 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Spina Bifida and Anencephaly Prevalence --- United States, 1991--2001Prepared by SummarySpina bifida and anencephaly are serious birth defects. To reduce the occurrence of these birth defects, the Food and Drug Administration authorized the fortification of all enriched cereal grain products with folic acid in March 1996, with compliance mandatory by January 1998. This report reviews data reported to CDC's National Center for Health Statistics (NCHS) regarding spina bifida and anencephaly prevalence for live births in the United States during 1991--2001. Since 1989, NCHS has compiled birth defect data from checkboxes that appear on birth certificates. For consistency in trends, this report uses data for 1991--2001 from all U.S. reporting areas except Maryland, New Mexico, and New York. Data for 2001 are preliminary. During1996--2001, a 23% decline occurred in neural tube defects (spina bifida and anencephaly combined). Spina bifida declined 24% during this period, and anencephaly declined 21%. The United States has experienced declines in spina bifida and anencephaly cases since folic acid fortification of all enriched cereal grain products. The observed declines have translated into approximately 920 infants being born without these serious defects each year. Continued monitoring of the occurrence of spina bifida and anencephaly will be necessary to evaluate the effectiveness of folic acid fortification.IntroductionIn 1992, the U.S. Public Health Service recommended that women of childbearing age increase consumption of the vitamin folic acid to reduce the number of spina bifida and anencephaly cases in the United States (1). By 1998, <30% of women were following this recommendation (2). In 1996, the Food and Drug Administration (FDA) mandated that all enriched cereal grain products be fortified with folic acid (3). An optional period for folic acid cereal grain enrichment started in March 1996, and mandatory fortification began in January 1998. The National Health and Nutrition Examination Survey (NHANES) documented that these public health actions were effective in increasing folate status among U.S. women of childbearing age from NHANES III (1989--1994) to NHANES 1999 (4). In 2001, researchers from CDC determined that the overall birth prevalence of these two neural tube defects declined 19% after mandatory folic acid fortification (5). Since 1989, birth certificates in the United States have included 21 checkboxes for birth defects, including spina bifida and anencephaly, and these data are collected by the National Vital Statistics System, a component of CDC's National Center for Health Statistics (NCHS). This system has been a useful data source to determine the effect of folic acid fortification and other sources of folic acid supplementation (5). MethodsNCHS receives birth certificate data collected by state vital statistics offices for the approximately four million births occurring in the United States annually. Data for Maryland, New Mexico, and New York, which had incomplete reporting or did not require reporting for spina bifida and anencephaly for part of the observation period, were excluded from this analysis. This analysis includes trends during 1991--2001 in frequency and prevalence for spina bifida and anencephaly. Included with the birth prevalence are 95% confidence intervals (CI) to determine statistical significance in changes over time. Data for 1991--2000 are final but are preliminary for 2001. The 2001 preliminary data are based on >96% of all births in 2001. More detailed explanations of final and preliminary birth data have been published in other reports (6,7). ResultsA 23% decline occurred in neural tube defects (spina bifida and anencephaly combined) in 2001, compared with 1996; births in 1996 were conceived before folic acid fortification was authorized. The prevalence of spina bifida reported on birth certificates declined from 24.88 (95% CI = 23.25--26.52) per 100,000 live births in 1991 to 20.09 (95% CI = 18.63--21.54) in 2001 (Table, Figure). The birth prevalences for 1999, 2000, and 2001 were significantly lower than in 1996 (pre-fortification). The 2001 prevalence was 24% lower than the prevalence in 1996; after a significant increase in the prevalence during 1992--1995, a significant decline occurred during 1995--1998. The birth prevalence of spina bifida was stable during 1999--2001. The prevalence of anencephaly reported on birth certificates declined from 18.38 (95% CI = 16.97--19.78) per 100,000 live births in 1991 to 9.40 (95% CI = 8.40--10.39) in 2001. After a decline in the early part of the decade, the anencephaly prevalence was stable during the mid-1990s. The prevalence of anencephaly in 2001 was 21% lower than in 1996. The birth prevalence did not change significantly during 1998--2001, the period of optional and mandatory folic acid fortification. DiscussionSpina bifida and anencephaly are serious birth defects that occur when the neural tube fails to close properly during fetal development. Anencephaly is a lethal defect, and spina bifida results in serious long-term morbidity and disability. Before folic acid fortification, approximately 4,000 pregnancies resulted in 2,500--3,000 births in the United States each year affected by one of these two neural tube defects (1,8) U.S. birth certificate data in this report demonstrate a 24% decline in spina bifida in 2001 births (based on preliminary data) compared with the occurrence of spina bifida in 1996 (before folic acid fortification). A decline of 21% in anencephaly was observed during the same period. This decline is similar to those observed by using the first 5 quarters postfortification, which was previously published (5). Although prevalence for birth defects (including spina bifida and anencephaly) as reported from birth certificate data has been underreported, the collection over time is considered to be stable (5,9). Further support of the findings from birth certificate data has come from population-based surveillance systems. Data from 24 birth defect surveillance systems indicated a 31% decline in spina bifida and a 16% decline in anencephaly when comparing the postfolic acid fortification years (October 1998--December 1999) with the pre-fortification years (January 1995--December 1996) (10). Nine of these birth defect surveillance systems ascertain defects that are prenatally diagnosed and terminated, allowing them to capture additional defects. Larger declines after folic acid fortification were observed for both spina bifida and anencephaly when analysis was limited to these nine surveillance systems with enhanced ascertainment (10). Maryland and New York were excluded in this report to provide consistent trend data, because they only began reporting both spina bifida and anencephaly in 1996. The inclusion of data from these two states does not have a significant effect on the overall prevalence for spina bifida or anencephaly, nor does it change the direction or pattern of change in the trend. New Mexico data are not available because the state does not require reporting of these defects on birth certificates. The declines observed in both birth certificate data and birth defects surveillance system data are less than the decline of >50% that was predicted on the basis of certain observational studies (1). Possible explanations for this difference include 1) the majority of folate-preventable neural tube defects in the United States might have been eliminated before fortification or 2) subpopulations of U.S. women might have not received adequate folic acid from either folic acid fortification or folic acid supplementation. Alternatively, the findings from the observational studies might not be applicable to populationwide interventions (e.g., folic acid fortification), possibly because of biases or uncontrolled confounding that might have been present in the observational studies. The 23% decline in neural tube defects (spina bifida and anencephaly combined) indicated by birth certificate data translates to approximately 920 additional babies without neural tube defects being born in the United States each year. Although this decline represents a lower reduction than those predicted on the basis of earlier studies, by September 2002, neural tube defects will have been prevented in nearly 4,000 U.S. children after folic acid fortification. Attaining and sustaining these substantial declines in neural tube defects is an important public health achievement. References
Table 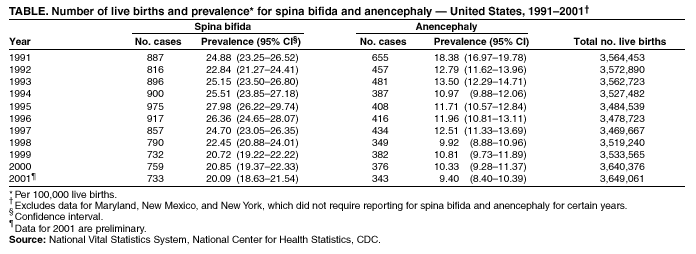 Return to top. Figure 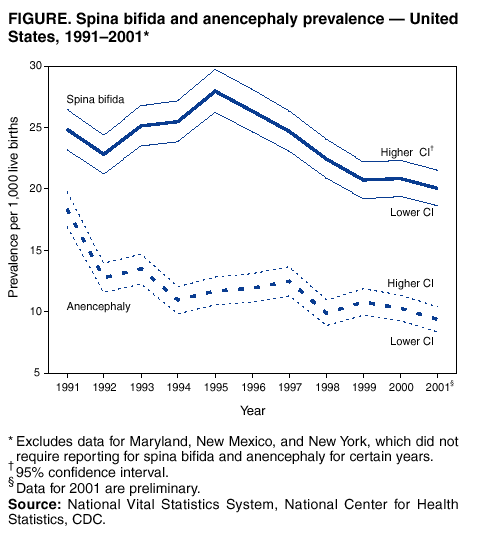 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 8/30/2002 |
|||||||||
This page last reviewed 8/30/2002
|