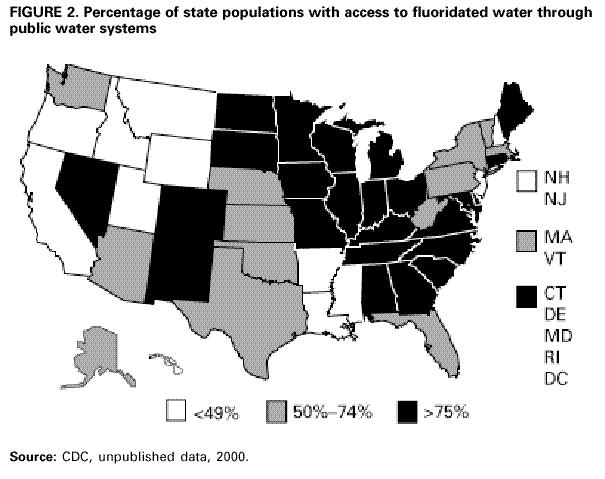 Return to top. Table 3 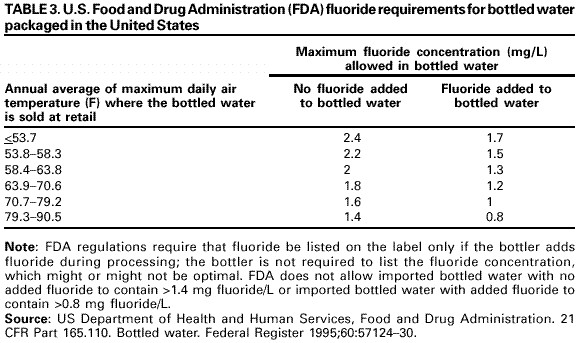 Return to top. Table 4 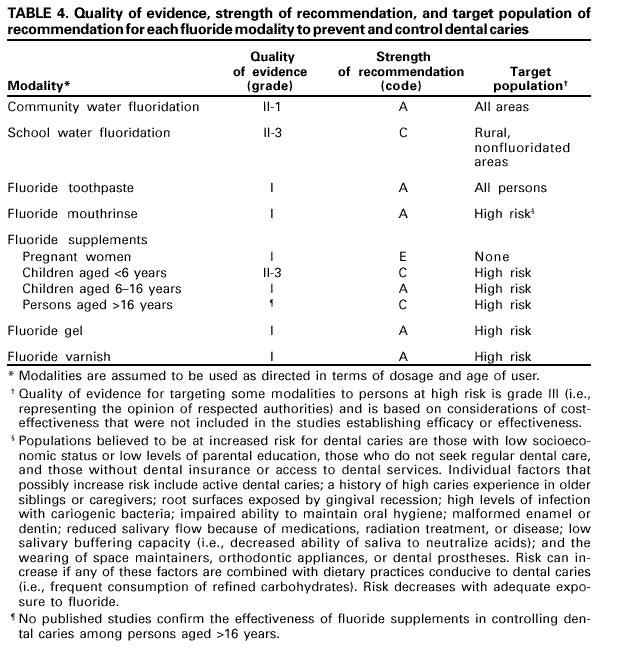 Return to top. Box 1  Return to top. Box 2  Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 8/22/2001 |
|||||||||
This page last reviewed 8/22/2001
|