 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Folic Acid Awareness and Use Among Women with a History of a Neural Tube Defect Pregnancy --- Texas, 2000--2001
Prepared by SummaryThe use of folic acid is a critical component in preventing birth defects. Health-care providers should take advantage of all health-care visits to counsel not only women at high risk (i.e., those with a history of having an infant with a neural tube defect [NTD]) but all women regarding the importance of folic acid use. A study conducted in Texas confirmed that white and Hispanic mothers were equally likely to recall receiving postpartum advice to use folic acid; however, Hispanic women were much less likely to use folic acid, compared with white women. This report covers data from May 2000 through November 2001. A study was conducted in Texas to determine whether women at high risk recall and follow recommendations to use folic acid. The study included 195 women at high risk and 223 control mothers who gave birth to infants without birth defects. These women participated in a telephone interview for a population-based case-control study of NTDs. Approximately 56.4% (110 of 195) of mothers who had infants affected by an NTD recalled receiving postpartum advice to use folic acid, compared with 25.6% (57 of 223) of control mothers (p < 0.01). Among nonpregnant case mothers, 54 (32.7%) of 165 reported regular use of supplements containing folic acid, and 53 (25.2%) of 210 nonpregnant control mothers reported this behavior (p = 0.11). Among case mothers, use of folic acid was significantly higher for whites (64.7%) versus Hispanics (16.5%) (p < 0.001); for women with some college education (57.1%) versus no college education (20.2%; p < 0.001); for women who were trying to get pregnant (66.7%) versus those using birth control (38.3%) or reporting using no contraceptive method (18.8%) (p = 0.001); and for women who reported receiving advice to use folic acid (40.9%) versus those who did not (22.2%; p = 0.01). Findings from this study support the need to implement NTD recurrence prevention activities in Texas. Data also identify a need for educational strategies in Texas that target Hispanic women at high risk, especially those who primarily speak Spanish. Further efforts should be made to determine why Hispanic women have low rates of folic acid use (e.g., the cost of vitamins and language and cultural barriers). On the basis of a review of research and current practice, recommendations developed by the Public Health Service include 1) women at risk for a recurrent NTD-affected pregnancy should take 0.4 mg of folic acid per day; and 2) if a woman at high risk is planning a pregnancy, she should consult her physician regarding taking the higher dose of 4.0 mg per day. BackgroundFor women who previously have had a neural tube defect (NTD)-affected infant, the risk for having another is 1%--3%, or 10--30 times the risk among the general population (1,2). From 1993 through 1999, a CDC-funded, population-based intervention study of these women at high risk was conducted among a primarily Hispanic population residing in Texas counties bordering Mexico (3). The success of this intervention indicated that NTD recurrence prevention activities should possibly be expanded in Texas. To determine the need for statewide NTD recurrence prevention, this statewide study was conducted to assess awareness and supplementation practices among women who recently delivered NTD-affected infants. MethodsThis study is an add-on component to the National Birth Defects Prevention Study, a multistate, case-control study of risk factors for 35 major birth defects sponsored by CDC (4). Eight states, including Texas, participated in the study, using a standardized, 60-minute, computer-assisted telephone interview and informed consent. In Texas, mothers who participated in the national interview and who had children with NTDs (i.e., spina bifida and anencephaly) were invited to participate in an additional 20-minute interview (Texas Interview for Prevention of Central Nervous System Birth Defects [TIP-CNS]). The current study includes 195 mothers of infants with NTDs and 223 control mothers who participated in both of these interviews. All data regarding folic acid awareness and use of folic acid at the time of the interview derived from the TIP-CNS interview. Telephone interviews for the study participants were conducted during May 2000--November 2001. Eligible women were contacted by telephone and invited to participate in the interview, which was administered in English or Spanish. The median time between delivery or pregnancy termination and the interview was 15 months (range: 3.4 months--34.4 months) for case mothers and 14.1 months (range: 1.4 months--32.9 months) for control mothers. Estimated participation rates were 57% (195 of 342) for case mothers and 51% (223 of 437) for control mothers. Cases were ascertained by the population-based Texas Birth Defects Monitoring Program, an active surveillance system for all birth defects diagnosed among elective pregnancy terminations, fetal deaths, live births, and infants (aged <1 year) (5). Cases were identified by an active search of medical records in all hospitals in Texas. Chromosomal abnormalities and syndromes are ascertained by the monitoring program but were not included in this study. Controls were live infants without malformations who were randomly selected from the same Texas hospitals from which the cases were ascertained, in proportion to the live birth proportion contributed by that hospital during the previous year. Women who stated that they took folic acid or multivitamins the majority of days or every day were considered to be regular users of folic acid supplements. Women who responded that they took folic acid occasionally or never were classified as nonusers. ResultsMothers of infants affected by NTDs were much more likely to remember receiving postpartum advice to use folic acid from a physician or health-care worker, (n = 195; 56.4%), compared with control mothers, (n = 223; 25.6%; p < 0.01) (Table 1). Recall of advice regarding folic acid among case mothers did not differ by type of defect, race/ethnicity, education, pregnancy/contraceptive status, or outcome of pregnancy. Demographic patterns (e.g., race/ethnicity and education) for recall among control mothers were similar to those for case mothers. To determine the proportion of women who were complying with the recommendation to use folic acid before they become pregnant, women who were pregnant at the time of the interview were excluded from the analyses (Table 2). Among nonpregnant case mothers, 54 (32.7%) of 165 reported regular use of multivitamins or folic acid at the time of the interview, compared with 53 (25.2%) of 210 control mothers. Among nonpregnant case mothers, regular use of folic acid or multivitamins varied substantially across ethnic groups: 33 (64.7%) of 51 white women, 9 (20.0%) of 45 English-speaking Hispanic women, and 7 (13.5%) of 52 Spanish-speaking Hispanic women (p < 0.001) (Table 2). A similar difference was observed among nonpregnant control women, (p < 0.001) (Table 2). Among nonpregnant case and control mothers, those with some college education were more than twice as likely to use folic acid regularly than those with no college education (p < 0.001 for case and control mothers) (Table 2). In addition, both case and control mothers who reported having received postpartum advice to use folic acid were nearly twice as likely to be taking supplemental folic acid at the time of the interview, compared with mothers who did not recall such advice (p = 0.01 for case mothers; p = 0.02 for control mothers). Nonpregnant case mothers who reported that they were trying to get pregnant were much more likely to report regular use of folic acid: 10 (66.7%) of 15 were regular users, compared with 31 (38.3%) of 81 women using birth control, and 13 (18.8%) of 69 women who did not use birth control (p = 0.001) (Table 2). This last category includes women who were sexually inactive and women who were sexually active but elected not to use birth control. The interview questions did not distinguish between these two categories. In contrast, among control mothers, the corresponding rates were not significantly different; rates were 28.6%, 25.3%, and 25.0%, respectively (p = 0.98). Recall of receiving advice to use folic acid did not vary by time between delivery and interview (categories examined: 1--6 months, 7--12 months, 13--18 months, and 19--35 months). Therefore, time from interview is not likely to be a bias in our results. No appreciable change occurred in these findings after adjustment for maternal age, ethnic group, educational level, contraceptive status, pregnancy outcome, and case-control status. DiscussionOf study subjects who had a previous NTD-affected pregnancy, 43% did not recall receiving advice regarding taking folic acid supplements of any type, and only approximately one third (32.7%) of nonpregnant subjects were using folic acid supplements regularly. Given the 1%--3% level of risk for NTD recurrence among this population, the percentage of women receiving this counseling must be improved. Other studies based on different methods have reported similar findings (6--9). This survey has certain limitations. The participation rates in this study were low, but they were similar to those of other recently conducted case-control studies (10). Women of lower educational status might be underrepresented, because they move more often and are less likely to have a telephone. They also have lower rates of awareness and use of folic acid (11). Therefore, rates of awareness and use of folic acid among all women in Texas with NTD-affected pregnancies might be lower than the rates in this report. In addition, differences across ethnic groups might be larger than the differences in this report. Health-care providers should take advantage of all health-care visits to counsel not only women at high risk but all women regarding the importance of folic acid use. Although white mothers and Hispanic mothers were equally likely to recall receiving postpartum advice to use folic acid, Hispanic women were much less likely to use folic acid, compared with white women. Therefore, further efforts should be made to explore the reasons that Hispanic women have such low rates of folic acid use, including the cost of vitamins and language and cultural barriers. A substantial number of unintended pregnancies occur among women using hormonal contraceptives (12). Therefore, women should be advised to take folic acid every day regardless of whether they use contraceptives. Women who were not trying to become pregnant and were not using contraception were the least likely to use folic acid. Reaching these women is vital, because they have a high risk of becoming pregnant and because, on average, their economic status is lower (12), placing them at a higher risk for having an infant with an NTD (13). The low rates of awareness and use of folic acid among women at high risk and the complex decision-making processes involved in reproductive health support the need for programs to identify women at high risk and to counsel them regarding folic acid use. Such programs use educational approaches specifically tailored for women at high risk and are a key adjunct to counseling by physicians and other health-care providers. These findings are important to consider as Texas develops its statewide NTD recurrence prevention strategy. References
Table 1 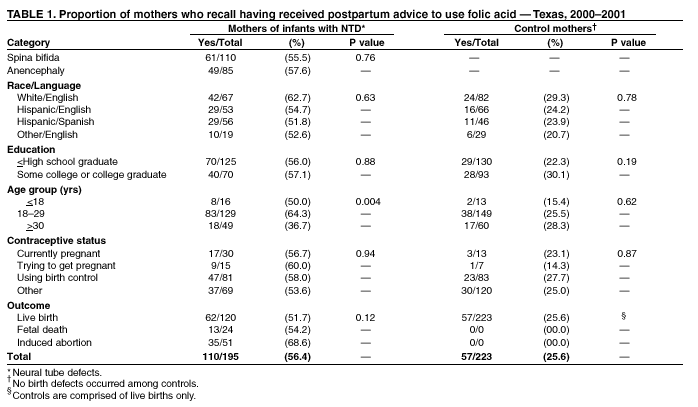 Return to top. Table 2 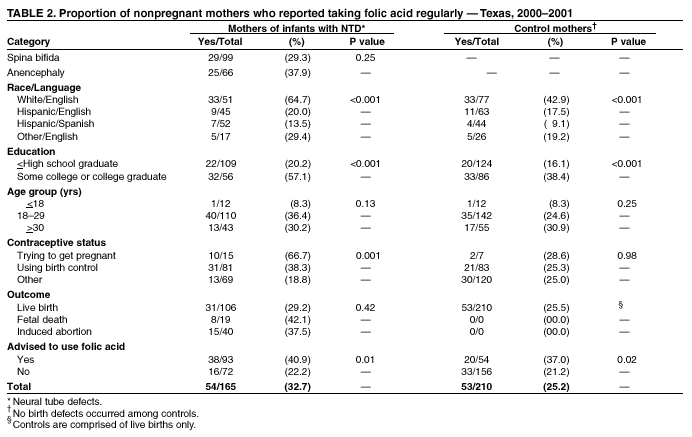 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 8/30/2002 |
|||||||||
This page last reviewed 8/30/2002
|