 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Case Definitions for Chemical PoisoningPrepared by The material in this report originated in the National Center for Environmental Health, Henry Falk, MD, Director; and the Division of Environmental Hazards and Health Effects, Michael McGeehin, PhD, Director. Corresponding preparer: Martin Belson, MD, Medical Toxicologist, Acting Team Leader, Environmental Toxins and Chemicals Team, Health Studies Branch, CDC/NCEH/DEHHE, 4770 Buford Highway, MS F-46, Atlanta, GA 30341; Telephone: 770-488-3425; Fax: 770-488-3450; E-mail: [email protected]. Summary When human illness results from an unintentional or intentional release of a toxin (chemicals produced by metabolism in an organism [e.g., ricin]) or a toxicant (natural or synthetic chemicals not metabolically produced by an organism [e.g., nerve agents]) into the environment, uniform reporting is necessary to direct appropriate resources, assess the extent of morbidity and mortality, track poisoned persons, and monitor response to intervention. In this report, CDC presents case definitions to facilitate uniform reporting among local, state, and federal public health agencies of illness resulting from a chemical release. The report also explains the rationale for the structure of the case definitions, the audience for whom it is intended, the setting in which the case definitions might be used, and reasons each chemical presented in the report was selected. Clinical knowledge and diagnostic tools (e.g., biologic laboratory tests) for detecting chemical poisoning are likely to improve over time. CDC will create new case definitions and revise existing definitions to meet the needs related to emerging threats and to enhance case definition sensitivity and specificity, when possible, with developing clinical information. IntroductionToxins are chemicals that are produced by organisms as a result of cellular metabolism (e.g., marine toxins such as saxitoxin or plant toxins such as ricin). Toxicants are synthetic (i.e., manufactured) or naturally found chemicals that are not produced by organisms as a result of cellular metabolism (e.g., nerve agents or arsenic). When illness results from an intentional or unintentional chemical release (either known or suspected on the basis of a credible threat) into the environment, uniform reporting is paramount to direct appropriate resources, assess the extent of morbidity and mortality, track poisoned persons, and monitor response to intervention. In this report, CDC presents case definitions to facilitate uniform reporting of illness resulting from a chemical (i.e., toxin and toxicant) release. How This Report Is OrganizedThe report provides an overview of 1) the settings in which the case definitions might be used, 2) the structure of the case definitions, 3) the rationale for choosing the particular chemicals, and 4) plans for revising the report. A list and description of the terms used in the report are also provided. In addition, case definitions, which include reference citations, are presented for the selected chemicals. How To Use the Information in This ReportThe case definitions in this report should be used by clinicians and public health officials in two settings: 1) after a credible threat of a chemical release or 2) after a known chemical release. The list of chemicals that have the potential for use as a terrorist weapon is extensive, and clinical presentation of poisoning from chemicals can be similar to that of common diseases (e.g., gastroenteritis). Therefore, use of these case definitions as a surveillance tool, in the absence of a credible threat or a known chemical release, typically results in excessive false-positive reports and is not recommended by CDC. Case definitions are not sufficient for establishing a medical diagnosis and should not be relied upon to initiate therapy. They are also not meant to be used for persons who are exposed to a chemical agent but remain asymptomatic. Clinical manifestations of poisonings might vary as a result of interindividual differences (e.g., previous medical history, genetic differences, sex, or age), route of exposure, amount and duration of exposure, and length of time since the exposure. In addition, simultaneous exposure to >2 chemicals can result in symptoms that are not typical for either agent alone. Use of additional clinical, epidemiologic, and laboratory data might enable a physician to make a medical diagnosis, although the formal surveillance case definition might not be met. Health-care providers should report suspect cases of intentional chemical exposure to their local poison-control center and to a public health agency. Local and state public health officials should notify CDC and law enforcement officials if they identify persons who might have been exposed to intentional chemical poisoning. Structure of the Case DefinitionCDC modeled the structure of the chemical poisoning case definitions in this report after the infectious disease case definitions that were previously developed by CDC and the Council of State and Territorial Epidemiologists (CSTE) (1,2). However, case definitions for chemical poisoning were modified to address the clinical and diagnostic challenges unique to chemical poisoning. A description of terminology used in the case definitions is presented in this report. Each case definition is composed of three sections: 1) clinical description, 2) laboratory criteria for diagnosis, and 3) case classification. Individual case definitions differ in the structure of the clinical description and the laboratory criteria for diagnosis. However, for all case definitions, the clinical description and the laboratory criteria for diagnosis will determine the case classification. CDC used an algorithmic method to determine the structure of the clinical description and the laboratory criteria and to determine how the user might classify a case by using the case definition (Figure). For case classifications, a case that is being considered as a chemical poisoning case is categorized as "suspected," "probable," or "confirmed." A suspected case is one in which any potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent; however, no specific credible threat exists. A probable case is 1) one in which a person has an illness that is clinically compatible with poisoning from a particular chemical agent and in which a credible threat exists (e.g., clinically compatible illness in an employee of a facility where a specific threat of a chemical release is made) or 2) one in which epidemiologic data link the person to a confirmed case (e.g., clinically compatible illness in a person who was at the same location as the subject of a case confirmed by biologic or environmental testing). A confirmed case is one in which a suspected or a probable case of exposure has been substantiated with laboratory testing of environmental or biologic specimens. One of the key elements in determining whether a potentially exposed person will be categorized as a suspected case-patient or a probable case-patient is deciding whether the person's illness is clinically compatible with exposure to a particular chemical. Providing the user of these case definitions with a specific set of clinical criteria (i.e., clinical criteria that objectively allow the user to decide whether the case is clinically compatible) is often not possible, because manifestations of chemical poisonings can vary on the basis of individual differences of the exposed persons (e.g., previous medical history, genetic differences, sex, or age), route of exposure, amount and duration of exposure, and length of time since the exposure. Therefore, the structure of the clinical description includes multiple possible clinical manifestations. If a valid laboratory test is available to confirm the exposure for a particular agent (e.g., cyanide), the clinical description summarizes the most notable features of acute poisoning from that particular chemical, on the basis of the medical literature. If no available or valid laboratory method is available to detect the chemical in biologic or environmental specimens, the case will never be confirmed and will remain either in the suspected or probable category. Therefore, making an association between the clinical presentation and the suspected agent will primarily depend on the clinical description and the presence of a credible threat. For these agents (e.g., tetrodotoxin), the clinical description of the case definition includes specific criteria for clinical compatibility (including nonconfirmatory or nonspecific laboratory parameters [e.g., electrolytes and renal function tests]) that should be met before a case can be categorized as suspected or probable for chemical poisoning. Medical toxicologists and epidemiologists at CDC used clinical information from the literature on each agent to develop the specific criteria included in the clinical description for that agent. However, CDC recognizes that the criteria do not provide positive or negative predictive value for confirming or excluding poisoning from a particular chemical. In certain instances, suspected or probable cases might exist for which laboratory (biologic or environmental) testing was not performed by the clinician or public health official. Reasons for not performing laboratory testing might include a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical or a 100% certainty of the etiology of the agent, as might be the case with agricultural workers who are known to have been exposed to a particular fumigant and who then develop clinically compatible illness. For example, in the case of a Bulgarian dissident reported to have been poisoned with ricin, no laboratory confirmation ever occurred (3). If the case definitions in this report are strictly followed, this case might never be a confirmed case, although a predominant amount of evidence existed for ricin poisoning, and ricin poisoning is accepted as the cause of death. This case and similar scenarios may be considered as confirmed. A suspected or probable case can become a confirmed case when excess exposure is verified by laboratory evidence (i.e., levels above the 95th percentile in CDC population studies or above a reference range). Laboratory evidence can be obtained from either biologic specimens (e.g., blood or urine) or environmental samples (e.g., water, air, soil, or a contaminated product such as food). Testing for chemicals in either environmental or biologic specimens is not universally available. In addition, results from field tests conducted by using hand-held assays intended for screening environmental samples and research tests are not considered confirmatory. CDC recommends that laboratory testing be used in conjunction with a state or CDC public health investigation for confirming exposure only when a valid laboratory test is available through 1) commercial resources, 2) the Laboratory Response Network (LRN), or 3) one of the following federal agencies (Appendix):
LRN includes multiple state laboratories capable of identifying select microbiologic agents, but only a limited number of state laboratories are capable of testing biologic specimens for chemical warfare agents. Data for validation of commercially available analyses of certain chemicals in either biologic or environmental samples might be difficult for nonlaboratorians to access. If an intentional release occurs, CDC personnel will be able to advise local and state public health partners on whether valid analyses for biologic samples for specific chemicals exist. However, CDC does not provide guidance concerning commercial laboratory methods; for guidance regarding environmental or food samples, consultation with EPA and FDA is recommended. Laboratorians should ask their referral laboratories to provide confirmation that a method is analytically valid for precision, detection limits, and accuracy. Laboratorians should also ask their laboratories to confirm whether applications are environmental or clinical, for example. A chemical agent probably will be detected in biologic specimens in traceable quantities in the absence of clinical findings. However, signs and symptoms consistent with poisoning should develop before an exposed person is considered a case-patient. Because timely laboratory confirmation might not be available, clinicians should not wait for laboratory verification to report suspected or probable cases to appropriate public health agencies. Early involvement of public health agencies will enable monitoring of trends, detection of covert events in multiple locations, mobilization of resources (e.g., National Pharmaceutical Stockpile, laboratory resources, or legal investigation), and containment of further exposure. State health departments should continue to promptly report suspected cases to CDC, and records should be updated with the appropriate classification status when additional surveillance information becomes available. Chemicals with Potential for Terrorist Use and Plans for Revision of This ReportThe substantial number of chemicals with potential for terrorist use precludes the development of a case definition for each possible agent. Therefore, certain agents with a potential for use as a terrorist weapon are not included in this report. Medical toxicologists at CDC's NCEH chose the chemicals presented in this report on the basis of knowledge of their accessibility, deliverability, lethality, potential to cause social disruption, or historic use. In certain cases, a category of agents with similar properties is represented (e.g., caustics/corrosives). This report underwent an extensive review process by CDC's Office of Security and Emergency Preparedness and Office of Terrorism Preparedness and Emergency Response, and by CDC's stakeholders (e.g., FDA, EPA, and CSTE). This report is designed to be updated and revised as new information becomes available. CDC plans to compose, in conjuction with state public health agencies and other organizations (e.g., FDA or EPA), new case definitions and revise existing definitions to reflect information concerning emerging threats and agents, improvements in diagnostic technology, and increasing clinical knowledge regarding a particular chemical. In addition, when a chemical is released or the threat of a release exists, CDC will review literature regarding the implicated chemical and might update the case definition. The most up-to-date versions of case definitions and other public health documents will be posted on CDC's Emergency Preparedness and Response Internet site (http://www.bt.cdc.gov/agent/agentlistchem.asp). Terms Used in This ReportClinically compatible case. A case in which a person has signs and symptoms compatible with poisoning by a particular agent. Epidemiologically linked case. A case that meets one of the following criteria:
Or,
Valid laboratory test. A biologic laboratory test that has been analytically, and in part, clinically validated. A test should be considered valid before it can be considered confirmatory. Analytical validation requires development of a definable and repeatable calibration-response relationship (e.g., linearity), demonstration studies of accuracy and imprecision, interference testing, and establishment of the limits of detection. Minimal clinical validation might include previous application to human situations and an understanding of background levels in noncases. Further clinical validation should include estimates of prevalence at known thresholds; studies of applied sensitivity, specificity, and predictive value; and demonstration of concentration-effect relationships. For clinical laboratories, the individual laboratory, in conjunction with guidelines established by the Clinical Laboratory Improvement Act, is responsible for ensuring validation. For environmental laboratories, the typical requirements for competence of testing are set by the International Organization for Standardization (IOS Standard 17025). Commercially available test. A test that is available to health investigators through either fee-for-service pathways or state public health and LRN laboratories that satisfy validation requirements. Typically, commercial regional laboratories can assist with only a limited number of the chemical measurements given in the case definitions (e.g., blood cyanide). Laboratory confirmation. Laboratory evidence of exposure (i.e., levels above known background levels) either through a biologic specimen (e.g., blood or urine) or environmental samples (e.g., samples of water, air, soil, or a contaminated product such as food). A valid laboratory test should be available commercially, through federal agencies (i.e., CDC, FDA, or EPA), or through LRN. Suspected case. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable case. A clinically compatible case in which a high index of suspicion (i.e., a credible threat) exists for exposure to a particular agent, or a case with an epidemiologic link to a laboratory-confirmed case. Confirmed case. A clinically compatible case with laboratory confirmation by using either biologic or environmental samples. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Case Definitions for Potential Terrorism Agents: Toxins and Toxicants*Adamsite (Diphenylaminechloroarsine or DM)Clinical DescriptionThe majority of exposures occur by inhalation and typically lead to symptoms of ocular, nasal, and respiratory tract irritation. Nonspecific gastrointestinal symptoms (e.g., vomiting or diarrhea) might also occur. The effects of adamsite poisoning take minutes to begin and might last for hours (4). If a rapid onset of manifestations of one of the following respiratory effects occurs, the clinical description for adamsite poisoning has been met: nose or throat irritation, cough, or dyspnea. Laboratory Classification for DiagnosisBiologic. No biologic marker is available for adamsite exposure. Environmental. No method is available to detect adamsite in environmental samples. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for adamsite exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests (not available for adamsite) have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. AmmoniaClinical DescriptionThe majority of exposures occur by inhalation and typically lead to symptoms of ocular, nasal, and respiratory irritation. Signs and symptoms of poisoning might include eye redness and lacrimation, nose and throat irritation, cough, suffocation or choking sensation, and dyspnea (5--7). Laboratory Criteria for DiagnosisBiologic. No biologic marker is available for ammonia exposure. Environmental. Detection of ammonia in environmental samples, as determined by NIOSH. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for ammonia exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests of environmental samples have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Arsenic (Inorganic)Clinical DescriptionAcute ingestion of toxic amounts of inorganic arsenic typically causes severe gastrointestinal signs and symptoms (e.g., vomiting, abdominal pain, and diarrhea). These signs and symptoms might rapidly lead to dehydration and shock. Different clinical manifestations might follow, including dysrhythmias (prolonged QT, T-wave changes), altered mental status, and multisystem organ failure that might ultimately result in death (8--11). Laboratory Criteria for DiagnosisBiologic. A case in which elevated urinary arsenic levels (>50 µg/L for a spot or >50 µg total for a 24-hour urine) exist, as determined by commercial laboratory tests. Speciation is required in all cases where total urine arsenic is elevated to differentiate the amount of organic and inorganic arsenic. Or, Environmental. Detection of arsenic in environmental samples above typical background levels, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for arsenic exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. ArsineClinical DescriptionInhalation of arsine gas causes no immediate symptoms. Signs and symptoms occur 2--24 hours after exposure and result from massive hemolysis. These signs and symptoms include generalized weakness, dark urine, jaundice, and dyspnea. Oliguria and renal failure often occur 1--3 days after exposure (12--14). Laboratory Criteria for DiagnosisBiologic. No specific test is available for arsine exposure; however, exposure to arsine might be indicated by detection of elevated arsenic levels in urine (>50 µg/L for a spot or >50 µg for a 24-hour urine) and signs of hemolysis (e.g., hemoglobinuria, anemia, or low haptoglobin). Environmental. Detection of arsine in environmental samples, as determined by NIOSH. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for arsine exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. BariumClinical Description Ingestion of certain forms of barium (e.g., barium carbonate or barium fluoride) in toxic amounts leads to gastrointestinal symptoms (e.g., vomiting, abdominal pain, and watery diarrhea). Within 1--4 hours of ingestion, profound hypokalemia develops in certain instances, and potassium levels <1.0 mmol/L are associated with generalized muscle weakness that might progress to paralysis of the limbs and respiratory muscles (15--19). Barium sulfate is not absorbed when taken by mouth and is therefore commonly used as a contrast agent for radiographic procedures. Laboratory Criteria for Diagnosis Biologic. A case in which an elevated spot urine barium level (>7 µg/L) exists (20), as determined by commercial laboratory tests. Or, Environmental. Elevation of barium compounds in environmental samples, as determined by NIOSH or FDA. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for barium exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. BrevetoxinClinical Description After oral ingestion, brevetoxin poisoning is characterized by a combination of gastrointestinal and neurologic signs and symptoms. The incubation period ranges from 15 minutes to 18 hours. Gastrointestinal symptoms include abdominal pain, vomiting, and diarrhea. Neurologic symptoms include paresthesias, reversal of hot and cold temperature sensation, vertigo, and ataxia. Inhalational exposure to brevetoxin results in cough, dyspnea, and bronchospasm (21--24). Laboratory Classification for Diagnosis Biologic. Brevetoxin can be detected by an enzyme-linked immunosorbent assay (ELISA) method in biologic samples; however, ELISA of biologic samples is not a certified method for detection of brevetoxin. Environmental. Any concentration of brevetoxin in environmental samples (25), as detected by a commercial laboratory. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for brevetoxin exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. BromineClinical Description The majority of exposures to bromine occur by inhalation and typically lead to symptoms of ocular, nasal, and respiratory irritation. Signs and symptoms of poisoning include eye redness and lacrimation, nose and throat irritation, cough, and dyspnea. Ingestion of liquid bromine can cause abdominal pain and hemorrhagic gastroenteritis with secondary shock. Signs and symptoms might also include brown discoloration of mucous membranes and the tongue (26,27). Laboratory Criteria for Diagnosis Biologic. No specific test for bromine is available; however, detection of elevated bromide levels in serum (reference level is 50--100 mg/L) might indicate that an exposure has occurred. Environmental. Detection of bromine in environmental samples, as determined by NIOSH. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for bromine exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. 3-Quinuclidinyl Benzilate (BZ)Clinical Description BZ toxicity, which might occur by inhalation, ingestion, or skin absorption, is an anticholinergic syndrome consisting of a combination of signs and symptoms that might include hallucinations; agitation; mydriasis (dilated pupils); blurred vision; dry, flushed skin; urinary retention; ileus; tachycardia; hypertension; and elevated temperature (>101ºF). The onset of incapacitation is dose-dependent. It might occur as early as 1 hour after exposure and continue up to 48 hours (28). Laboratory Criteria for Diagnosis Biologic. A case in which BZ is detected in urine (29), as determined by CDC. Environmental. No method is available for detecting BZ in environmental samples. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for BZ exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on biologic samples have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Carbon MonoxideClinical Description The predominant manifestations of carbon monoxide poisoning are cardiovascular and neurologic effects. Inhalation of carbon monoxide gas typically leads to headache, dizziness, and confusion, which might progress to dyspnea, tachypnea, syncope, and metabolic acidosis (30--32). Laboratory Criteria for Diagnosis Biologic. A case in which carboxyhemoglobin concentration exists >5% in venous or arterial blood in nonsmokers and >10% in smokers, as determined by hospital or commercial laboratory tests. The typical range of carboxyhemoglobin concentrations in smokers is 6%--10% (32). Environmental. No confirmatory test is available for carbon monoxide in environmental samples. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for carbon monoxide exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on biologic samples have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Caustic or Corrosive AgentsClinical Description Ingestion of caustic or corrosive agents (e.g., phosphoric acid or sulfuric acid) can cause direct injury to tissue upon exposure, which might lead to the following signs and symptoms: oral pain, ulcerations, drooling, dysphagia, vomiting, and abdominal pain. Dermal and ocular exposure might result in local irritation or burn injury. Inhalation of corrosive gases might result in upper and lower respiratory irritation, leading to stridor, dyspnea, wheezing, and pulmonary edema (33--36). Laboratory Criteria for Diagnosis Biologic. No biologic marker for exposure to a caustic or corrosive agent is available. Environmental. Detection of caustic or corrosive agents in environmental samples, as determined by NIOSH or FDA. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for a caustic exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. ChlorineClinical Description The majority of exposures occur by inhalation and typically lead to symptoms of ocular, nasal, and respiratory irritation. Signs and symptoms of poisoning might include eye redness and lacrimation, nose and throat irritation, cough, suffocation or choking sensation, and dyspnea. For cutaneous exposures, burning, blistering, and frostbite injury to the skin are possible (37,38). Laboratory Criteria for Diagnosis Biologic. No biologic marker for chlorine exposure is available. Environmental. Detection of chlorine in environmental samples, as determined by NIOSH. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for chlorine exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. ColchicineClinical Description Ingestion of colchicine typically leads to profuse vomiting and diarrhea, which can be bloody, followed by hypovolemic shock and multisystem organ failure within 24--72 hours. Coma, convulsions, and sudden death might also occur. Subsequent complications include bone marrow suppression with resultant leukopenia, thrombocytopenia (nadir in 4--7 days), and possibly sepsis (39). Laboratory Criteria for Diagnosis Biologic. A case in which colchicine is detected in urine, serum, or plasma (40), as determined by a commercial laboratory. Or, Environmental. Detection of colchicine in environmental samples, as determined by FDA. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for colchicine exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. CyanideClinical Description Inhalation of cyanide gas or ingestion of cyanide salts typically leads to lethargy or coma (possibly sudden collapse), dyspnea, tachypnea, tachycardia, and hypotension. Severe poisoning results in bradypnea, bradycardia, cardiovascular collapse, and death. Nonspecific laboratory findings include metabolic and lactic acidosis (41--43). Laboratory Criteria for Diagnosis Biologic. A case in which cyanide concentration is higher than the normal reference range (0.02--0.05 µg/mL) in whole blood (43), as determined by a commercial laboratory. Or, Environmental. Detection of cyanide in environmental samples, as determined by NIOSH or FDA. Case Classification Suspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for cyanide exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. DigitalisClinical Description Signs and symptoms of acute digitalis (digoxin or digitoxin) poisoning by ingestion include primarily gastrointestinal effects (nausea and vomiting), hyperkalemia, and cardiovascular effects (bradydysrhythmias [heart rate <60 or atrioventricular block] or tachydysrhythmias [ventricular tachycardia/fibrillation or atrial tachycardia with 2:1 block]) (44--46). Laboratory Criteria for DiagnosisBiologic. A case in which digitalis in serum samples is detected, as determined by a commercial laboratory.
Or, Environmental. Detection of digitalis in environmental samples, as determined by FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for digitalis exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Elemental White or Yellow PhosphorusClinical DescriptionIngestion of elemental white or yellow phosphorus typically causes severe vomiting and diarrhea, which are both described as "smoking," "luminescent," and having a garlic-like odor. Other signs and symptoms of severe poisoning might include dysrhythmias, coma, hypotension, and death. Contact with skin might cause severe burns within minutes to hours (48--51). Laboratory Criteria for DiagnosisBiologic. No specific test for elemental white or yellow phosphorus is available; however, an elevated serum phosphate level might indicate that an exposure has occurred. Although phosphate production is a by-product of elemental phosphorus metabolism in humans, a normal phosphate concentration does not rule out an elemental phosphorus exposure. Environmental. Detection of elemental phosphorus in environmental samples, as determined by NIOSH, and an elevated phosphorus level in food, as determined by FDA, might also indicate that an exposure has occurred. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for elemental white or yellow phosphorus exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Hydrofluoric AcidClinical DescriptionDepending on the concentration of a dermal exposure, affected skin can initially look completely normal but often will become painful and appear pale or white, possibly leading to necrosis. Inhalational poisoning might result in dyspnea, chest pain, stridor, and wheezing. Oral poisoning can result in vomiting (possibly bloody), abdominal pain, and bloody diarrhea (52--54). Systemic poisoning might occur after oral, dermal, or inhalational exposure. Systemic signs and symptoms include hypocalcemia and hyperkalemia, which leads to dysrhythmias, seizures, and possibly death. Laboratory Classification for DiagnosisBiologic. No specific test for hydrofluoric acid is available; however, hypocalcemia, hyperkalemia, and an elevated concentration of fluoride in the serum might indicate that an exposure has occurred. Normal serum fluoride levels are <20 mcg/L, but levels vary substantially on the basis of dietary intake and environmental levels. Environmental. Detection of hydrofluoric acid in environmental samples, as determined by NIOSH. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for hydrofluoric acid exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Long-Acting Anticoagulant (Super Warfarin)Clinical DescriptionAfter an acute unintentional ingestion of a long-acting anticoagulant, the majority of patients are entirely asymptomatic. After a substantial ingestion of a long-acting anticoagulant, clinical signs of coagulopathy typically occur within 24--72 hours postexposure. Coagulopathy might manifest as epistaxis, gingival bleeding, hematemesis, hematuria, hematochezia, menometrorrhagia, ecchymosis, petechial hemorrhages, intracranial hemorrhages, or bleeding that is not in proportion with the level of the injury (55--57). Laboratory Criteria for DiagnosisBiologic. The criteria for diagnosis of a long-acting anticoagulant is the presence of one of the following factors:
Or, Environmental. Detection of a long-acting anticoagulant in environmental samples, as determined by FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for a long-acting anticoagulant exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Mercury (Elemental)Clinical DescriptionInhalation exposure is the most typical route of elemental mercury toxicity. Acute toxicity might result in fever, fatigue, and clinical signs of pneumonitis. Chronic exposure results in neurologic, dermatologic, and renal manifestations. Signs and symptoms might include neuropsychiatric disturbances (e.g., memory loss, irritability, or depression), tremor, paresthesias, gingivostomatitis, flushing, discoloration and desquamation of the hands and feet, and hypertension (58--61). Laboratory Criteria for DiagnosisBiologic. A case in which elevated urinary or whole blood mercury levels (>10 µg/L) (20,58) exist, as determined by a commercial laboratory. No definitive correlation exists between either blood or urine mercury levels and mercury toxicity. Or, Environmental. Detection of mercury in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for elemental mercury exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Mercury (Inorganic)Clinical DescriptionIngestion is the most typical route of exposure to cause toxicity from inorganic mercury. Signs and symptoms might include profuse vomiting and diarrhea that is often bloody, followed by hypovolemic shock, oliguric renal failure, and possibly death. Survivors of acute poisoning or persons chronically exposed to inorganic mercury might develop neurologic, dermatologic, and renal manifestations that might include neuropsychiatric disturbances (e.g., memory loss, irritability, or depression), tremor, paresthesias, gingivostomatitis, flushing, discoloration and desquamation of the hands and feet, and hypertension (58,61,62). Laboratory Criteria for DiagnosisBiologic. A case in which elevated urinary or whole blood mercury levels (>10 µg/L) (20,58) exist, as determined by a commercial laboratory. No definitive correlation exists between either blood or urine mercury levels and mercury toxicity. Or, Environmental. Detection of mercury in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for inorganic mercury exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Mercury (Organic)Clinical DescriptionAlthough ingestion of organic mercury is the most typical route of organic mercury toxicity, toxicity might also result from inhalation and dermal exposures, particularly with dimethylmercury. Symptoms of toxicity are typically delayed for >1 month after organic mercury exposure and usually involve the central nervous system. These symptoms might include paresthesias, headaches, ataxia, dysarthria, visual field constriction, blindness, and hearing impairment (58,63--66). Laboratory Criteria for DiagnosisBiologic. A case in which whole blood mercury levels (>10 µg/L) (20,58) are detected, as determined by a commercial laboratory. Urine mercury levels are not useful in evaluating organic mercury poisoning. Or, Environmental. Detection of mercury in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for organic mercury exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Methyl BromideClinical DescriptionMethyl bromide poisoning primarily occurs after inhalational exposure, but concurrent dermal exposure might also occur. Methyl bromide is an ocular, dermal, and mucous membrane irritant. Onset of symptoms might be delayed 1--48 hours. Symptoms of inhalational exposure are typically cough and dyspnea, which can develop into pneumonitis and pulmonary edema but might be delayed up to 4--5 days. Severe poisoning can result in seizures, coma, and death (67--71). Laboratory Criteria for DiagnosisBiologic. No specific test for methyl bromide is available; however, detection of elevated bromide levels in serum (reference level: 50--100 mg/L) might indicate that an exposure has occurred. Detection of bromide below toxic levels does not rule out methyl bromide poisoning. Or, Environmental. Detection of methyl bromide in environmental samples, as determined by NIOSH. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for a methyl bromide exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Methyl IsocyanateClinical DescriptionExposure to methyl isocyanate typically occurs through inhalation or dermal absorption. Toxicity might develop over 1--4 hours after exposure. Signs and symptoms of methyl isocyanate typically include cough, dyspnea, chest pain, lacrimation, eyelid edema, and unconsciousness. These effects might progress over the next 24--72 hours to include acute lung injury, cardiac arrest, and death (72--75). Laboratory Criteria for DiagnosisBiologic. No biologic marker for methyl isocyanate exposure is available. Environmental. Detection of methyl isocyanate in environmental samples, as determined by NIOSH. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for methyl isocyanate exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Nerve Agents or OrganophosphatesClinical DescriptionNerve agent or organophosphate toxicity might result from multiple routes of exposure and is a cholinergic syndrome consisting of excess respiratory and oral secretions, diarrhea and vomiting, diaphoresis, convulsions, altered mental status, miosis, bradycardia, and generalized weakness that can progress to paralysis and respiratory arrest (76--78). In certain cases, excessive autonomic activity from stimulation of nicotinic receptors will offset the cholinergic syndrome and will include mydriasis, fasciculations, tachycardia, and hypertension. Laboratory Criteria for DiagnosisBiologic. A case in which nerve agents in urine are detected, as determined by CDC or one of five LRN laboratories that have this capacity. Decreased plasma or red blood cell cholinesterase levels based on a specific commercial laboratory reference range might indicate a nerve agent or organophosphate exposure; however, the normal range levels for cholinesterase are wide, which makes interpretation of levels difficult without a baseline measurement or repeat measurements over time. Or, Environmental. Detection of organophosphate pesticides in environmental samples, as determined by FDA. However, a confirmation test for nerve agents in environmental samples is not available. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for nerve agent or organophosphate pesticide exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. NicotineClinical DescriptionAfter oral ingestion of nicotine, signs and symptoms of nicotine poisoning mimic those for nerve agent or organophosphate poisoning and typically include excess oral secretions, bronchorrhea, diaphoresis, vomiting (common, especially among children), diarrhea, abdominal cramping, confusion, and convulsions. Although tachycardia and hypertension are common, bradycardia and hypotension might also occur as a result of a severe poisoning (79,80). Laboratory Criteria for DiagnosisBiologic. A case in which increased nicotine or cotinine (the nicotine metabolite) is detected in urine, or increased serum nicotine levels occur, as determined by a commercial laboratory or CDC. Or, Environmental. Detection of nicotine in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for nicotine exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Opioids (Fentanyl, Etorphine, or Others)Clinical DescriptionExposure to opioids typically occurs through ingestion but potentially can result from inhalation, if opioids are aerosolized. Clinical effects of opioid poisoning result from central nervous system and respiratory system depression manifesting as lethargy or coma, decreased respiratory rate, miosis, and possibly apnea (81,82). Laboratory Criteria for DiagnosisBiologic. A case in which opioids are detected in urine, as determined by hospital or commercial laboratory tests. Fentanyl derivatives and certain other synthetic opioids (e.g., oxycodone) might not be detected by routine toxicologic screens. Or, Environmental. Detection of opioids in environmental samples, as determined by FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for opioid exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. ParaquatClinical DescriptionIngestion of paraquat typically results in gastrointestinal illness, including oropharyngeal ulcerations, vomiting, and diarrhea, which might contain blood. Patients might have dyspnea and hemoptysis as a result of pulmonary edema or hemorrhage, which can progress to fibrosis over the course of days to weeks (83--85). Laboratory Criteria for DiagnosisBiologic. A case in which paraquat in urine, plasma, or serum is detected, as determined by a commercial laboratory. Or, Environmental. Detection of paraquat in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for paraquat exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. PhosgeneClinical DescriptionThe majority of exposures to phosgene occur by inhalation. In high concentrations, exposure might lead to symptoms of ocular, nasal, and throat irritation. Lower respiratory irritation is the most consistent finding after phosgene exposure. If one of the following lower respiratory signs and symptoms is reported, the clinical description for phosgene poisoning has been met (86,87): chest tightness or cough, dyspnea, or pulmonary edema, which might be delayed <48 hours after exposure. Laboratory Criteria for DiagnosisBiologic. No biologic marker exists for phosgene exposure. Environmental. Confirmation of phosgene in environmental samples is not available. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for phosgene exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests (not available for phosgene) have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. PhosphineClinical DescriptionThe majority of exposures to phosphine occur by inhalation. Severe poisoning might result in multiorgan involvement (e.g., convulsions, cardiac dysrhythmias, and shock). If one of the following lower respiratory signs and symptoms is reported, the clinical description for phosphine poisoning has been met (88--91): chest tightness or cough, dyspnea, or pulmonary edema, which might have a delayed onset. Laboratory Criteria for DiagnosisBiologic. No biologic marker for phosphine exposure is available. Finding measurable amounts of urinary phosphorus and phosphorus-containing compounds is not a reliable indicator of exposure. Environmental. Confirmation of phosphine in environmental samples is not available. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for phosphine exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests (not available for phosphine) have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Ricin (Ingestion)Clinical DescriptionIngestion of ricin typically leads to profuse vomiting and diarrhea, which might be bloody, followed by hypovolemic shock and multisystem organ dysfunction. Weakness and influenza-like symptoms, fever, myalgia, and arthralgia, might also be reported (92--95). Laboratory Criteria for DiagnosisBiologic. CDC can assess selected specimens on a provisional basis for urinary ricinine, an alkaloid in the castor bean plant. Only urinary ricinine testing is available at CDC for clincial specimens. Or, Environmental. Detection of ricin in environmental samples, as determined by CDC or FDA. Ricin can be detected qualitatively by time-resolved fluoroimmunoassay (TRFIA) and polymerase chain reaction (PCR) in environmental specimens (e.g., filters, swabs, or wipes). Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for ricin exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Ricin (Inhalation)Clinical DescriptionInhalation of ricin typically leads to cough and respiratory distress followed by pulmonary edema, respiratory failure, and multisystem organ dysfunction. Weakness and influenza-like symptoms of fever, myalgia, and arthralgia might also be reported (92--95). Laboratory Criteria for DiagnosisBiologic. CDC can assess selected specimens on a provisional basis for urinary ricinine, an alkaloid in the castor bean plant. Only urinary ricinine testing is available at CDC for clincial specimens. Or, Environmental. Detection of ricin in environmental samples, as determined by CDC or FDA. Ricin can be detected qualitatively by TRFIA and PCR in environmental specimens (e.g., filters, swabs, or wipes). Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for ricin exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Riot-Control AgentsClinical DescriptionCutaneous exposures of riot-control agents might produce dermal burns and rash (96--101). However, the majority of exposures to riot-control agents occur by inhalation. If a rapid onset of the following signs and symptoms occurs, the clinical description for an exposure to a riot-control agent has been met: 1) lacrimation and 2) one respiratory effect (i.e., nose or throat irritation, cough, or suffocation or choking sensation). Laboratory Criteria for DiagnosisBiologic. No biologic marker for exposure to riot-control agents is available. Environmental. No method is available for detecting riot-control agents in environmental samples. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for riot-control--agent exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests (not available for riot-control agents) have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. SaxitoxinClinical DescriptionExposure to saxitoxin might cause numbness of the oral mucosa within 30 minutes after ingestion. In severe poisoning, signs and symptoms typically progress rapidly, including parasthesias, a floating sensation, muscle weakness, vertigo, and cranial nerve dysfunction. Respiratory failure and death might occur from paralysis (102--106). Laboratory Classification for DiagnosisBiologic. A case in which saxitoxin in urine is detected, as determined by a commercial laboratory. Or, Environmental. Detection of saxitoxin in ingested compounds or seafood, as determined by a commercial laboratory or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for saxitoxin exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Sodium AzideClinical DescriptionThe majority of exposures to sodium azide occur by inhalation. Signs and symptoms of sodium azide poisoning include lethargy or coma (possibly sudden collapse), dyspnea, tachypnea, tachycardia, and hypotension. Nausea and vomiting also might occur, especially after ingestion. Exposure to dust or gas might produce conjunctivitis and nasal and bronchial irritation. Nonspecific laboratory findings include metabolic and lactic acidosis (107--108). Laboratory Criteria for DiagnosisBiologic. A case in which sodium azide in serum is detected, as determined by a commercial laboratory. Or, Environmental. Detection of sodium azide in environmental samples, as determined by FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for sodium azide exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Sodium Monofluoroacetate (Compound 1080)Clinical DescriptionExposure to sodium monoflouroacetate might cause systemic toxicity by different routes of exposure. Clinical effects usually develop within 30 minutes to 2.5 hours of exposure but might be delayed as long as 20 hours. The predominant manifestations of sodium monoflouroacetate poisoning are metabolic, cardiovascular, and neurologic signs and symptoms. Effects of acute exposure might include metabolic acidosis, hypotension, dysrhythmias, seizures, coma, and respiratory depression (109--111). Laboratory Criteria for DiagnosisBiologic. No biologic marker for sodium monoflouroacetate is available. Or, Environmental. Detection of sodium monoflouroacetate in environmental samples, as determined by FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for a sodium monofluoroacetate exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case with laboratory confirmation from environmental samples. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. StrychnineClinical DescriptionThe major identifying clinical features of strychnine poisoning through ingestion are severe, painful spasms of the neck, back, and limbs and convulsions with an intact sensorium. Symptoms might progress to coma. Tachycardia and hypertension are also common effects (112--115). Laboratory Criteria for DiagnosisBiologic. A case in which strychnine in urine or serum is detected, as determined by a commercial laboratory. Or, Environmental. Detection of strychnine in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for strychnine exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests of biologic and environmental samples have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Sulfuryl FluorideClinical DescriptionSulfuryl fluoride poisoning usually occurs after inhalational exposure. The predominant manifestations of sulfuryl fluoride poisoning are respiratory irritation and neurologic symptoms. Effects of acute exposure usually include lacrimation, nose or throat irritation, cough, dyspnea, paresthesias, and seizures (116--118). Laboratory Criteria for DiagnosisBiologic. No specific test for sulfuryl fluoride exposure is available. However, an elevated fluoride concentration in the serum, hypocalcemia, and hyperkalemia might indicate that an exposure has occurred. Normal serum fluoride levels are <20 mcg/L but varies substantially on the basis of dietary intake and environmental levels. Environmental. Detection of sulfuryl fluoride in environmental samples, as determined by NIOSH. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for a sulfuryl fluoride exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on environmental samples are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. TetrodotoxinClinical DescriptionThe consumption of toxic amounts of tetrodotoxin results primarily in neurologic and gastrointestinal signs and symptoms. In severe poisoning, dysrhythmias, hypotension, and even death might occur (119--120). If a rapid onset of one of the following neurologic and gastrointestinal signs or symptoms occurs, the clinical description for tetrodotoxin poisoning has been met: 1) oral paresthesias (might progress to include the arms and legs), 2) cranial nerve dysfunction, 3) weakness (might progress to paralysis), or 4) nausea or vomiting. Laboratory Classification for DiagnosisBiologic. No biologic marker for tetrodotoxin exposure is available. Environmental. No method for detection of tetrodotoxin in environmental samples is available commercially. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for tetrodotoxin exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests (not available for tetrodotoxin) are confirmatory. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. ThalliumClinical DescriptionIngestion of toxic amounts of thallium might cause gastrointestinal signs and symptoms, most commonly abdominal pain. Subacute symptoms (onset of days to weeks) after a substantial, acute exposure or a chronic exposure to limited amounts of thallium might include severely painful ascending neuropathy, ataxia, seizure, alopecia, and neurocognitive deficits (121--123). Laboratory Criteria for DiagnosisBiologic. A case in which elevated spot urine thallium levels are detected (reference level: <0.5 µg/L) (20), as determined by a commercial laboratory. Or, Environmental. Detection of thallium in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for thallium exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests of biologic and environmental samples have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Toxic AlcoholsClinical DescriptionIngestion of toxic alcohols (methanol, ethylene glycol, or other glycols) might result in symptoms similar to those of ethanol inebriation (vomiting, lethargy, or coma). A high anion gap metabolic acidosis is common. Renal failure is common after ethylene glycol and diethylene glycol toxicity, whereas optic neuritis and visual impairment are unique to methanol toxicity (124--127). Laboratory Criteria for DiagnosisBiologic. A case in which glycols or methanol in whole blood is detected, as determined by hospital or commercial laboratory tests. Or, Environmental. Detection of glycols or methanol in environmental samples, as determined by NIOSH or FDA. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for toxic alcohol exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Trichothecene MycotoxinsClinical DescriptionTrichothecene mycotoxins might be weaponized and dispersed through the air or mixed in food or beverages. Initially, route-specific effects are typically prominent. Dermal exposure leads to burning pain, redness, and blisters, and oral exposure leads to vomiting and diarrhea. Ocular exposure might result in blurred vision, and inhalational exposure might cause nasal irritation and cough. Systemic symptoms can develop with all routes of exposure and might include weakness, ataxia, hypotension, coagulopathy, and death (128). Laboratory Criteria for DiagnosisBiologic. Selected commercial laboratories are offering immunoassays to identify trichothecenes or trichothecene-specific antibodies in human blood or urine (129--130). However, these procedures have not been analytically validated and are not recommended. Environmental. Detection of trichothecene mycotoxins in environmental samples, as determined by FDA. As a result of indoor air-quality investigations involving mold and potentially mold-related health effects, mycotoxin analyses of bulk environmental samples are now commercially available through environmental microbiology laboratories in the United States (131). Studies have not been done to determine the background level of trichothecenes in nonmoldy homes and office buildings or nonagricultural outdoor environments. Therefore, the simple detection of trichothecenes in environmental samples does not invariably indicate an intentional contamination. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists. Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for trichothecene mycotoxins exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests of environmental samples have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. Vesicant (Mustards, Dimethyl Sulfate, and Lewisite)Clinical DescriptionThe most common clinical effects after exposure to vesicants include dermal (skin erythema and blistering), respiratory (cough, dyspnea, pneumonitis, and acute lung injury), ocular (conjunctivitis and burns), and gastrointestinal (vomiting) signs and symptoms. The effects of the majority of vesicants manifest rapidly (within minutes). However, clinical findings might be delayed for hours after exposure (e.g., sulfur mustard) (132--135). Laboratory Criteria for DiagnosisBiologic. A case in which sulfur mustard in biologic samples is detected, as determined by CDC or one of five LRN laboratories that have this capacity, and a case in which nitrogen mustard and lewisite are detected in biologic samples, as determined by CDC. Environmental. Confirmation of the detection of vesicants in environmental samples is not available. Case ClassificationSuspected. A case in which a potentially exposed person is being evaluated by health-care workers or public health officials for poisoning by a particular chemical agent, but no specific credible threat exists Probable. A clinically compatible case in which a high index of suspicion (credible threat or patient history regarding location and time) exists for vesicant exposure, or an epidemiologic link exists between this case and a laboratory-confirmed case. Confirmed. A clinically compatible case in which laboratory tests on biologic samples have confirmed exposure. The case can be confirmed if laboratory testing was not performed because either a predominant amount of clinical and nonspecific laboratory evidence of a particular chemical was present or a 100% certainty of the etiology of the agent is known. ConclusionWhen illness results from an intentional or unintentional chemical release (either known or suspected on the basis of a credible threat) into the environment, uniform reporting is paramount for directing appropriate resources, assessing the extent of morbidity and mortality, tracking poisoned persons, and monitoring response to intervention. The case definitions presented in this report facilitate uniform reporting of illness resulting from a chemical (i.e., toxin and toxicant) release. Health-care providers should report suspected cases of intentional chemical exposure to their local poison-control center (telephone: 800-222-1222) and to a public health agency. Local and state public health officials should notify CDC and law enforcement officials if they identify persons who they suspect have been exposed to intentional chemical poisoning. Acknowledgments The authors acknowledge contributions from Sandra V. McNeel, DVM, and Henry A. Anderson, MD, both with CSTE. References
* Toxic syndrome descriptions for the toxins and toxicants presented in this report are available at http://www.bt.cdc.gov/agent/agentlistchem.asp. These descriptions provide a comprehensive list of signs and symptoms for a particular agent, a differential diagnosis, and background information on the toxin/toxicant. Figure 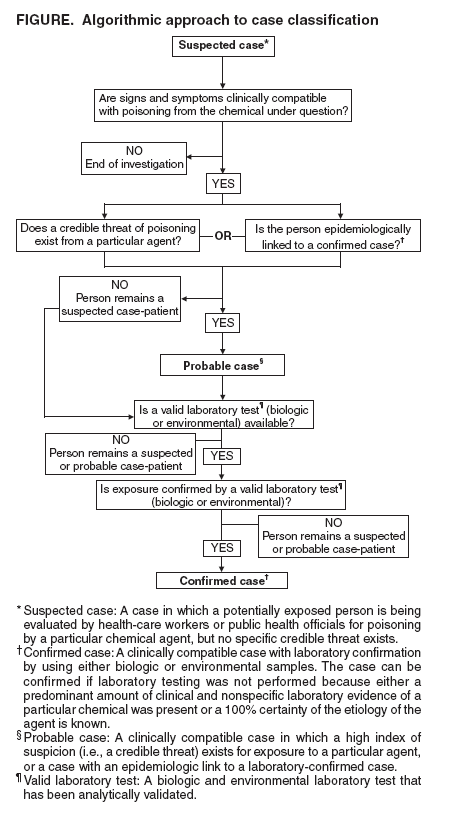 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 1/7/2005 |
|||||||||
This page last reviewed 1/7/2005
|