 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Guideline for Hand Hygiene in Health-Care SettingsRecommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task ForcePrepared by The material in this report originated in the National Center for Infectious Diseases, James M. Hughes, M.D., Director; and the Division of Healthcare Quality Promotion, Steve Solomon, M.D., Acting Director. SummaryThe Guideline for Hand Hygiene in Health-Care Settings provides health-care workers (HCWs) with a review of data regarding handwashing and hand antisepsis in health-care settings. In addition, it provides specific recommendations to promote improved hand-hygiene practices and reduce transmission of pathogenic microorganisms to patients and personnel in health-care settings. This report reviews studies published since the 1985 CDC guideline (Garner JS, Favero MS. CDC guideline for handwashing and hospital environmental control, 1985. Infect Control 1986;7:231--43) and the 1995 APIC guideline (Larson EL, APIC Guidelines Committee. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control 1995;23:251--69) were issued and provides an in-depth review of hand-hygiene practices of HCWs, levels of adherence of personnel to recommended handwashing practices, and factors adversely affecting adherence. New studies of the in vivo efficacy of alcohol-based hand rubs and the low incidence of dermatitis associated with their use are reviewed. Recent studies demonstrating the value of multidisciplinary hand-hygiene promotion programs and the potential role of alcohol-based hand rubs in improving hand-hygiene practices are summarized. Recommendations concerning related issues (e.g., the use of surgical hand antiseptics, hand lotions or creams, and wearing of artificial fingernails) are also included. Part I. Review of the Scientific Data Regarding Hand Hygiene
For generations, handwashing with soap and water has been considered a measure of personal hygiene (1). The concept of cleansing hands with an antiseptic agent probably emerged in the early 19th century. As early as 1822, a French pharmacist demonstrated that solutions containing chlorides of lime or soda could eradicate the foul odors associated with human corpses and that such solutions could be used as disinfectants and antiseptics (2). In a paper published in 1825, this pharmacist stated that physicians and other persons attending patients with contagious diseases would benefit from moistening their hands with a liquid chloride solution (2). In 1846, Ignaz Semmelweis observed that women whose babies were delivered by students and physicians in the First Clinic at the General Hospital of Vienna consistently had a higher mortality rate than those whose babies were delivered by midwives in the Second Clinic (3). He noted that physicians who went directly from the autopsy suite to the obstetrics ward had a disagreeable odor on their hands despite washing their hands with soap and water upon entering the obstetrics clinic. He postulated that the puerperal fever that affected so many parturient women was caused by "cadaverous particles" transmitted from the autopsy suite to the obstetrics ward via the hands of students and physicians. Perhaps because of the known deodorizing effect of chlorine compounds, as of May 1847, he insisted that students and physicians clean their hands with a chlorine solution between each patient in the clinic. The maternal mortality rate in the First Clinic subsequently dropped dramatically and remained low for years. This intervention by Semmelweis represents the first evidence indicating that cleansing heavily contaminated hands with an antiseptic agent between patient contacts may reduce health-care--associated transmission of contagious diseases more effectively than handwashing with plain soap and water. In 1843, Oliver Wendell Holmes concluded independently that puerperal fever was spread by the hands of health personnel (1). Although he described measures that could be taken to limit its spread, his recommendations had little impact on obstetric practices at the time. However, as a result of the seminal studies by Semmelweis and Holmes, handwashing gradually became accepted as one of the most important measures for preventing transmission of pathogens in health-care facilities. In 1961, the U. S. Public Health Service produced a training film that demonstrated handwashing techniques recommended for use by health-care workers (HCWs) (4). At the time, recommendations directed that personnel wash their hands with soap and water for 1--2 minutes before and after patient contact. Rinsing hands with an antiseptic agent was believed to be less effective than handwashing and was recommended only in emergencies or in areas where sinks were unavailable. In 1975 and 1985, formal written guidelines on handwashing practices in hospitals were published by CDC (5,6). These guidelines recommended handwashing with non-antimicrobial soap between the majority of patient contacts and washing with antimicrobial soap before and after performing invasive procedures or caring for patients at high risk. Use of waterless antiseptic agents (e.g., alcohol-based solutions) was recommended only in situations where sinks were not available. In 1988 and 1995, guidelines for handwashing and hand antisepsis were published by the Association for Professionals in Infection Control (APIC) (7,8). Recommended indications for handwashing were similar to those listed in the CDC guidelines. The 1995 APIC guideline included more detailed discussion of alcohol-based hand rubs and supported their use in more clinical settings than had been recommended in earlier guidelines. In 1995 and 1996, the Healthcare Infection Control Practices Advisory Committee (HICPAC) recommended that either antimicrobial soap or a waterless antiseptic agent be used for cleaning hands upon leaving the rooms of patients with multidrug-resistant pathogens (e.g., vancomycin-resistant enterococci [VRE] and methicillin-resistant Staphylococcus aureus [MRSA]) (9,10). These guidelines also provided recommendations for handwashing and hand antisepsis in other clinical settings, including routine patient care. Although the APIC and HICPAC guidelines have been adopted by the majority of hospitals, adherence of HCWs to recommended handwashing practices has remained low (11,12). Recent developments in the field have stimulated a review of the scientific data regarding hand hygiene and the
development of new guidelines designed to improve hand-hygiene practices in health-care facilities. This literature review and accompanying recommendations have been prepared by a Hand Hygiene Task Force, comprising representatives from HICPAC, the
Society for Healthcare Epidemiology of America (SHEA), APIC, and the Infectious Diseases Society of America (IDSA).
To understand the objectives of different approaches to hand cleansing, a knowledge of normal bacterial skin flora
is essential. Normal human skin is colonized with bacteria; different areas of the body have varied total aerobic bacterial counts (e.g., 1 x 106 colony forming units
(CFUs)/cm2 on the scalp, 5 x
105 CFUs/cm2 in the axilla, 4 x 104 CFUs/cm2 on the abdomen, and 1 x 104 CFUs/cm2 on the forearm)
(13). Total bacterial counts on the hands of medical personnel have
ranged from 3.9 x 104 to 4.6 x
106 (14--17). In 1938, bacteria recovered from the hands were divided into two categories:
transient and resident (14). Transient flora, which colonize the superficial layers of the skin, are more amenable to removal by routine handwashing. They are often acquired by HCWs during direct contact with patients or contact with
contaminated environmental surfaces within close proximity of the patient. Transient flora are the organisms most frequently associated with health-care--associated infections. Resident flora, which are attached to deeper layers of the skin, are more resistant to removal. In addition, resident flora (e.g., coagulase-negative staphylococci and diphtheroids) are less likely to be associated with such infections. The hands of HCWs may become persistently colonized with pathogenic flora (e.g.,
S. aureus), gram-negative bacilli, or yeast. Investigators have documented that, although the number of transient and resident flora varies considerably from person to person, it is often relatively constant for any specific person
(14,18).
The primary function of the skin is to reduce water loss, provide protection against abrasive action and microorganisms, and act as a permeability barrier to the environment. The basic structure of skin includes, from outer- to inner-most layer, the superficial region (i.e., the stratum corneum or horny layer, which is 10- to 20-µm thick), the viable epidermis (50- to 100-µm thick), the dermis (1- to 2-mm thick), and the hypodermis (1- to 2-mm thick). The barrier to percutaneous absorption lies within the stratum corneum, the thinnest and smallest compartment of the skin. The stratum corneum contains the corneocytes (or horny cells), which are flat, polyhedral-shaped nonnucleated cells, remnants of the terminally differentiated keratinocytes located in the viable epidermis. Corneocytes are composed primarily of insoluble bundled keratins surrounded by a cell envelope stabilized by cross-linked proteins and covalently bound lipid. Interconnecting the corneocytes of the stratum corneum are polar structures (e.g., corneodesmosomes), which contribute to stratum corneum cohesion. The intercellular region of the stratum corneum is composed of lipid primarily generated from the exocytosis of lamellar bodies during the terminal differentiation of the keratinocytes. The intercellular lipid is required for a competent skin barrier and forms the only continuous domain. Directly under the stratum corneum is a stratified epidermis, which is composed primarily of 10--20 layers of keratinizing epithelial cells that are responsible for the synthesis of the stratum corneum. This layer also contains melanocytes involved in skin pigmentation; Langerhans cells, which are important for antigen presentation and immune responses; and Merkel cells, whose precise role in sensory reception has yet to be fully delineated. As keratinocytes undergo terminal differentiation, they begin to flatten out and assume the dimensions characteristic of the corneocytes (i.e., their diameter changes from 10--12 µm to 20--30 µm, and their volume increases by 10- to 20-fold). The viable epidermis does not contain a vascular network, and the keratinocytes obtain their nutrients from below by passive diffusion through the interstitial fluid. The skin is a dynamic structure. Barrier function does not simply arise from the dying, degeneration, and compaction of the underlying epidermis. Rather, the processes of cornification and desquamation are intimately linked; synthesis of the stratum corneum occurs at the same rate as loss. Substantial evidence now confirms that the formation of the skin barrier is under homeostatic control, which is illustrated by the epidermal response to barrier perturbation by skin stripping or solvent extraction. Circumstantial evidence indicates that the rate of keratinocyte proliferation directly influences the integrity of the skin barrier. A general increase in the rate of proliferation results in a decrease in the time available for 1) uptake of nutrients (e.g., essential fatty acids), 2) protein and lipid synthesis, and 3) processing of the precursor molecules required for skin-barrier function. Whether chronic but quantitatively smaller increases in rate of epidermal proliferation also lead to changes in skin-barrier function remains unclear. Thus, the extent to which the decreased barrier function caused by irritants is caused by an increased epidermal proliferation also is unknown. The current understanding of the formation of the stratum corneum has come from studies of the epidermal responses
to perturbation of the skin barrier. Experimental manipulations that disrupt the skin barrier include 1) extraction of skin lipids with apolar solvents, 2) physical stripping of the stratum corneum using adhesive tape, and 3) chemically induced irritation. All of these experimental manipulations lead to a decreased skin barrier as determined by transepidermal water loss (TEWL). The most studied experimental system is the treatment of mouse skin with acetone. This experiment results in a marked and immediate increase in TEWL, and therefore a decrease in skin-barrier function. Acetone treatment selectively
removes glycerolipids and sterols from the skin, which indicates that these lipids are necessary, though perhaps not sufficient in themselves, for barrier function. Detergents act like acetone on the intercellular lipid domain. The return to normal
barrier function is biphasic: 50%--60% of barrier recovery typically occurs within 6 hours, but complete normalization of
barrier function requires 5--6 days.
Alcohol-based hand rub. An alcohol-containing preparation designed for application to the hands for reducing the number of viable microorganisms on the hands. In the United States, such preparations usually contain 60%--95% ethanol
or isopropanol.
Evidence of Transmission of Pathogens on HandsTransmission of health-care--associated pathogens from one patient to another via the hands of HCWs requires the following sequence of events:
Health-care--associated pathogens can be recovered not only from infected or draining wounds, but also from frequently colonized areas of normal, intact patient skin (20-- 31). The perineal or inguinal areas are usually most heavily colonized, but the axillae, trunk, and upper extremities (including the hands) also are frequently colonized (23,25,26,28,30--32). The number of organisms (e.g., S. aureus, Proteus mirabilis, Klebsiella spp., and Acinetobacter spp.) present on intact areas of the skin of certain patients can vary from 100 to 106/cm2 (25,29,31,33). Persons with diabetes, patients undergoing dialysis for chronic renal failure, and those with chronic dermatitis are likely to have areas of intact skin that are colonized with S. aureus (34--41). Because approximately 106 skin squames containing viable microorganisms are shed daily from normal skin (42), patient gowns, bed linen, bedside furniture, and other objects in the patient's immediate environment can easily become contaminated with patient flora (30,43--46). Such contamination is particularly likely to be caused by staphylococci or enterococci, which are resistant to dessication. Data are limited regarding the types of patient-care activities that result in transmission of patient flora to the hands of personnel (26,45--51). In the past, attempts have been made to stratify patient-care activities into those most likely to cause hand contamination (52), but such stratification schemes were never validated by quantifying the level of bacterial contamination that occurred. Nurses can contaminate their hands with 100--1,000 CFUs of Klebsiella spp. during "clean" activities (e.g., lifting a patient; taking a patient's pulse, blood pressure, or oral temperature; or touching a patient's hand, shoulder, or groin) (48). Similarly, in another study, hands were cultured of nurses who touched the groins of patients heavily colonized with P. mirabilis (25); 10--600 CFUs/mL of this organism were recovered from glove juice samples from the nurses' hands. Recently, other researchers studied contamination of HCWs' hands during activities that involved direct patient-contact wound care, intravascular catheter care, respiratory-tract care, and the handling of patient secretions (51). Agar fingertip impression plates were used to culture bacteria; the number of bacteria recovered from fingertips ranged from 0 to 300 CFUs. Data from this study indicated that direct patient contact and respiratory-tract care were most likely to contaminate the fingers of caregivers. Gram-negative bacilli accounted for 15% of isolates and S. aureus for 11%. Duration of patient-care activity was strongly associated with the intensity of bacterial contamination of HCWs' hands. HCWs can contaminate their hands with gram-negative bacilli, S. aureus, enterococci, or Clostridium difficile by performing "clean procedures" or touching intact areas of the skin of hospitalized patients (26,45,46,53). Furthermore, personnel caring for infants with respiratory syncytial virus (RSV) infections have acquired RSV by performing certain activities (e.g., feeding infants, changing diapers, and playing with infants) (49). Personnel who had contact only with surfaces contaminated with the infants' secretions also acquired RSV by contaminating their hands with RSV and inoculating their oral or conjunctival mucosa. Other studies also have documented that HCWs may contaminate their hands (or gloves) merely by touching inanimate objects in patient rooms (46,53--56). None of the studies concerning hand contamination of hospital personnel were designed to determine if the contamination resulted in transmission of pathogens to susceptible patients. Other studies have documented contamination of HCWs' hands with potential health-care--associated pathogens, but
did not relate their findings to the specific type of preceding patient contact (15,17,57--62). For example, before glove use
was common among HCWs, 15% of nurses working in an isolation unit carried a median of 1 x 104 CFUs of S. aureus on their hands
(61). Of nurses working in a general hospital, 29% had S. aureus on their hands (median count: 3,800 CFUs),
whereas 78% of those working in a hospital for dermatology patients had the organism on their hands (median count: 14.3 x 106 CFUs). Similarly, 17%--30% of nurses carried gram-negative bacilli on their hands (median counts: 3,400--38,000 CFUs). One study found that S. aureus could be recovered from the hands of 21% of intensive-care--unit personnel and that 21% of physician and 5% of nurse carriers had >1,000 CFUs of the organism on their hands
(59). Another study found lower levels of colonization on the hands of personnel working in a neurosurgery unit, with an average of 3 CFUs of S. aureus and 11 CFUs of gram-negative bacilli
(16). Serial cultures revealed that 100% of HCWs carried gram-negative bacilli at least once, and
64% carried S. aureus at least once.
Several investigators have studied transmission of infectious agents by using different experimental models. In one study, nurses were asked to touch the groins of patients heavily colonized with gram-negative bacilli for 15 seconds --- as though they were taking a femoral pulse (25). Nurses then cleaned their hands by washing with plain soap and water or by using an alcohol hand rinse. After cleaning their hands, they touched a piece of urinary catheter material with their fingers, and the catheter segment was cultured. The study revealed that touching intact areas of moist skin of the patient transferred enough organisms to the nurses' hands to result in subsequent transmission to catheter material, despite handwashing with plain soap and water. The transmission of organisms from artificially contaminated "donor" fabrics to clean "recipient" fabrics via hand contact also has been studied. Results indicated that the number of organisms transmitted was greater if the donor fabric or the
hands were wet upon contact (63). Overall, only 0.06% of the organisms obtained from the contaminated donor fabric were transferred to recipient fabric via hand contact.
Staphylococcus saprophyticus, Pseudomonas aeruginosa,
and Serratia spp. were also transferred in greater numbers than was Escherichia coli from contaminated fabric to clean fabric after hand contact
(64). Organisms are transferred to various types of surfaces in much larger numbers (i.e., >104) from wet hands than from hands that are thoroughly dried
(65).
Hand antisepsis reduces the incidence of health-care--associated infections (66,67). An intervention trial using historical controls demonstrated in 1847 that the mortality rate among mothers who delivered in the First Obstetrics Clinic at the General Hospital of Vienna was substantially lower when hospital staff cleaned their hands with an antiseptic agent than when they washed their hands with plain soap and water (3). In the 1960s, a prospective, controlled trial sponsored by the National Institutes of Health and the Office of the Surgeon General demonstrated that infants cared for by nurses who did not wash their hands after handling an index infant colonized with S. aureus acquired the organism more often and more rapidly than did infants cared for by nurses who used hexachlorophene to clean their hands between infant contacts (68). This trial provided evidence that, when compared with no handwashing, washing hands with an antiseptic agent between patient contacts reduces transmission of health-care--associated pathogens. Trials have studied the effects of handwashing with plain soap and water versus some form of hand antisepsis on health-care--associated infection rates (69,70). Health-care--associated infection rates were lower when antiseptic handwashing was performed by personnel (69). In another study, antiseptic handwashing was associated with lower health-care--associated infection rates in certain intensive-care units, but not in others (70). Health-care--associated infection rates were lower after antiseptic handwashing using a chlorhexidine-containing detergent compared with handwashing with plain soap or use of an alcohol-based hand rinse (71). However, because only a minimal amount of the alcohol rinse was used during periods when the combination regimen also was in use and because adherence to policies was higher when chlorhexidine was available, determining which factor (i.e., the hand-hygiene regimen or differences in adherence) accounted for the lower infection rates was difficult. Investigators have determined also that health-care--associated acquisition of MRSA was reduced when the antimicrobial soap used for hygienic handwashing was changed (72,73). Increased handwashing frequency among hospital staff has been associated with decreased transmission of Klebsiella spp. among patients (48); these studies, however, did not quantitate the level of handwashing among personnel. In a recent study, the acquisition of various health-care--associated pathogens was reduced when hand antisepsis was performed more frequently by hospital personnel (74); both this study and another (75) documented that the prevalence of health-care--associated infections decreased as adherence to recommended hand-hygiene measures improved. Outbreak investigations have indicated an association between infections and understaffing or overcrowding; the association was consistently linked with poor adherence to hand hygiene. During an outbreak investigation of risk factors for central venous catheter-associated bloodstream infections
(76), after adjustment for confounding factors, the patient-to-nurse
ratio remained an independent risk factor for bloodstream infection, indicating that nursing staff reduction below a
critical threshold may have contributed to this outbreak by jeopardizing adequate catheter care. The understaffing of nurses can facilitate the spread of MRSA in intensive-care settings
(77) through relaxed attention to basic control measures (e.g., hand hygiene). In an outbreak of Enterobacter
cloacae in a neonatal intensive-care unit
(78), the daily number of hospitalized children was above the maximum capacity of the unit, resulting in an available space per child below current recommendations. In parallel, the number of staff members on duty was substantially less than the number necessitated by
the workload, which also resulted in relaxed attention to basic infection-control measures. Adherence to hand-hygiene
practices before device contact was only 25% during the workload peak, but increased to 70% after the end of the understaffing and overcrowding period. Surveillance documented that being hospitalized during this period was associated with a
fourfold
increased risk of acquiring a health-care--associated infection. This study not only demonstrates the association
between workload and infections, but it also highlights the intermediate cause of antimicrobial spread: poor adherence to hand-hygiene policies.
Current Methods Investigators use different methods to study the in vivo efficacy of handwashing, antiseptic handwash, and surgical hand antisepsis protocols. Differences among the various studies include 1) whether hands are purposely contaminated with bacteria before use of test agents, 2) the method used to contaminate fingers or hands, 3) the volume of hand-hygiene product applied to the hands, 4) the time the product is in contact with the skin, 5) the method used to recover bacteria from the skin after the test solution has been used, and 6) the method of expressing the efficacy of the product (i.e., either percent reduction in bacteria recovered from the skin or log reduction of bacteria released from the skin). Despite these differences, the majority of studies can be placed into one of two major categories: studies focusing on products to remove transient flora and studies involving products that are used to remove resident flora from the hands. The majority of studies of products for removing transient flora from the hands of HCWs involve artificial contamination of the volunteer's skin with a defined inoculum of a test organism before the volunteer uses a plain soap, an antimicrobial soap, or a waterless antiseptic agent. In contrast, products tested for the preoperative cleansing of surgeons' hands (which must comply with surgical hand-antisepsis protocols) are tested for their ability to remove resident flora from without artificially contaminating the volunteers' hands. In the United States, antiseptic handwash products intended for use by HCWs are regulated by FDA's Division of Over-the-Counter Drug Products (OTC). Requirements for in vitro and in vivo testing of HCW handwash products and surgical hand scrubs are outlined in the FDA Tentative Final Monograph for Healthcare Antiseptic Drug Products (TFM) (19). Products intended for use as HCW handwashes are evaluated by using a standardized method (19). Tests are performed in accordance with use directions for the test material. Before baseline bacterial sampling and before each wash with the test material, 5 mL of a standardized suspension of Serratia marcescens are applied to the hands and then rubbed over the surfaces of the hands. A specified volume of the test material is dispensed into the hands and is spread over the hands and lower one third of the forearms. A small amount of tap water is added to the hands, and hands are completely lathered for a specified time, covering all surfaces of the hands and the lower third of the forearms. Volunteers then rinse hands and forearms under 40ºC tap water for 30 seconds. Ten washes with the test formulation are required. After the first, third, seventh, and tenth washes, rubber gloves or polyethylene bags used for sampling are placed on the right and left hands, and 75 mL of sampling solution is added to each glove; gloves are secured above the wrist. All surfaces of the hand are massaged for 1 minute, and samples are obtained aseptically for quantitative culture. No neutralizer of the antimicrobial is routinely added to the sampling solution, but if dilution of the antimicrobial in the sampling fluid does not result in demonstrable neutralization, a neutralizer specific for the test formulation is added to the sampling solution. For waterless formulations, a similar procedure is used. TFM criteria for efficacy are as follows: a 2-log10 reduction of the indicator organism on each hand within 5 minutes after the first use, and a 3-log10 reduction of the indicator organism on each hand within 5 minutes after the tenth use (19). Products intended for use as surgical hand scrubs have been evaluated also by using a standardized method (19). Volunteers clean under fingernails with a nail stick and clip their fingernails. All jewelry is removed from hands and arms. Hands and two thirds of forearms are rinsed with tap water (38ºC--42ºC) for 30 seconds, and then they are washed with a non-antimicrobial soap for 30 seconds and are rinsed for 30 seconds under tap water. Baseline microbial hand counts can then be determined. Next, a surgical scrub is performed with the test formulation using directions provided by the manufacturer. If no instructions are provided with the formulation, two 5-minute scrubs of hands and forearms followed by rinsing are performed. Reduction from baseline microbial hand counts is determined in a series of 11 scrubs conducted during 5 days. Hands are sampled at 1 minute, 3 hours, and 6 hours after the first scrubs on day 1, day 2, and day 5. After washing, volunteers wear rubber gloves; 75 mL of sampling solution are then added to one glove, and all surfaces of the hands are massaged for 1 minute. Samples are then taken aseptically and cultured quantitatively. The other glove remains on the other hand for 6 hours and is sampled in the same manner. TFM requires that formulations reduce the number of bacteria 1 log10 on each hand within 1 minute of product application and that the bacterial cell count on each hand does not subsequently exceed baseline within 6 hours on day 1; the formulation must produce a 2-log10 reduction in microbial flora on each hand within 1 minute of product application by the end of the second day of enumeration and a 3-log10 reduction of microbial flora on each hand within 1 minute of product use by the end of the fifth day when compared with the established baseline (19). The method most widely used in Europe to evaluate the efficacy of hand-hygiene agents is European Standard 1500--1997 (EN 1500---Chemical disinfectants and antiseptics. Hygienic hand-rub test method and requirements) (79). This method requires 12--15 test volunteers and an 18- to 24-hour growth of broth culture of E. coli K12. Hands are washed with a soft soap, dried, and then immersed halfway to the metacarpals in the broth culture for 5 seconds. Hands are removed from the broth culture, excess fluid is drained off, and hands are dried in the air for 3 minutes. Bacterial recovery for the initial value is obtained by kneading the fingertips of each hand separately for 60 seconds in 10 mL of tryptic soy broth (TSB) without neutralizers. The hands are removed from the broth and disinfected with 3 mL of the hand-rub agent for 30 seconds in a set design. The same operation is repeated with total disinfection time not exceeding 60 seconds. Both hands are rinsed in running water for 5 seconds and water is drained off. Fingertips of each hand are kneaded separately in 10 mL of TSB with added neutralizers. These broths are used to obtain the final value. Log10 dilutions of recovery medium are prepared and plated out. Within 3 hours, the same volunteers are tested with the reference disinfectant (60% 2-propanol [isopropanol]) and the test product. Colony counts are performed after 24 and 48 hours of incubation at 36ºC. The average colony count of both left and right hand is used for evaluation. The log-reduction factor is calculated and compared with the initial and final values. The reduction factor of the test product should be superior or the same as the reference alcohol-based rub for acceptance. If a difference exists, then the results are analyzed statistically using the Wilcoxon test. Products that have log reductions substantially less than that observed with the reference alcohol-based hand rub (i.e., approximately 4 log10 reduction) are classified as not meeting the standard. Because of different standards for efficacy, criteria cited in FDA TFM and the European EN 1500 document for establishing alcohol-based hand rubs vary (1,19,79). Alcohol-based hand rubs that meet TFM criteria for efficacy may not necessarily meet the EN 1500 criteria for efficacy (80). In addition, scientific studies have not established the extent to which counts of bacteria or other microorganisms on the hands need to be reduced to minimize transmission of pathogens in health-care facilities (1,8); whether bacterial counts on the hands must be reduced by 1 log10 (90% reduction), 2 log10 (99%), 3 log10 (99.9%), or 4 log10 (99.99%) is unknown. Several other methods also have been used to measure the efficacy of antiseptic agents against various viral pathogens (81--83). Shortcomings of Traditional Methodologies Accepted methods of evaluating hand-hygiene products intended for use by HCWs require that test volunteers wash
their hands with a plain or antimicrobial soap for 30 seconds or 1 minute, despite the observation in the majority of studies that
the average duration of handwashing by hospital personnel is <15 seconds
(52,84--89). A limited number of investigators
have used 15-second handwashing or hygienic hand-wash protocols
(90--94). Therefore, almost no data exist regarding the
efficacy of plain or antimicrobial soaps under conditions in which they are actually used by HCWs. Similarly, certain
accepted methods for evaluating waterless antiseptic agents for use as antiseptic hand rubs require that 3 mL of alcohol be rubbed into the hands for 30 seconds, followed by a repeat application for the same duration. This type of protocol also does not
reflect actual usage patterns among HCWs. Furthermore, volunteers used in evaluations of products are usually surrogates for HCWs, and their hand flora may not reflect flora found on the hands of personnel working in health-care settings. Further studies should be conducted among practicing HCWs using standardized protocols to obtain more realistic views of
microbial colonization and risk of bacterial transfer and cross-transmission
(51).
Plain (Non-Antimicrobial) Soap Soaps are detergent-based products that contain esterified fatty acids and sodium or potassium hydroxide. They are available in various forms including bar soap, tissue, leaflet, and liquid preparations. Their cleaning activity can be attributed to their detergent properties, which result in removal of dirt, soil, and various organic substances from the hands. Plain soaps have minimal, if any, antimicrobial activity. However, handwashing with plain soap can remove loosely adherent transient flora. For example, handwashing with plain soap and water for 15 seconds reduces bacterial counts on the skin by 0.6--1.1 log10, whereas washing for 30 seconds reduces counts by 1.8--2.8 log10 (1). However, in several studies, handwashing with plain soap failed to remove pathogens from the hands of hospital personnel (25,45). Handwashing with plain soap can result in paradoxical increases in bacterial counts on the skin (92,95--97). Non-antimicrobial soaps may be associated with considerable skin irritation and dryness (92,96,98), although adding emollients to soap preparations may reduce their propensity to cause irritation. Occasionally, plain soaps have become contaminated, which may lead to colonization of hands of personnel with gram-negative bacilli (99). Alcohols The majority of alcohol-based hand antiseptics contain either isopropanol, ethanol, n-propanol, or a combination of two of these products. Although n-propanol has been used in alcohol-based hand rubs in parts of Europe for many years, it is not listed in TFM as an approved active agent for HCW handwashes or surgical hand-scrub preparations in the United States. The majority of studies of alcohols have evaluated individual alcohols in varying concentrations. Other studies have focused on combinations of two alcohols or alcohol solutions containing limited amounts of hexachlorophene, quaternary ammonium compounds, povidone-iodine, triclosan, or chlorhexidine gluconate (61,93,100--119). The antimicrobial activity of alcohols can be attributed to their ability to denature proteins (120). Alcohol solutions containing 60%--95% alcohol are most effective, and higher concentrations are less potent (120--122) because proteins are not denatured easily in the absence of water (120). The alcohol content of solutions may be expressed as percent by weight (w/w), which is not affected by temperature or other variables, or as percent by volume (vol/vol), which can be affected by temperature, specific gravity, and reaction concentration (123). For example, 70% alcohol by weight is equivalent to 76.8% by volume if prepared at 15ºC, or 80.5% if prepared at 25ºC (123). Alcohol concentrations in antiseptic hand rubs are often expressed as percent by volume (19). Alcohols have excellent in vitro germicidal activity against gram-positive and gram-negative vegetative bacteria, including multidrug-resistant pathogens (e.g., MRSA and VRE), Mycobacterium tuberculosis, and various fungi (120--122,124--129). Certain enveloped (lipophilic) viruses (e.g., herpes simplex virus, human immunodeficiency virus [HIV], influenza virus, respiratory syncytial virus, and vaccinia virus) are susceptible to alcohols when tested in vitro (120,130,131) (Table 1). Hepatitis B virus is an enveloped virus that is somewhat less susceptible but is killed by 60%--70% alcohol; hepatitis C virus also is likely killed by this percentage of alcohol (132). In a porcine tissue carrier model used to study antiseptic activity, 70% ethanol and 70% isopropanol were found to reduce titers of an enveloped bacteriophage more effectively than an antimicrobial soap containing 4% chlorhexidine gluconate (133). Despite its effectiveness against these organisms, alcohols have very poor activity against bacterial spores, protozoan oocysts, and certain nonenveloped (nonlipophilic) viruses. Numerous studies have documented the in vivo antimicrobial activity of alcohols. Alcohols effectively reduce bacterial counts on the hands (14,121,125,134). Typically, log reductions of the release of test bacteria from artificially contaminated hands average 3.5 log10 after a 30-second application and 4.0--5.0 log10 after a 1-minute application (1). In 1994, the FDA TFM classified ethanol 60%--95% as a Category I agent (i.e., generally safe and effective for use in antiseptic handwash or HCW hand-wash products) (19). Although TFM placed isopropanol 70%--91.3% in category IIIE (i.e., insufficient data to classify as effective), 60% isopropanol has subsequently been adopted in Europe as the reference standard against which alcohol-based hand-rub products are compared (79). Alcohols are rapidly germicidal when applied to the skin, but they have no appreciable persistent (i.e., residual) activity. However, regrowth of bacteria on the skin occurs slowly after use of alcohol-based hand antiseptics, presumably because of the sublethal effect alcohols have on some of the skin bacteria (135,136). Addition of chlorhexidine, quaternary ammonium compounds, octenidine, or triclosan to alcohol-based solutions can result in persistent activity (1). Alcohols, when used in concentrations present in alcohol-based hand rubs, also have in vivo activity against several nonenveloped viruses (Table 2). For example, 70% isopropanol and 70% ethanol are more effective than medicated soap or nonmedicated soap in reducing rotavirus titers on fingerpads (137,138). A more recent study using the same test methods evaluated a commercially available product containing 60% ethanol and found that the product reduced the infectivity titers of three nonenveloped viruses (i.e., rotavirus, adenovirus, and rhinovirus) by >3 logs (81). Other nonenveloped viruses such as hepatitis A and enteroviruses (e.g., poliovirus) may require 70%--80% alcohol to be reliably inactivated (82,139). However, both 70% ethanol and a 62% ethanol foam product with emollients reduced hepatitis A virus titers on whole hands or fingertips more than nonmedicated soap; both were equally as effective as antimicrobial soap containing 4% chlorhexidine gluconate in reducing reduced viral counts on hands (140). In the same study, both 70% ethanol and the 62% ethanol foam product demonstrated greater virucidal activity against poliovirus than either non-antimicrobial soap or a 4% chlorhexidine gluconate-containing soap (140). However, depending on the alcohol concentration, the amount of time that hands are exposed to the alcohol, and viral variant, alcohol may not be effective against hepatitis A and other nonlipophilic viruses. The inactivation of nonenveloped viruses is influenced by temperature, disinfectant-virus volume ratio, and protein load (141). Ethanol has greater activity against viruses than isopropanol. Further in vitro and in vivo studies of both alcohol-based formulations and antimicrobial soaps are warranted to establish the minimal level of virucidal activity that is required to interrupt direct contact transmission of viruses in health-care settings. Alcohols are not appropriate for use when hands are visibly dirty or contaminated with proteinaceous materials. However, when relatively small amounts of proteinaceous material (e.g., blood) are present, ethanol and isopropanol may reduce viable bacterial counts on hands more than plain soap or antimicrobial soap (142). Alcohol can prevent the transfer of health-care--associated pathogens (25,63,64). In one study, gram-negative bacilli were transferred from a colonized patient's skin to a piece of catheter material via the hands of nurses in only 17% of experiments after antiseptic hand rub with an alcohol-based hand rinse (25). In contrast, transfer of the organisms occurred in 92% of experiments after handwashing with plain soap and water. This experimental model indicates that when the hands of HCWs are heavily contaminated, an antiseptic hand rub using an alcohol-based rinse can prevent pathogen transmission more effectively than can handwashing with plain soap and water. Alcohol-based products are more effective for standard handwashing or hand antisepsis by HCWs than soap or antimicrobial soaps (Table 3) (25,53,61,93,106--112,119,143--152). In all but two of the trials that compared alcohol-based solutions with antimicrobial soaps or detergents, alcohol reduced bacterial counts on hands more than washing hands with soaps or detergents containing hexachlorophene, povidone-iodine, 4% chlorhexidine, or triclosan. In studies examining antimicrobial-resistant organisms, alcohol-based products reduced the number of multidrug-resistant pathogens recovered from the hands of HCWs more effectively than did handwashing with soap and water (153--155). Alcohols are effective for preoperative cleaning of the hands of surgical personnel (1,101,104,113--119,135,143,147,156--159) (Tables 4 and 5). In multiple studies, bacterial counts on the hands were determined immediately after using the product and again 1--3 hours later; the delayed testing was performed to determine if regrowth of bacteria on the hands is inhibited during operative procedures. Alcohol-based solutions were more effective than washing hands with plain soap in all studies, and they reduced bacterial counts on the hands more than antimicrobial soaps or detergents in the majority of experiments (101,104,113--119,135,143,147,157--159 ). In addition, the majority of alcohol-based preparations were more effective than povidone-iodine or chlorhexidine. The efficacy of alcohol-based hand-hygiene products is affected by several factors, including the type of alcohol used, concentration of alcohol, contact time, volume of alcohol used, and whether the hands are wet when the alcohol is applied. Applying small volumes (i.e., 0.2--0.5 mL) of alcohol to the hands is not more effective than washing hands with plain soap and water (63,64). One study documented that 1 mL of alcohol was substantially less effective than 3 mL (91). The ideal volume of product to apply to the hands is not known and may vary for different formulations. However, if hands feel dry after rubbing hands together for 10--15 seconds, an insufficient volume of product likely was applied. Because alcohol-impregnated towelettes contain a limited amount of alcohol, their effectiveness is comparable to that of soap and water (63,160,161). Alcohol-based hand rubs intended for use in hospitals are available as low viscosity rinses, gels, and foams. Limited data are available regarding the relative efficacy of various formulations. One field trial demonstrated that an ethanol gel was slightly more effective than a comparable ethanol solution at reducing bacterial counts on the hands of HCWs (162). However, a more recent study indicated that rinses reduced bacterial counts on the hands more than the gels tested (80). Further studies are warranted to determine the relative efficacy of alcohol-based rinses and gels in reducing transmission of health-care--associated pathogens. Frequent use of alcohol-based formulations for hand antisepsis can cause drying of the skin unless emollients, humectants, or other skin-conditioning agents are added to the formulations. The drying effect of alcohol can be reduced or eliminated by adding 1%--3% glycerol or other skin-conditioning agents (90,93,100,101,106,135,143,163,164). Moreover, in several recent prospective trials, alcohol-based rinses or gels containing emollients caused substantially less skin irritation and dryness than the soaps or antimicrobial detergents tested (96,98,165,166). These studies, which were conducted in clinical settings, used various subjective and objective methods for assessing skin irritation and dryness. Further studies are warranted to establish whether products with different formulations yield similar results. Even well-tolerated alcohol hand rubs containing emollients may cause a transient stinging sensation at the site of any broken skin (e.g., cuts and abrasions). Alcohol-based hand-rub preparations with strong fragrances may be poorly tolerated by HCWs with respiratory allergies. Allergic contact dermatitis or contact urticaria syndrome caused by hypersensitivity to alcohol or to various additives present in certain alcohol hand rubs occurs only rarely (167,168). Alcohols are flammable. Flash points of alcohol-based hand rubs range from 21ºC to 24ºC, depending on the type and concentration of alcohol present (169). As a result, alcohol-based hand rubs should be stored away from high temperatures or flames in accordance with National Fire Protection Agency recommendations. In Europe, where alcohol-based hand rubs have been used extensively for years, the incidence of fires associated with such products has been low (169). One recent U.S. report described a flash fire that occurred as a result of an unusual series of events, which included an HCW applying an alcohol gel to her hands, immediately removing a polyester isolation gown, and then touching a metal door before the alcohol had evaporated (170). Removing the polyester gown created a substantial amount of static electricity that generated an audible static spark when the HCW touched the metal door, igniting the unevaporated alcohol on her hands (170). This incident emphasizes the need to rub hands together after application of alcohol-based products until all the alcohol has evaporated. Because alcohols are volatile, containers should be designed to minimize evaporation. Contamination of alcohol-based solutions has seldom been reported. One report documented a cluster of pseudoinfections caused by contamination of ethyl alcohol by Bacillus cereus spores (171). Chlorhexidine Chlorhexidine gluconate, a cationic bisbiguanide, was developed in England in the early 1950s and was introduced into the United States in the 1970s (8,172). Chlorhexidine base is only minimally soluble in water, but the digluconate form is water-soluble. The antimicrobial activity of chlorhexidine is likely attributable to attachment to, and subsequent disruption of, cytoplasmic membranes, resulting in precipitation of cellular contents (1,8). Chlorhexidine's immediate antimicrobial activity occurs more slowly than that of alcohols. Chlorhexidine has good activity against gram-positive bacteria, somewhat less activity against gram-negative bacteria and fungi, and only minimal activity against tubercle bacilli (1,8,172). Chlorhexidine is not sporicidal (1,172). It has in vitro activity against enveloped viruses (e.g., herpes simplex virus, HIV, cytomegalovirus, influenza, and RSV) but substantially less activity against nonenveloped viruses (e.g., rotavirus, adenovirus, and enteroviruses) (130,131,173). The antimicrobial activity of chlorhexidine is only minimally affected by the presence of organic material, including blood. Because chlorhexidine is a cationic molecule, its activity can be reduced by natural soaps, various inorganic anions, nonionic surfactants, and hand creams containing anionic emulsifying agents (8,172,174). Chlorhexidine gluconate has been incorporated into a number of hand-hygiene preparations. Aqueous or detergent formulations containing 0.5% or 0.75% chlorhexidine are more effective than plain soap, but they are less effective than antiseptic detergent preparations containing 4% chlorhexidine gluconate (135,175). Preparations with 2% chlorhexidine gluconate are slightly less effective than those containing 4% chlorhexidine (176). Chlorhexidine has substantial residual activity (106,114--116,118,135,146,175). Addition of low concentrations (0.5%--1.0%) of chlorhexidine to alcohol-based preparations results in greater residual activity than alcohol alone (116,135). When used as recommended, chlorhexidine has a good safety record (172). Minimal, if any, absorption of the compound occurs through the skin. Care must be taken to avoid contact with the eyes when using preparations with >1% chlorhexidine, because the agent can cause conjunctivitis and severe corneal damage. Ototoxicity precludes its use in surgery involving the inner or middle ear. Direct contact with brain tissue and the meninges should be avoided. The frequency of skin irritation is concentration-dependent, with products containing 4% most likely to cause dermatitis when used frequently for antiseptic handwashing (177); allergic reactions to chlorhexidine gluconate are uncommon (118,172). Occasional outbreaks of nosocomial infections have been traced to contaminated solutions of chlorhexidine (178--181). Chloroxylenol Chloroxylenol, also known as parachlorometaxylenol (PCMX), is a halogen-substituted phenolic compound that has been used as a preservative in cosmetics and other products and as an active agent in antimicrobial soaps. It was developed in Europe in the late 1920s and has been used in the United States since the 1950s (182). The antimicrobial activity of PCMX likely is attributable to inactivation of bacterial enzymes and alteration of cell walls (1). It has good in vitro activity against gram-positive organisms and fair activity against gram-negative bacteria, mycobacteria, and certain viruses (1,7,182). PCMX is less active against P. aeruginosa, but addition of ethylene-diaminetetraacetic acid (EDTA) increases its activity against Pseudomonas spp. and other pathogens. A limited number of articles focusing on the efficacy of PCMX-containing preparations intended for use by HCWs have been published in the last 25 years, and the results of studies have sometimes been contradictory. For example, in studies in which antiseptics were applied to abdominal skin, PCMX had the weakest immediate and residual activity of any of the agents studied (183). However, when 30-second handwashes were performed using 0.6% PCMX, 2% chlorhexidine gluconate, or 0.3% triclosan, the immediate effect of PCMX was similar to that of the other agents. When used 18 times per day for 5 consecutive days, PCMX had less cumulative activity than did chlorhexidine gluconate (184). When PCMX was used as a surgical scrub, one report indicated that 3% PCMX had immediate and residual activity comparable to 4% chlorhexidine gluconate (185), whereas two other studies demonstrated that the immediate and residual activity of PCMX was inferior to both chlorhexidine gluconate and povidone-iodine (176,186). The disparity between published studies may be associated with the various concentrations of PCMX included in the preparations evaluated and with other aspects of the formulations tested, including the presence or absence of EDTA (7,182). PCMX is not as rapidly active as chlorhexidine gluconate or iodophors, and its residual activity is less pronounced than that observed with chlorhexidine gluconate (7,182). In 1994, FDA TFM tentatively classified PCMX as a Category IIISE active agent (i.e., insufficient data are available to classify this agent as safe and effective) (19). Further evaluation of this agent by the FDA is ongoing. The antimicrobial activity of PCMX is minimally affected by the presence of organic matter, but it is neutralized by nonionic surfactants. PCMX, which is absorbed through the skin (7,182), is usually well-tolerated, and allergic reactions associated with its use are uncommon. PCMX is available in concentrations of 0.3%--3.75%. In-use contamination of a PCMX-containing preparation has been reported (187). Hexachlorophene Hexachlorophene is a bisphenol composed of two phenolic groups and three chlorine moieties. In the 1950s and early 1960s, emulsions containing 3% hexachlorophene were widely used for hygienic handwashing, as surgical scrubs, and for routine bathing of infants in hospital nurseries. The antimicrobial activity of hexachlorophene results from its ability to inactivate essential enzyme systems in microorganisms. Hexachlorophene is bacteriostatic, with good activity against S. aureus and relatively weak activity against gram-negative bacteria, fungi, and mycobacteria (7). Studies of hexachlorophene as a hygienic handwash and surgical scrub demonstrated only modest efficacy after a single handwash (53,143,188). Hexachlorophene has residual activity for several hours after use and gradually reduces bacterial counts on hands after multiple uses (i.e., it has a cumulative effect) (1,101,188,189). With repeated use of 3% hexachlorophene preparations, the drug is absorbed through the skin. Infants bathed with hexachlorophene and personnel regularly using a 3% hexachlorophene preparation for handwashing have blood levels of 0.1--0.6 ppm hexachlorophene (190). In the early 1970s, certain infants bathed with hexachlorophene developed neurotoxicity (vacuolar degeneration) (191). As a result, in 1972, the FDA warned that hexachlorophene should no longer be used routinely for bathing infants. However, after routine use of hexachlorophene for bathing infants in nurseries was discontinued, investigators noted that the incidence of health-care--associated S. aureus infections in hospital nurseries increased substantially (192,193). In several instances, the frequency of infections decreased when hexachlorophene bathing of infants was reinstituted. However, current guidelines still recommend against the routine bathing of neonates with hexachlorophene because of its potential neurotoxic effects (194). The agent is classified by FDA TFM as not generally recognized as safe and effective for use as an antiseptic handwash (19). Hexachlorophene should not be used to bathe patients with burns or extensive areas of susceptible, sensitive skin. Soaps containing 3% hexachlorophene are available by prescription only (7). Iodine and Iodophors Iodine has been recognized as an effective antiseptic since the 1800s. However, because iodine often causes irritation and discoloring of skin, iodophors have largely replaced iodine as the active ingredient in antiseptics. Iodine molecules rapidly penetrate the cell wall of microorganisms and inactivate cells by forming complexes with amino acids and unsaturated fatty acids, resulting in impaired protein synthesis and alteration of cell membranes (195). Iodophors are composed of elemental iodine, iodide or triiodide, and a polymer carrier (i.e., the complexing agent) of high molecular weight. The amount of molecular iodine present (so-called "free" iodine) determines the level of antimicrobial activity of iodophors. "Available" iodine refers to the total amount of iodine that can be titrated with sodium thiosulfate (196). Typical 10% povidone-iodine formulations contain 1% available iodine and yield free iodine concentrations of 1 ppm (196). Combining iodine with various polymers increases the solubility of iodine, promotes sustained release of iodine, and reduces skin irritation. The most common polymers incorporated into iodophors are polyvinyl pyrrolidone (i.e., povidone) and ethoxylated nonionic detergents (i.e., poloxamers) (195,196). The antimicrobial activity of iodophors also can be affected by pH, temperature, exposure time, concentration of total available iodine, and the amount and type of organic and inorganic compounds present (e.g., alcohols and detergents). Iodine and iodophors have bactericidal activity against gram-positive, gram-negative, and certain spore-forming bacteria (e.g., clostridia and Bacillus spp.) and are active against mycobacteria, viruses, and fungi (8,195,197--200). However, in concentrations used in antiseptics, iodophors are not usually sporicidal (201). In vivo studies have demonstrated that iodophors reduce the number of viable organisms that are recovered from the hands of personnel (113,145,148,152,155). Povidone-iodine 5%--10% has been tentatively classified by FDA TFM as a Category I agent (i.e., a safe and effective agent for use as an antiseptic handwash and an HCW handwash) (19). The extent to which iodophors exhibit persistent antimicrobial activity after they have been washed off the skin is unclear. In one study, persistent activity was noted for 6 hours (176); however, several other studies demonstrated persistent activity for only 30--60 minutes after washing hands with an iodophor (61,117,202). In studies in which bacterial counts were obtained after gloves were worn for 1--4 hours after washing, iodophors have demonstrated poor persistent activity (1,104,115,189,203--208). The in vivo antimicrobial activity of iodophors is substantially reduced in the presence of organic substances (e.g., blood or sputum) (8). The majority of iodophor preparations used for hand hygiene contain 7.5%--10% povidone-iodine. Formulations with lower concentrations also have good antimicrobial activity because dilution can increase free iodine concentrations (209). However, as the amount of free iodine increases, the degree of skin irritation also may increase (209). Iodophors cause less skin irritation and fewer allergic reactions than iodine, but more irritant contact dermatitis than other antiseptics commonly used for hand hygiene (92). Occasionally, iodophor antiseptics have become contaminated with gram-negative bacilli as a result of poor manufacturing processes and have caused outbreaks or pseudo-outbreaks of infection (196). Quaternary Ammonium Compounds Quaternary ammonium compounds are composed of a nitrogen atom linked directly to four alkyl groups, which may vary in their structure and complexity (210). Of this large group of compounds, alkyl benzalkonium chlorides are the most widely used as antiseptics. Other compounds that have been used as antiseptics include benzethonium chloride, cetrimide, and cetylpyridium chloride (1). The antimicrobial activity of these compounds was first studied in the early 1900s, and a quaternary ammonium compound for preoperative cleaning of surgeons' hands was used as early as 1935 (210). The antimicrobial activity of this group of compounds likely is attributable to adsorption to the cytoplasmic membrane, with subsequent leakage of low molecular weight cytoplasmic constituents (210). Quaternary ammonium compounds are primarily bacteriostatic and fungistatic, although they are microbicidal against certain organisms at high concentrations (1); they are more active against gram-positive bacteria than against gram-negative bacilli. Quaternary ammonium compounds have relatively weak activity against mycobacteria and fungi and have greater activity against lipophilic viruses. Their antimicrobial activity is adversely affected by the presence of organic material, and they are not compatible with anionic detergents (1,210). In 1994, FDA TFM tentatively classified benzalkonium chloride and benzethonium chloride as Category IIISE active agents (i.e., insufficient data exists to classify them as safe and effective for use as an antiseptic handwash) (19). Further evaluation of these agents by FDA is in progress. Quaternary ammonium compounds are usually well tolerated. However, because of weak activity against gram-negative bacteria, benzalkonium chloride is prone to contamination by these organisms. Several outbreaks of infection or pseudoinfection have been traced to quaternary ammonium compounds contaminated with gram-negative bacilli (211--213). For this reason, in the United States, these compounds have been seldom used for hand antisepsis during the last 15--20 years. However, newer handwashing products containing benzalkonium chloride or benzethonium chloride have recently been introduced for use by HCWs. A recent study of surgical intensive-care unit personnel found that cleaning hands with antimicrobial wipes containing a quaternary ammonium compound was about as effective as using plain soap and water for handwashing; both were less effective than decontaminating hands with an alcohol-based hand rub (214). One laboratory-based study reported that an alcohol-free hand-rub product containing a quaternary ammonium compound was efficacious in reducing microbial counts on the hands of volunteers (215). Further studies of such products are needed to determine if newer formulations are effective in health-care settings. Triclosan Triclosan (chemical name: 2,4,4' --trichloro-2'-hydroxy-diphenyl ether) is a nonionic, colorless substance that was developed in the 1960s. It has been incorporated into soaps for use by HCWs and the public and into other consumer products. Concentrations of 0.2%--2% have antimicrobial activity. Triclosan enters bacterial cells and affects the cytoplasmic membrane and synthesis of RNA, fatty acids, and proteins (216). Recent studies indicate this agent's antibacterial activity is attributable to binding to the active site of enoyl-acyl carrier protein reductase (217,218). Triclosan has a broad range of antimicrobial activity, but it is often bacteriostatic (1). Minimum inhibitory concentrations (MICs) range from 0.1 to 10 ug/mL, whereas minimum bactericidal concentrations are 25--500 ug/mL. Triclosan's activity against gram-positive organisms (including MRSA) is greater than against gram-negative bacilli, particularly P. aeruginosa (1,216). The agent possesses reasonable activity against mycobacterial and Candida spp., but it has limited activity against filamentous fungi. Triclosan (0.1%) reduces bacterial counts on hands by 2.8 log10 after a 1-minute hygienic handwash (1). In several studies, log reductions have been lower after triclosan is used than when chlorhexidine, iodophors, or alcohol-based products are applied (1,61,149,184,219). In 1994, FDA TFM tentatively classified triclosan <1.0% as a Category IIISE active agent (i.e., insufficient data exist to classify this agent as safe and effective for use as an antiseptic handwash) (19). Further evaluation of this agent by the FDA is underway. Like chlorhexidine, triclosan has persistent activity on the skin. Its activity in hand-care products is affected by pH, the presence of surfactants, emollients, or humectants and by the ionic nature of the particular formulation (1,216). Triclosan's activity is not substantially affected by organic matter, but it can be inhibited by sequestration of the agent in micelle structures formed by surfactants present in certain formulations. The majority of formulations containing <2% triclosan are well-tolerated and seldom cause allergic reactions. Certain reports indicate that providing hospital personnel with a triclosan-containing preparation for hand antisepsis has led to decreased MRSA infections (72,73). Triclosan's lack of potent activity against gram-negative bacilli has resulted in occasional reports of contamination (220). Other Agents Approximately 150 years after puerperal-fever--related maternal mortality rates were demonstrated by Semmelweis to be reduced by use of a hypochlorite hand rinse, the efficacy of rubbing hands for 30 seconds with an aqueous hypochlorite solution was studied once again (221). The solution was demonstrated to be no more effective than distilled water. The regimen used by Semmelweis, which called for rubbing hands with a 4% [w/w] hypochlorite solution until the hands were slippery (approximately 5 minutes), has been revisited by other researchers (222). This more current study indicated that the regimen was 30 times more effective than a 1-minute rub using 60% isopropanol. However, because hypochlorite solutions are often irritating to the skin when used repeatedly and have a strong odor, they are seldom used for hand hygiene. Certain other agents are being evaluated by FDA for use in health-care-related antiseptics
(19). However, the efficacy of these agents has not been evaluated adequately for use in handwashing preparations intended for use by HCWs. Further evaluation of these agents is warranted. Products that use different concentrations of traditional antiseptics (e.g., low concentrations
of iodophor) or contain novel compounds with antiseptic properties are likely to be introduced for use by HCWs. For
example, preliminary studies have demonstrated that adding silver-containing polymers to an ethanol carrier (i.e., Surfacine®) results in a preparation that has persistent antimicrobial activity on animal and human skin
(223). New compounds with good in vitro activity must be tested in vivo to determine their abilities to reduce transient and resident skin flora on the hands of HCWs.
The widespread prevalence of health-care--associated diarrhea caused by
Clostridium difficile and the recent occurrence in
the United States of human Bacillus
anthracis infections associated with contaminated items sent through the postal system
has raised concern regarding the activity of antiseptic agents against spore-forming bacteria. None of the agents
(including alcohols, chlorhexidine, hexachlorophene, iodophors, PCMX, and triclosan) used in antiseptic handwash or antiseptic
hand-rub preparations are reliably sporicidal against
Clostridium spp. or Bacillus spp.
(120,172,224,225). Washing hands with non-antimicrobial or antimicrobial soap and water may help to physically remove spores from the surface of contaminated hands. HCWs should be encouraged to wear gloves when caring for patients with
C. difficile-associated diarrhea
(226). After gloves are removed, hands should be washed with a non-antimicrobial or an antimicrobial soap and water or disinfected with
an alcohol-based hand rub. During outbreaks of C.
difficile-related infections, washing hands with a non-antimicrobial
or
antimicrobial soap and water after removing gloves is prudent. HCWs with suspected or documented exposure to
B. anthracis-contaminated items also should be encouraged to wash their hands with a non-antimicrobial or antimicrobial soap and water.
Reduced susceptibility of bacteria to antiseptic agents can either be an intrinsic characteristic of a species or can be
an acquired trait (227). Several reports have described strains of bacteria that appear to have acquired reduced susceptibility
(when defined by MICs established in vitro) to certain antiseptics (e.g., chlorhexidine, quaternary ammonium compounds,
and triclosan) (227--230). However, because the antiseptic concentrations that are actually used by HCWs are often substantially higher than the MICs of strains with reduced antiseptic susceptibility, the clinical relevance of the in vitro findings is questionable. For example, certain strains of MRSA have chlorhexidine and quaternary ammonium
compound MICs that are several-fold higher than
methicillin-susceptible strains, and certain strains of S. aureus have elevated MICs to triclosan
(227,228). However, such strains were readily inhibited by the concentrations of these antiseptics that are actually used by practicing HCWs (227,228). The description of a triclosan-resistant bacterial enzyme has raised the question of whether resistance to this agent may develop more readily than to other antiseptic agents
(218). In addition, exposing
Pseudomonas strains containing the MexAB-OprM efflux system to triclosan may select for mutants that are resistant to multiple antibiotics, including fluoroquinolones
(230). Further studies are needed to determine whether reduced susceptibility to antiseptic
agents is of epidemiologic significance and whether resistance to antiseptics has any influence on the prevalence of antibiotic-resistant strains (227).
Since the late 1800s, when Lister promoted the application of carbolic acid to the hands of surgeons before procedures, preoperative cleansing of hands and forearms with an antiseptic agent has been an accepted practice (231). Although no randomized, controlled trials have been conducted to indicate that surgical-site infection rates are substantially lower when preoperative scrubbing is performed with an antiseptic agent rather than a non-antimicrobial soap, certain other factors provide a strong rationale for this practice. Bacteria on the hands of surgeons can cause wound infections if introduced into the operative field during surgery (232); rapid multiplication of bacteria occurs under surgical gloves if hands are washed with a non-antimicrobial soap. However, bacterial growth is slowed after preoperative scrubbing with an antiseptic agent (14,233). Reducing resident skin flora on the hands of the surgical team for the duration of a procedure reduces the risk of bacteria being released into the surgical field if gloves become punctured or torn during surgery (1,156,169). Finally, at least one outbreak of surgical-site infections occurred when surgeons who normally used an antiseptic surgical scrub preparation began using a non-antimicrobial product (234). Antiseptic preparations intended for use as surgical hand scrubs are evaluated for their ability to reduce the number of bacteria released from hands at different times, including 1) immediately after scrubbing, 2) after wearing surgical gloves for 6 hours (i.e., persistent activity), and 3) after multiple applications over 5 days (i.e., cumulative activity). Immediate and persistent activity are considered the most important in determining the efficacy of the product. U.S. guidelines recommend that agents used for surgical hand scrubs should substantially reduce microorganisms on intact skin, contain a nonirritating antimicrobial preparation, have broad-spectrum activity, and be fast-acting and persistent (19,235). Studies have demonstrated that formulations containing 60%--95% alcohol alone or 50%--95% when combined with limited amounts of a quaternary ammonium compound, hexachlorophene, or chlorhexidine gluconate, lower bacterial counts on the skin immediately postscrub more effectively than do other agents (Table 4). The next most active agents (in order of decreasing activity) are chlorhexidine gluconate, iodophors, triclosan, and plain soap (104,119,186,188, 203,204,206,208,236). Because studies of PCMX as a surgical scrub have yielded contradictory results, further studies are needed to establish how the efficacy of this compound compares with the other agents (176,185,186). Although alcohols are not considered to have persistent antimicrobial activity, bacteria appear to reproduce slowly on the hands after a surgical scrub with alcohol, and bacterial counts on hands after wearing gloves for 1--3 hours seldom exceed baseline (i.e., prescrub) values (1). However, a recent study demonstrated that a formulation containing 61% ethanol alone did not achieve adequate persistent activity at 6 hours postscrub (237). Alcohol-based preparations containing 0.5% or 1% chlorhexidine gluconate have persistent activity that, in certain studies, has equaled or exceeded that of chlorhexidine gluconate-containing detergents (1,118,135,237).* Persistent antimicrobial activity of detergent-based surgical scrub formulations is greatest for those containing 2% or 4% chlorhexidine gluconate, followed by hexachlorophene, triclosan, and iodophors (1,102,113--115,159,189,203, 204,206--208,236). Because hexachlorophene is absorbed into the blood after repeated use, it is seldom used as a surgical scrub. Surgical staff have been traditionally required to scrub their hands for 10 minutes preoperatively, which frequently leads to skin damage. Several studies have demonstrated that scrubbing for 5 minutes reduces bacterial counts as effectively as a 10-minute scrub (117,238,239). In other studies, scrubbing for 2 or 3 minutes reduced bacterial counts to acceptable levels (156,205,207,240,241). Studies have indicated that a two-stage surgical scrub using an antiseptic detergent, followed by application of an alcohol-containing preparation, is effective. For example, an initial 1- or 2-minute scrub with 4% chlorhexidine gluconate or povidone-iodine followed by application of an alcohol-based product has been as effective as a 5-minute scrub with an antiseptic detergent (114,242). Surgical hand-antisepsis protocols have required personnel to scrub with a brush. But this practice can damage the skin
of personnel and result in increased shedding of bacteria from the hands
(95,243). Scrubbing with a disposable sponge
or combination sponge-brush has reduced bacterial counts on the hands as effectively as scrubbing with a brush
(244--246). However, several studies indicate that neither a brush nor a sponge is necessary to reduce bacterial counts on the hands of surgical personnel to acceptable levels, especially when
alcohol-based products are used (102,117,159,165,233,237,
247,248). Several of these studies performed cultures immediately or at 45--60 minutes postscrub (102,117, 233,247,248), whereas in other studies, cultures were obtained 3 and 6 hours postscrub (159,237). For example, a recent laboratory-based study
using volunteers demonstrated that brushless application of a preparation containing 1% chlorhexidine gluconate plus 61% ethanol yielded lower bacterial counts on the hands of participants than using a sponge/brush to apply a 4% chlorhexidine-containing detergent preparation
(237).
Comparing studies related to the in vivo efficacy of plain soap, antimicrobial soaps, and alcohol-based hand rubs
is problematic, because certain studies express efficacy as the percentage reduction in bacterial counts achieved, whereas
others give log10 reductions in counts achieved. However, summarizing the relative efficacy of agents tested in each study can
provide an overview of the in vivo activity of various formulations intended for handwashing, hygienic handwash, antiseptic hand
rub, or surgical hand antisepsis (Tables 2--4).
Frequency and Pathophysiology of Irritant Contact Dermatitis In certain surveys, approximately 25% of nurses report symptoms or signs of dermatitis involving their hands, and as many as 85% give a history of having skin problems (249). Frequent and repeated use of hand-hygiene products, particularly soaps and other detergents, is a primary cause of chronic irritant contact dermatitis among HCWs (250). The potential of detergents to cause skin irritation can vary considerably and can be ameliorated by the addition of emollients and humectants. Irritation associated with antimicrobial soaps may be caused by the antimicrobial agent or by other ingredients of the formulation. Affected persons often complain of a feeling of dryness or burning; skin that feels "rough;" and erythema, scaling, or fissures. Detergents damage the skin by causing denaturation of stratum corneum proteins, changes in intercellular lipids (either depletion or reorganization of lipid moieties), decreased corneocyte cohesion, and decreased stratum corneum water-binding capacity (250,251). Damage to the skin also changes skin flora, resulting in more frequent colonization by staphylococci and gram-negative bacilli (17,90). Although alcohols are among the safest antiseptics available, they can cause dryness and irritation of the skin (1,252). Ethanol is usually less irritating than n-propanol or isopropanol (252). Irritant contact dermatitis is more commonly reported with iodophors (92). Other antiseptic agents that can cause irritant contact dermatitis (in order of decreasing frequency) include chlorhexidine, PCMX, triclosan, and alcohol-based products. Skin that is damaged by repeated exposure to detergents may be more susceptible to irritation by alcohol-based preparations (253). The irritancy potential of commercially prepared hand-hygiene products, which is often determined by measuring transepidermal water loss, may be available from the manufacturer. Other factors that can contribute to dermatitis associated with frequent handwashing include using hot water for handwashing, low relative humidity (most common in winter months), failure to use supplementary hand lotion or cream, and the quality of paper towels (254,255). Shear forces associated with wearing or removing gloves and allergy to latex proteins may also contribute to dermatitis of the hands of HCWs. Allergic Contact Dermatitis Associated with Hand-Hygiene Products Allergic reactions to products applied to the skin (i.e., contact allergies) may present as delayed type reactions (i.e., allergic contact dermatitis) or less commonly as immediate reactions (i.e., contact urticaria). The most common causes of contact allergies are fragrances and preservatives; emulsifiers are less common causes (256--259). Liquid soaps, hand lotions or creams, and "udder ointments" may contain ingredients that cause contact allergies among HCWs (257,258). Allergic reactions to antiseptic agents, including quaternary ammonium compounds, iodine or iodophors, chlorhexidine, triclosan, PCMX, and alcohols have been reported (118,167,172,256,260--265). Allergic contact dermatitis associated with alcohol-based hand rubs is uncommon. Surveillance at a large hospital in Switzerland, where a commercial alcohol hand rub has been used for >10 years, failed to identify a single case of documented allergy to the product (169). In late 2001, a Freedom of Information Request for data in the FDA's Adverse Event Reporting System regarding adverse reactions to popular alcohol hand rubs in the United States yielded only one reported case of an erythematous rash reaction attributed to such a product (John M. Boyce, M.D., Hospital of St. Raphael, New Haven, Connecticut, personal communication, 2001). However, with increasing use of such products by HCWs, true allergic reactions to such products likely will be encountered. Allergic reactions to alcohol-based products may represent true allergy to alcohol, allergy to an impurity or
aldehyde metabolite, or allergy to another constituent of the product
(167). Allergic contact dermatitis or immediate contact urticarial reactions may be caused by ethanol or isopropanol (167). Allergic reactions can be caused by compounds that may be
present as inactive ingredients in alcohol-based hand rubs, including fragrances, benzyl alcohol, stearyl or isostearyl alcohol, phenoxyethanol, myristyl alcohol, propylene glycol, parabens, and benzalkonium chloride (167,256,266--270).
Potential strategies for minimizing hand-hygiene--related irritant contact dermatitis among HCWs include reducing the frequency of exposure to irritating agents (particularly anionic detergents), replacing products with high irritation potential with preparations that cause less damage to the skin, educating personnel regarding the risks of irritant contact dermatitis, and providing caregivers with moisturizing skin-care products or barrier creams (96,98,251,271--273). Reducing the frequency of exposure of HCWs to hand-hygiene products would prove difficult and is not desirable because of the low levels of adherence to hand-hygiene policies in the majority of institutions. Although hospitals have provided personnel with non-antimicrobial soaps in hopes of minimizing dermatitis, frequent use of such products may cause greater skin damage, dryness, and irritation than antiseptic preparations (92,96,98). One strategy for reducing the exposure of personnel to irritating soaps and detergents is to promote the use of alcohol-based hand rubs containing various emollients. Several recent prospective, randomized trials have demonstrated that alcohol-based hand rubs containing emollients were better tolerated by HCWs than washing hands with non-antimicrobial soaps or antimicrobial soaps (96,98,166). Routinely washing hands with soap and water immediately after using an alcohol hand rub may lead to dermatitis. Therefore, personnel should be reminded that it is neither necessary nor recommended to routinely wash hands after each application of an alcohol hand rub. Hand lotions and creams often contain humectants and various fats and oils that can increase skin hydration and replace altered or depleted skin lipids that contribute to the barrier function of normal skin (251,271). Several controlled trials have demonstrated that regular use (e.g., twice a day) of such products can help prevent and treat irritant contact dermatitis caused by hand-hygiene products (272,273). In one study, frequent and scheduled use of an oil-containing lotion improved skin condition, and thus led to a 50% increase in handwashing frequency among HCWs (273). Reports from these studies emphasize the need to educate personnel regarding the value of regular, frequent use of hand-care products. Recently, barrier creams have been marketed for the prevention of hand-hygiene--related irritant contact dermatitis. Such products are absorbed to the superficial layers of the epidermis and are designed to form a protective layer that is not removed by standard handwashing. Two recent randomized, controlled trials that evaluated the skin condition of caregivers demonstrated that barrier creams did not yield better results than did the control lotion or vehicle used (272,273). As a result, whether barrier creams are effective in preventing irritant contact dermatitis among HCWs remains unknown. In addition to evaluating the efficacy and acceptability of hand-care products, product-selection committees should
inquire about the potential deleterious effects that oil-containing products may have on the integrity of rubber gloves and on
the efficacy of antiseptic agents used in the facility
(8,236).
When evaluating hand-hygiene products for potential use in health-care facilities, administrators or product-selection committees must consider factors that can affect the overall efficacy of such products, including the relative efficacy of antiseptic agents against various pathogens (Appendix) and acceptance of hand-hygiene products by personnel (274,275). Soap products that are not well-accepted by HCWs can be a deterrent to frequent handwashing (276). Characteristics of a product (either soap or alcohol-based hand rub) that can affect acceptance by personnel include its smell, consistency (i.e., "feel"), and color (92,277,278). For soaps, ease of lathering also may affect user preference. Because HCWs may wash their hands from a limited number of times per shift to as many as 30 times per shift, the tendency of products to cause skin irritation and dryness is a substantial factor that influences acceptance, and ultimate usage (61,98,274,275,277,279). For example, concern regarding the drying effects of alcohol was a primary cause of poor acceptance of alcohol-based hand-hygiene products in hospitals in the United States (5,143). However, several studies have demonstrated that alcohol-based hand rubs containing emollients are acceptable to HCWs (90,93,98,100,101,106, 143,163,164,166). With alcohol-based products, the time required for drying may also affect user acceptance. Studies indicate that the frequency of handwashing or antiseptic handwashing by personnel is affected by the accessibility of hand-hygiene facilities (280--283). In certain health-care facilities, only one sink is available in rooms housing several patients, or sinks are located far away from the door of the room, which may discourage handwashing by personnel leaving the room. In intensive-care units, access to sinks may be blocked by bedside equipment (e.g., ventilators or intravenous infusion pumps). In contrast to sinks used for handwashing or antiseptic handwash, dispensers for alcohol-based hand rubs do not require plumbing and can be made available adjacent to each patient's bed and at many other locations in patient-care areas. Pocket carriage of alcohol-based hand-rub solutions, combined with availability of bedside dispensers, has been associated with substantial improvement in adherence to hand-hygiene protocols (74,284). To avoid any confusion between soap and alcohol hand rubs, alcohol hand-rub dispensers should not be placed adjacent to sinks. HCWs should be informed that washing hands with soap and water after each use of an alcohol hand rub is not necessary and is not recommended, because it may lead to dermatitis. However, because personnel feel a "build-up" of emollients on their hands after repeated use of alcohol hand gels, washing hands with soap and water after 5--10 applications of a gel has been recommended by certain manufacturers. Automated handwashing machines have not been demonstrated to improve the quality or frequency of handwashing (88,285). Although technologically advanced automated handwashing devices and monitoring systems have been developed recently, only a minimal number of studies have been published that demonstrate that use of such devices results in enduring improvements in hand-hygiene adherence among HCWs. Further evaluation of automated handwashing facilities and monitoring systems is warranted. Dispenser systems provided by manufacturers or vendors also must be considered when evaluating hand-hygiene products. Dispensers may discourage use by HCWs when they 1) become blocked or partially blocked and do not deliver the product when accessed by personnel, and 2) do not deliver the product appropriately onto the hands. In one hospital where a viscous alcohol-based hand rinse was available, only 65% of functioning dispensers delivered product onto the caregivers' hands with one press of the dispenser lever, and 9% of dispensers were totally occluded (286). In addition, the volume delivered was often suboptimal, and the product was sometimes squirted onto the wall instead of the caregiver's hand. Only limited information is available regarding the cost of hand-hygiene products used in health-care facilities (165,287). These costs were evaluated in patient-care areas at a 450-bed community teaching hospital (287); the hospital spent $22,000 ($0.72 per patient-day) on 2% chlorhexidine-containing preparations, plain soap, and an alcohol hand rinse. (287) When hand-hygiene supplies for clinics and nonpatient care areas were included, the total annual budget for soaps and hand antiseptic agents was $30,000 (approximately $1 per patient-day). Annual hand-hygiene product budgets at other institutions vary considerably because of differences in usage patterns and varying product prices. One researcher (287) determined that if non-antimicrobial liquid soap were assigned an arbitrary relative cost of 1.0, the cost per liter would be 1.7 times as much for 2% chlorhexidine gluconate detergent, 1.6--2.0 times higher for alcohol-based hand-rub products, and 4.5 times higher for an alcohol-based foam product. A recent cost comparison of surgical scrubbing with an antimicrobial soap versus brushless scrubbing with an alcohol-based hand rub revealed that costs and time required for preoperative scrubbing were less with the alcohol-based product (165). In a trial conducted in two critical-care units, the cost of using an alcohol hand rub was half as much as using an antimicrobial soap for handwashing ($0.025 versus $0.05 per application, respectively) (166). To put expenditures for hand-hygiene products into perspective, health-care facilities should consider comparing
their budget for hand-hygiene products to estimated excess hospital costs resulting from health-care--associated infections.
The excess hospital costs associated with only four or five health-care--associated infections of average severity may equal the
entire annual budget for hand-hygiene products used in inpatient-care areas. Just one severe surgical site infection, lower
respiratory tract infection, or bloodstream infection may cost the hospital more than the entire annual budget for antiseptic agents
used for hand hygiene (287). Two studies provided certain quantitative estimates of the benefit of
hand-hygiene--promotion programs (72,74). One study demonstrated a cost saving of approximately $17,000 resulting from reduced use of vancomycin after the observed decrease in MRSA incidence in a 7-month period
(72). In another study that examined both direct
costs associated with the hand-hygiene promotion program (increased use of hand-rub solution and poster production) and
indirect costs associated with health-care--personnel time
(74), costs of the program were an estimated $57,000 or less per year
(an average of $1.42 per patient admitted). Supplementary costs associated with the increased use of alcohol-based
hand-rub solution averaged $6.07 per 100 patient-days. Based on conservative estimates of $2,100 saved per infection averted and on
the assumption that only 25% of the observed reduction in the infection rate was associated with improved hand-hygiene
practice, the program was substantially cost-effective. Thus, hospital administrators must consider that by purchasing more effective
or more acceptable hand-hygiene products to improve hand-hygiene practices, they
will avoid the occurrence of nosocomial infections;
preventing only a limited number of additional health-care--associated infections per year will lead to savings
that will exceed any incremental costs of improved hand-hygiene products.
In observational studies conducted in hospitals, HCWs washed their hands an average of five times per shift to as many as 30 times per shift (Table 6) (17,61,90,98,274,288); certain nurses washed their hands <100 times per shift (90). Hospitalwide surveillance of hand hygiene reveals that the average number of handwashing opportunities varies markedly between hospital wards. For example, nurses in pediatric wards had an average of eight opportunities for hand hygiene per hour of patient care compared with an average of 20 for nurses in intensive-care units (11). The duration of handwashing or hygienic handwash episodes by HCWs has averaged 6.6--24.0 seconds in observational studies (Table 7) (17,52,59,84--87,89,249,279). In addition to washing their hands for limited time periods, personnel often fail to cover all surfaces of their hands and fingers (288). Adherence of HCWs to Recommended Hand-Hygiene Practices Observational Studies of Hand-Hygiene Adherence. Adherence of HCWs to recommended hand-hygiene procedures has been poor, with mean baseline rates of 5%--81% (overall average: 40%) (Table 8) (71,74,86,87,276,280,281,283,285, 289--313). The methods used for defining adherence (or nonadherence) and those used for conducting observations vary considerably among studies, and reports do not provide detailed information concerning the methods and criteria used. The majority of studies were conducted with hand-hygiene adherence as the major outcome measure, whereas a limited number measured adherence as part of a broader investigation. Several investigators reported improved adherence after implementing various interventions, but the majority of studies had short follow-up periods and did not confirm whether behavioral improvements were long-lasting. Other studies established that sustained improvements in handwashing behavior occurred during a long-term program to improve adherence to hand-hygiene policies (74,75). Factors Affecting Adherence. Factors that may influence hand hygiene include those identified in epidemiologic studies and factors reported by HCWs as being reasons for lack of adherence to hand-hygiene recommendations. Risk factors for poor adherence to hand hygiene have been determined objectively in several observational studies or interventions to improve adherence (11,12,274,292,295,314--317). Among these, being a physician or a nursing assistant, rather than a nurse, was consistently associated with reduced adherence (Box 1). In the largest hospitalwide survey of hand-hygiene practices among HCWs (11), predictors of poor adherence to recommended hand-hygiene measures were identified. Predictor variables included professional category, hospital ward, time of day/week, and type and intensity of patient care, defined as the number of opportunities for hand hygiene per hour of patient care. In 2,834 observed opportunities for hand hygiene, average adherence was 48%. In multivariate analysis, nonadherence was lowest among nurses and during weekends (Odds Ratio [OR]: 0.6; 95% confidence interval [CI] = 0.4--0.8). Nonadherence was higher in intensive-care units compared with internal medicine wards (OR: 2.0; 95% CI = 1.3--3.1), during procedures that carried a high risk of bacterial contamination (OR: 1.8; 95% CI = 1.4--2.4), and when intensity of patient care was high (21--40 handwashing opportunities --- OR: 1.3; 95% CI = 1.0-1.7; 41--60 opportunities --- OR: 2.1; 95% CI = 1.5-2.9; >60 opportunities --- OR: 2.1; 95% CI = 1.3--3.5). The higher the demand for hand hygiene, the lower the adherence; on average, adherence decreased by 5% (+ 2%) for each increase of 10 opportunities per hour when the intensity of patient care exceeded 10 opportunities per hour. Similarly, the lowest adherence rate (36%) was found in intensive-care units, where indications for hand hygiene were typically more frequent (on average, 20 opportunities per patient-hour). The highest adherence rate (59%) was observed in pediatrics wards, where the average intensity of patient care was lower than in other hospital areas (an average of eight opportunities per patient-hour). The results of this study indicate that full adherence to previous guidelines may be unrealistic, and that facilitated access to hand hygiene could help improve adherence (11,12,318). Perceived barriers to adherence with hand-hygiene practice recommendations include skin irritation caused by hand-hygiene agents, inaccessible hand-hygiene supplies, interference with HCW-patient relationships, priority of care (i.e., the patients' needs are given priority over hand hygiene), wearing of gloves, forgetfulness, lack of knowledge of the guidelines, insufficient time for hand hygiene, high workload and understaffing, and the lack of scientific information indicating a definitive impact of improved hand hygiene on health-care--associated infection rates (11,274,292,295,315--317). Certain perceived barriers to adherence with hand-hygiene guidelines have been assessed or quantified in observational studies (12,274,292,295,314--317) (Box 1). Skin irritation by hand-hygiene agents constitutes a substantial barrier to appropriate adherence (319). Because soaps and detergents can damage skin when applied on a regular basis, HCWs must be better informed regarding the possible adverse effects associated with hand-hygiene agents. Lack of knowledge and education regarding this subject is a barrier to motivation. In several studies, alcohol-based hand rubs containing emollients (either isopropanol, ethanol, or n-propanol in 60%--90% vol/vol) were less irritating to the skin than the soaps or detergents tested. In addition, the alcohol-based products containing emollients that were tested were at least as tolerable and efficacious as the detergents tested. Also, studies demonstrate that several hand lotions have reduced skin scaling and cracking, which may reduce microbial shedding from the hands (67,272,273). Easy access to hand-hygiene supplies, whether sink, soap, medicated detergent, or alcohol-based hand-rub solution, is essential for optimal adherence to hand-hygiene recommendations. The time required for nurses to leave a patient's bedside, go to a sink, and wash and dry their hands before attending the next patient is a deterrent to frequent handwashing or hand antisepsis (11,318). Engineering controls could facilitate adherence, but careful monitoring of hand-hygiene behavior should be conducted to exclude the possible negative effect of newly introduced handwashing devices (88). The impact of wearing gloves on adherence to hand-hygiene policies has not been definitively established, because published studies have yielded contradictory results (87,290,301,320). Hand hygiene is required regardless of whether gloves are used or changed. Failure to remove gloves after patient contact or between "dirty" and "clean" body-site care on the same patient must be regarded as nonadherence to hand-hygiene recommendations (11). In a study in which experimental conditions approximated those occurring in clinical practice (321), washing and reusing gloves between patient contacts resulted in observed bacterial counts of 0--4.7 log on the hands after glove removal. Therefore, this practice should be discouraged; handwashing or disinfection should be performed after glove removal. Lack of 1) knowledge of guidelines for hand hygiene, 2) recognition of hand-hygiene opportunities during patient care, and 3) awareness of the risk of cross-transmission of pathogens are barriers to good hand-hygiene practices. Furthermore, certain HCWs believe they have washed their hands when necessary, even when observations indicate they have not (89,92,295,296,322). Perceived barriers to hand-hygiene behavior are linked not only to the institution, but also to HCWs' colleagues. Therefore, both institutional and small-group dynamics need to be considered when implementing a system change to secure an improvement in HCWs' hand-hygiene practice. Possible Targets for Hand-Hygiene Promotion Targets for the promotion of hand hygiene are derived from studies assessing risk factors for nonadherence, reported reasons for the lack of adherence to recommendations, and additional factors perceived as being important to facilitate appropriate HCW behavior. Although certain factors cannot be modified (Box 1), others can be changed. One factor that must be addressed is the time required for HCWs to clean their hands. The time required for traditional handwashing may render full adherence to previous guidelines unrealistic (11,12,318) and more rapid access to hand-hygiene materials could help improve adherence. One study conducted in an intensive-care unit demonstrated that it took nurses an average of 62 seconds to leave a patient's bedside, walk to a sink, wash their hands, and return to patient care (318). In contrast, an estimated one fourth as much time is required when using alcohol-based hand rub placed at each patient's bedside. Providing easy access to hand-hygiene materials is mandatory for appropriate hand-hygiene behavior and is achievable in the majority of health-care facilities (323). In particular, in high-demand situations (e.g., the majority of critical-care units), under hectic working conditions, and at times of overcrowding or understaffing, HCWs may be more likely to use an alcohol-based hand rub than to wash their hands (323). Further, using alcohol-based hand rubs may be a better option than traditional handwashing with plain soap and water or antiseptic handwash, because they not only require less time (166,318) but act faster (1) and irritate hands less often (1,67,96,98,166). They also were used in the only program that reported a sustained improvement in hand-hygiene adherence associated with decreased infection rates (74). However, making an alcohol-based hand rub available to personnel without providing ongoing educational and motivational activities may not result in long-lasting improvement in hand-hygiene practices (313). Because increased use of hand-hygiene agents might be associated with skin dryness, the availability of free skin-care lotion is recommended. Education is a cornerstone for improvement with hand-hygiene practices. Topics that must be addressed by educational programs include the lack of 1) scientific information for the definitive impact of improved hand hygiene on health-care--associated infection and resistant organism transmission rates; 2) awareness of guidelines for hand hygiene and insufficient knowledge concerning indications for hand hygiene during daily patient care; 3) knowledge concerning the low average adherence rate to hand hygiene by the majority of HCWs; and 4) knowledge concerning the appropriateness, efficacy, and understanding of the use of hand-hygiene and skin-care--protection agents. HCWs necessarily evolve within a group that functions within an institution. Possible targets for improvement in hand-hygiene behavior not only include factors linked to individual HCWs, but also those related to the group(s) and the
institution as a whole (317,323). Examples of possible targets for hand-hygiene promotion at the group level include education and performance feedback on hand-hygiene adherence; efforts to prevent high workload, downsizing, and understaffing;
and encouragement and provision of role models from key members in the work unit. At the institutional level, targets
for improvement include 1) written guidelines, hand-hygiene agents, skin-care promotions and agents, or hand-hygiene facilities; 2) culture or tradition of adherence; and 3) administrative leadership, sanction, support, and rewards. Several studies, conducted in various types of institutions, reported modest and even low levels of adherence to recommended hand-hygiene practices, indicating that such adherence varied by hospital ward and by type of HCW. These results indicate educational sessions may need to be designed specifically for certain types of personnel
(11,289,290,294,317,323).
In 1998, the prevailing behavioral theories and their applications with regard to the health professions were reviewed by researchers in an attempt to better understand how to target more successful interventions (317). The researchers proposed a hypothetical framework to enhance hand-hygiene practices and stressed the importance of considering the complexity of individual and institutional factors when designing behavioral interventions. Although behavioral theories and secondary interventions have primarily targeted individual workers, this practice might be insufficient to produce sustained change (317,324,325). Interventions aimed at improving hand-hygiene practices must account for different levels of behavior interaction (12,317,326). Thus, the interdependence of individual factors, environmental constraints, and the institutional climate must be taken into account in the strategic planning and development of hand-hygiene campaigns. Interventions to promote hand hygiene in hospitals should consider variables at all these levels. Various factors involved in hand-hygiene behavior include intention, attitude towards the behavior, perceived social norm, perceived behavioral control, perceived risk for infection, hand-hygiene practices, perceived role model, perceived knowledge, and motivation (317). The factors necessary for change include 1) dissatisfaction with the current situation, 2) perception of alternatives, and 3) recognition, both at the individual and institutional level, of the ability and potential to change. Although the latter implies education and motivation, the former two necessitate a system change. Among the reported reasons for poor adherence with
hand-hygiene recommendations (Box 1), certain ones are
clearly associated with the institution or system (e.g., lack of institutional priority for hand hygiene, administrative sanctions, and a safety climate). Although all of these reasons would require a system change in the majority of institutions, the third
requires management commitment, visible safety programs,
an acceptable level of work stress, a tolerant and supportive attitude
toward reported problems, and belief in the efficacy of preventive strategies
(12,317,325,327). Most importantly, an improvement
in infection-control practices requires 1) questioning basic beliefs, 2) continuous assessment of the group (or individual) stage
of behavioral change, 3) intervention(s) with an appropriate process of change, and 4) supporting individual and group
creativity (317). Because of the complexity of the process of change, single interventions often fail. Thus, a multimodal, multidisciplinary strategy is likely necessary
(74,75,317,323,326).
Hand-hygiene promotion has been challenging for >150 years. In-service education, information leaflets, workshops and lectures, automated dispensers, and performance feedback on hand-hygiene adherence rates have been associated with transient improvement (291,294--296,306,314). Several strategies for promotion of hand hygiene in hospitals have been published (Table 9). These strategies require education, motivation, or system change. Certain strategies are based on epidemiologic evidence, others on the authors' and other investigators' experience and review of current knowledge. Some strategies may be unnecessary in certain circumstances, but may be helpful in others. In particular, changing the hand-hygiene agent could be beneficial in institutions or hospital wards with a high workload and a high demand for hand hygiene when alcohol-based hand rubs are not available (11,73,78,328). However, a change in the recommended hand-hygiene agent could be deleterious if introduced during winter, at a time of higher hand-skin irritability, and if not accompanied by the provision of skin-care products (e.g., protective creams and lotions). Additional specific elements should be considered for inclusion in educational and motivational programs (Box 2). Several strategies that could potentially be associated with successful promotion of hand hygiene require a system change (Box 1). Hand-hygiene adherence and promotion involve factors at both the individual and system level. Enhancing individual and institutional attitudes regarding the feasibility of making changes (self-efficacy), obtaining active participation of personnel at both levels, and promoting an institutional safety climate represent challenges that exceed the current perception of the role of infection-control professionals. Whether increased education, individual reinforcement technique, appropriate rewarding, administrative sanction, enhanced self-participation, active involvement of a larger number of organizational leaders, enhanced perception of health threat, self-efficacy, and perceived social pressure (12,317,329,330), or combinations of these factors can improve HCWs' adherence with hand hygiene needs further investigation. Ultimately, adherence to recommended hand-hygiene practices should become part of a culture of patient safety where a set of interdependent quality elements interact to achieve a shared objective (331). On the basis of both these hypothetical considerations and successful, actual experiences in certain institutions, strategies to improve adherence to hand-hygiene practices should be both multimodal and multidisciplinary. However, strategies must
be further researched before they are implemented.
The lack of scientific information of the definitive impact of improved hand hygiene on health-care--associated infection rates is a possible barrier to appropriate adherence with hand-hygiene recommendations (Box 1). However, evidence supports the belief that improved hand hygiene can reduce health-care--associated infection rates. Failure to perform appropriate hand hygiene is considered the leading cause of health-care--associated infections and spread of multiresistant organisms and has been recognized as a substantial contributor to outbreaks. Of nine hospital-based studies of the impact of hand hygiene on the risk of health-care--associated infections (Table 10) (48,69--75,296), the majority demonstrated a temporal relationship between improved hand-hygiene practices and reduced infection rates. In one of these studies, endemic MRSA in a neonatal intensive-care unit was eliminated 7 months after introduction of a new hand antiseptic (1% triclosan); all other infection-control measures remained in place, including the practice of conducting weekly active surveillance by obtaining cultures (72). Another study reported an MRSA outbreak involving 22 infants in a neonatal unit (73). Despite intensive efforts, the outbreak could not be controlled until a new antiseptic was added (i.e., 0.3% triclosan); all previously used control measures remained in place, including gloves and gowns, cohorting, and obtaining cultures for active surveillance. The effectiveness of a longstanding, hospitalwide program to promote hand hygiene at the University of Geneva hospitals was recently reported (74). Overall adherence to hand-hygiene guidelines during routine patient care was monitored during hospitalwide observational surveys. These surveys were conducted biannually during December 1994--December 1997, before and during implementation of a hand-hygiene campaign that specifically emphasized the practice of bedside, alcohol-based hand disinfection. Individual-sized bottles of hand-rub solution were distributed to all wards, and custom-made holders were mounted on all beds to facilitate access to hand disinfection. HCWs were also encouraged to carry bottles in their pockets, and in 1996, a newly designed flat (instead of round) bottle was made available to further facilitate pocket carriage. The promotional strategy was multimodal and involved a multidisciplinary team of HCWs, the use of wall posters, the promotion of antiseptic hand rubs located at bedsides throughout the institution, and regular performance feedback to all HCWs (see http://www.hopisafe.ch for further details on methodology). Health-care--associated infection rates, attack rates of MRSA cross-transmission, and consumption of hand-rub disinfectant were measured. Adherence to recommended hand-hygiene practices improved progressively from 48% in 1994 to 66% in 1997 (p < 0.001). Whereas recourse to handwashing with soap and water remained stable, frequency of hand disinfection markedly increased during the study period (p < 0.001), and the consumption of alcohol-based hand-rub solution increased from 3.5 to 15.4 liters per 1,000 patient-days during 1993--1998 (p < 0.001). The increased frequency of hand disinfection was unchanged after adjustment for known risk factors of poor adherence. During the same period, both overall health-care--associated infection and MRSA transmission rates decreased (both p < 0.05). The observed reduction in MRSA transmission may have been affected by both improved hand-hygiene adherence and the simultaneous implementation of active surveillance cultures for detecting and isolating patients colonized with MRSA (332). The experience from the University of Geneva hospitals constitutes the first report of a hand-hygiene campaign with a sustained improvement over several years. An additional multimodal program also yielded sustained improvements in hand-hygiene practices over an extended period (75); the majority of studies have been limited to a 6- to 9-month observation period. Although these studies were not designed to assess the independent contribution of hand hygiene on the prevention
of health-care--associated infections, the results indicate that improved hand-hygiene practices reduce the risk of transmission
of pathogenic microorganisms. The beneficial effects of hand-hygiene promotion on the risk of cross-transmission also have
been reported in surveys conducted in schools and day care centers
(333--338), as well as in a community setting
(339--341).
Fingernails and Artificial Nails Studies have documented that subungual areas of the hand harbor high concentrations of bacteria, most frequently coagulase-negative staphylococci, gram-negative rods (including Pseudomonas spp.), Corynebacteria, and yeasts (14,342,343). Freshly applied nail polish does not increase the number of bacteria recovered from periungual skin, but chipped nail polish may support the growth of larger numbers of organisms on fingernails (344,345). Even after careful handwashing or the use of surgical scrubs, personnel often harbor substantial numbers of potential pathogens in the subungual spaces (346--348). Whether artificial nails contribute to transmission of health-care--associated infections is unknown. However, HCWs who wear artificial nails are more likely to harbor gram-negative pathogens on their fingertips than are those who have natural nails, both before and after handwashing (347--349). Whether the length of natural or artificial nails is a substantial risk factor is unknown, because the majority of bacterial growth occurs along the proximal 1 mm of the nail adjacent to subungual skin (345,347,348). Recently, an outbreak of P. aeruginosa in a neonatal intensive care unit was attributed to two nurses (one with long natural nails and one with long artificial nails) who carried the implicated strains of Pseudomonas spp. on their hands (350). Patients were substantially more likely than controls to have been cared for by the two nurses during the exposure period, indicating that colonization of long or artificial nails with Pseudomonas spp. may have contributed to causing the outbreak. Personnel wearing artificial nails also have been epidemiologically implicated in several other outbreaks of infection caused by gram-negative bacilli and yeast (351--353). Although these studies provide evidence that wearing artificial nails poses an infection hazard, additional studies are warranted. Gloving Policies CDC has recommended that HCWs wear gloves to 1) reduce the risk of personnel acquiring infections from patients, 2) prevent health-care worker flora from being transmitted to patients, and 3) reduce transient contamination of the hands of personnel by flora that can be transmitted from one patient to another (354). Before the emergence of the acquired immunodeficiency syndrome (AIDS) epidemic, gloves were worn primarily by personnel caring for patients colonized or infected with certain pathogens or by personnel exposed to patients with a high risk of hepatitis B. Since 1987, a dramatic increase in glove use has occurred in an effort to prevent transmission of HIV and other bloodborne pathogens from patients to HCWs (355). The Occupational Safety and Health Administration (OSHA) mandates that gloves be worn during all patient-care activities that may involve exposure to blood or body fluids that may be contaminated with blood (356). The effectiveness of gloves in preventing contamination of HCWs' hands has been confirmed in several clinical studies (45,51,58). One study found that HCWs who wore gloves during patient contact contaminated their hands with an average of only 3 CFUs per minute of patient care, compared with 16 CFUs per minute for those not wearing gloves (51). Two other studies, involving personnel caring for patients with C. difficile or VRE, revealed that wearing gloves prevented hand contamination among the majority of personnel having direct contact with patients (45,58). Wearing gloves also prevented personnel from acquiring VRE on their hands when touching contaminated environmental surfaces (58). Preventing heavy contamination of the hands is considered important, because handwashing or hand antisepsis may not remove all potential pathogens when hands are heavily contaminated (25,111). Several studies provide evidence that wearing gloves can help reduce transmission of pathogens in health-care settings. In a prospective controlled trial that required personnel to routinely wear vinyl gloves when handling any body substances, the incidence of C. difficile diarrhea among patients decreased from 7.7 cases/1,000 patient discharges before the intervention to 1.5 cases/1,000 discharges during the intervention (226). The prevalence of asymptomatic C. difficile carriage also decreased substantially on "glove" wards, but not on control wards. In intensive-care units where VRE or MRSA have been epidemic, requiring all HCWs to wear gloves to care for all patients in the unit (i.e., universal glove use) likely has helped control outbreaks (357,358). The influence of glove use on the hand-hygiene habits of personnel is not clear. Several studies found that personnel who wore gloves were less likely to wash their hands upon leaving a patient's room (290,320). In contrast, two other studies found that personnel who wore gloves were substantially more likely to wash their hands after patient care (87,301). The following caveats regarding use of gloves by HCWs must be considered. Personnel should be informed that gloves do not provide complete protection against hand contamination. Bacterial flora colonizing patients may be recovered from the hands of <30% of HCWs who wear gloves during patient contact (50,58). Further, wearing gloves does not provide complete protection against acquisition of infections caused by hepatitis B virus and herpes simplex virus (359,360). In such instances, pathogens presumably gain access to the caregiver's hands via small defects in gloves or by contamination of the hands during glove removal (50,321,359,361). Gloves used by HCWs are usually made of natural rubber latex and synthetic nonlatex materials (e.g., vinyl, nitrile, and neoprene [polymers and copolymers of chloroprene]). Because of the increasing prevalence of latex sensitivity among HCWs and patients, FDA has approved several powdered and powder-free latex gloves with reduced protein contents, as well as synthetic gloves that can be made available by health-care institutions for use by latex-sensitive employees. In published studies, the barrier integrity of gloves varies on the basis of type and quality of glove material, intensity of use, length of time used, manufacturer, whether gloves were tested before or after use, and method used to detect glove leaks (359,361--366). In published studies, vinyl gloves have had defects more frequently than latex gloves, the difference in defect frequency being greatest after use (359,361,364,367). However, intact vinyl gloves provide protection comparable to that of latex gloves (359). Limited studies indicate that nitrile gloves have leakage rates that approximate those of latex gloves (368--371). Having more than one type of glove available is desirable, because it allows personnel to select the type that best suits their patient-care activities. Although recent studies indicate that improvements have been made in the quality of gloves (366), hands should be decontaminated or washed after removing gloves (8,50,58,321,361). Gloves should not be washed or reused (321,361). Use of petroleum-based hand lotions or creams may adversely affect the integrity of latex gloves (372). After use of powdered gloves, certain alcohol hand rubs may interact with residual powder on the hands of personnel, resulting in a gritty feeling on the hands. In facilities where powdered gloves are commonly used, various alcohol-based hand rubs should be tested after removal of powdered gloves to avoid selecting a product that causes this undesirable reaction. Personnel should be reminded that failure to remove gloves between patients may contribute to transmission of organisms (358,373). Jewelry Several studies have demonstrated that skin underneath rings is more heavily colonized than comparable areas of skin
on fingers without rings (374--376). One study found that 40% of nurses harbored gram-negative bacilli (e.g.,
E. cloacae, Klebsiella, and
Acinetobacter) on skin under rings and that certain nurses carried the same organism under their rings for
several months (375). In a more recent study involving >60 intensive care unit nurses, multivariable analysis revealed that rings were the only substantial risk factor for carriage of gram-negative bacilli and
S. aureus and that the concentration of
organisms recovered correlated with the number of rings worn
(377). Whether the wearing of rings results in greater transmission
of pathogens is unknown. Two studies determined that mean bacterial colony counts on hands after handwashing were
similar among persons wearing rings and those not wearing rings
(376,378). Further studies are needed to establish if wearing
rings results in greater transmission of pathogens in health-care settings.
Although the number of published studies concerning hand hygiene has increased considerably in recent years, many questions regarding hand-hygiene products and strategies for improving adherence of personnel to recommended policies remain unanswered. Several concerns must still be addressed
by researchers in industry and by clinical investigators (Box 3).
Additional information regarding improving hand hygiene is available at
http://www.hopisafe.ch Part II. RecommendationsCategoriesThese recommendations are designed to improve hand-hygiene practices of HCWs and to reduce transmission of pathogenic microorganisms to patients and personnel in health-care settings. This guideline and its recommendations are not intended for use in food processing or food-service establishments, and are not meant to replace guidance provided by FDA's Model Food Code. As in previous CDC/HICPAC guidelines, each recommendation is categorized on the basis of existing scientific
data, theoretical rationale, applicability, and economic impact. The CDC/HICPAC system for categorizing recommendations is
as follows: Recommendations1. Indications for handwashing and hand antisepsis
2. Hand-hygiene technique
3. Surgical hand antisepsis
4. Selection of hand-hygiene agents
5. Skin care
6. Other Aspects of Hand Hygiene
7. Health-care worker educational and motivational programs
8. Administrative measures
Part III. Performance Indicators1. The following performance indicators are recommended for measuring improvements in HCWs' hand-hygiene adherence:
References
* In a recent randomized clinical trial, surgical site infection rates were monitored among patients who were operated on by surgical personnel who cleaned their hands preoperatively either by performing a traditional 5-minute surgical hand scrub using 4% povidone-iodine or 4% antisepsis antimicrobial soap, or by washing their hands for 1 minute with a non-antimicrobial soap followed by a 5-minute hand-rubbing technique using an alcohol-based hand rinse containing 0.2% mecetronium etilsulfate. The incidence of surgical site infections was virtually identical in the two groups of patients. (Source: Parienti JJ, Thibon P, Heller R, et al. for Members of the Antisepsie Chirurgicale des Mains Study Group. Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates: a randomized equivalence study. JAMA 2002;288:722--7). Table 1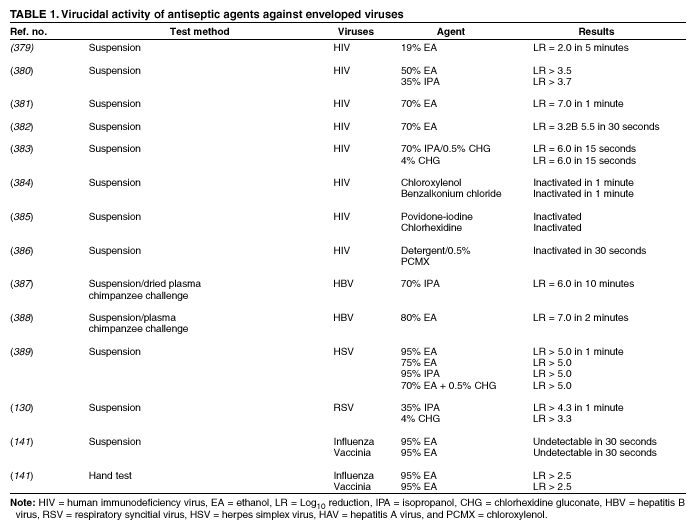 Return to top. Box 1  Return to top. Table 2 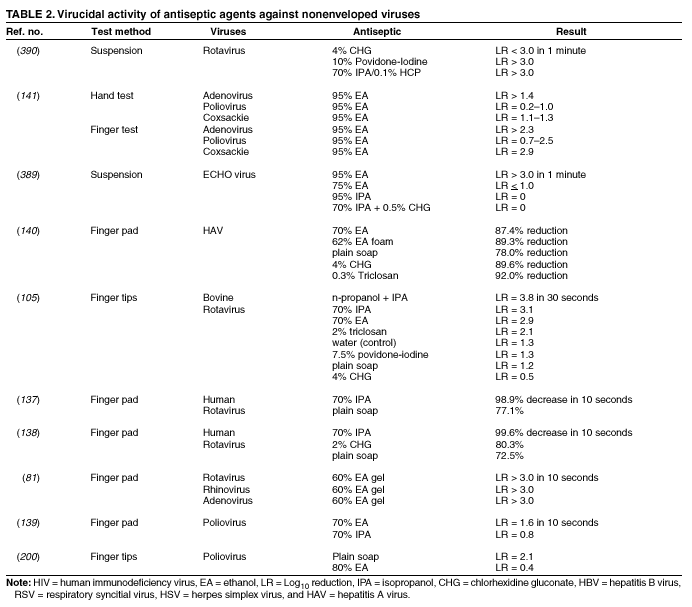 Return to top. Box 2  Return to top. Table 3 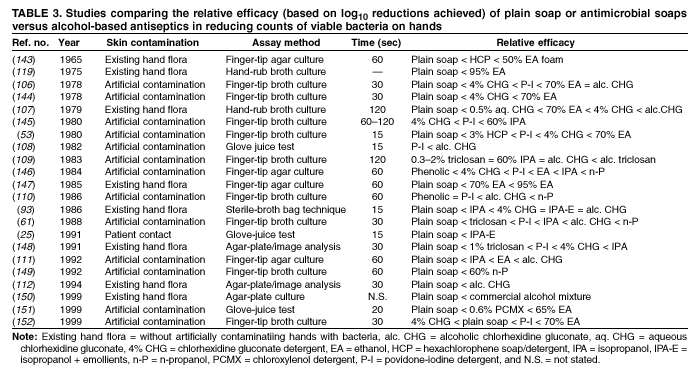 Return to top. Box 3 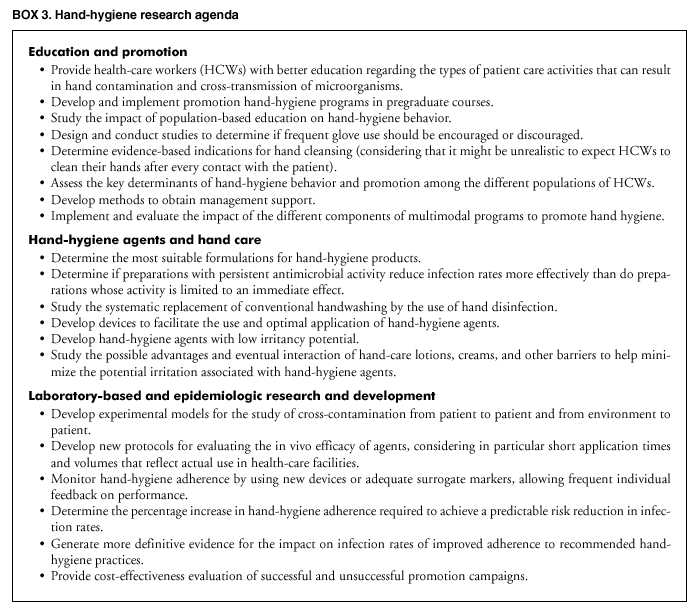 Return to top. Table 4 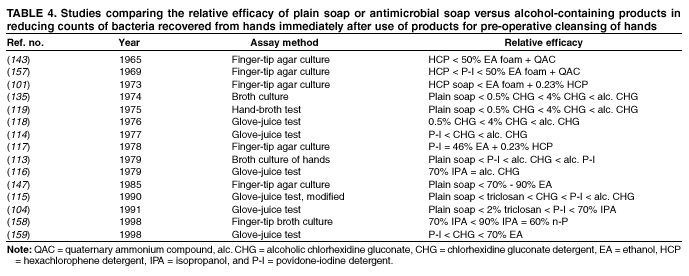 Return to top. Table 5 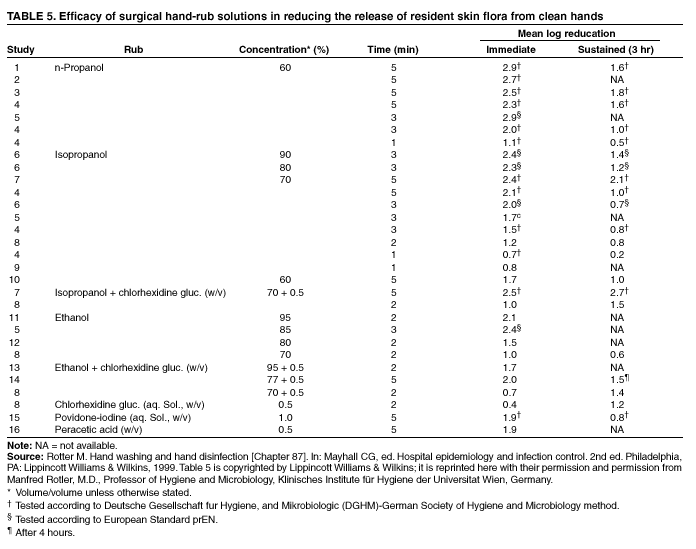 Return to top. Table 6 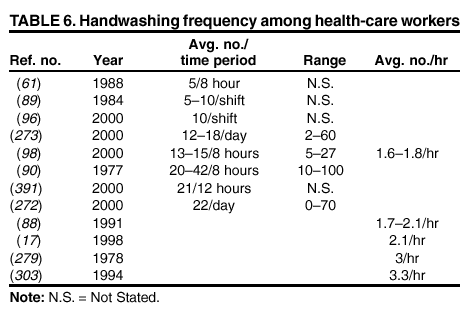 Return to top. Table 7 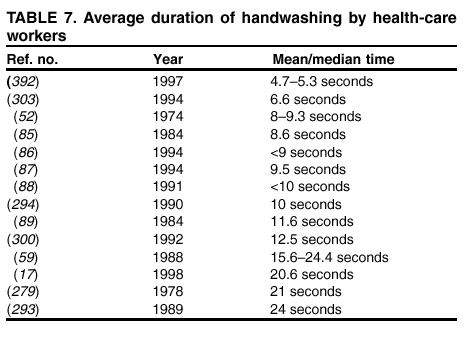 Return to top. Table 8 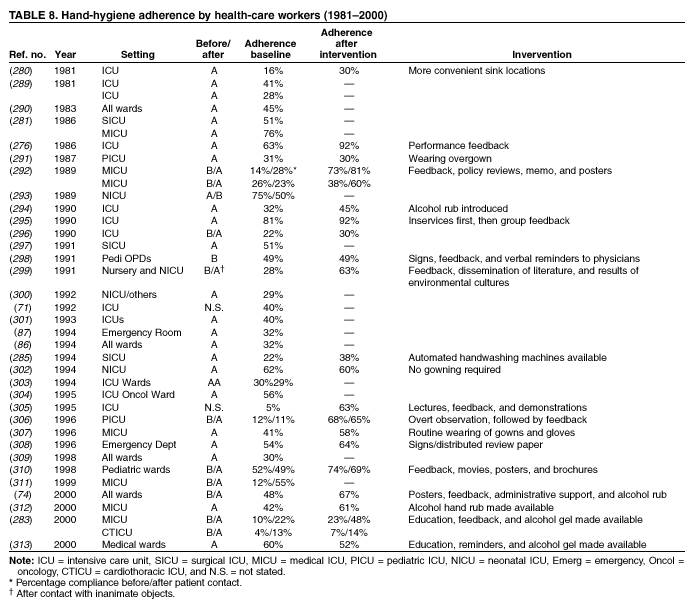 Return to top. Table 9 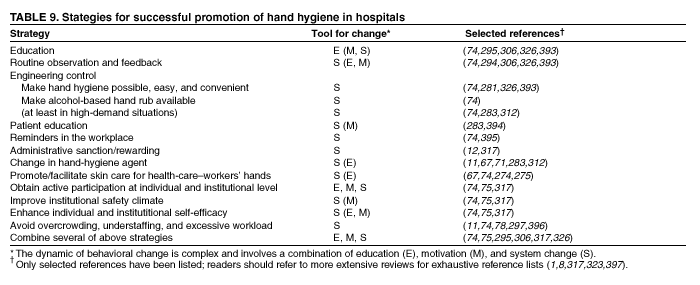 Return to top. Table 10 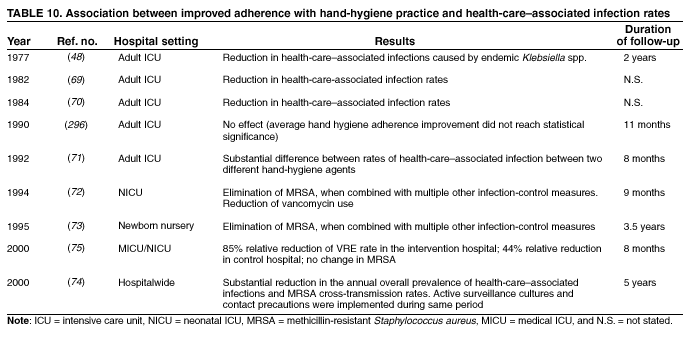 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 10/17/2002 |
|||||||||
This page last reviewed 10/17/2002
|