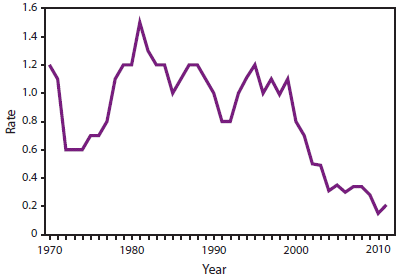
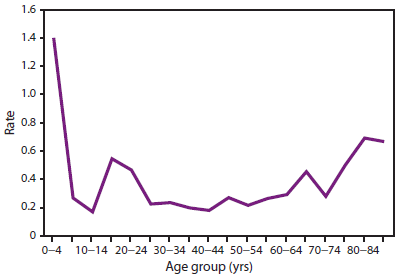
ACIP Membership List
As of June 2012
Chair: Carol Baker, MD, Baylor College of Medicine, Houston, Texas.
Executive Secretary: Larry Pickering, MD, National Center for Immunization and Respiratory Diseases, CDC, Atlanta, Georgia.
Members: Nancy Bennett, MD, University of Rochester School of Medicine and Dentistry, Rochester, New York; Joseph Bocchini, MD, Louisiana State University Health Sciences Center, Shreveport, Louisiana; Douglas Campos-Outcalt, MD, University of Arizona College of Medicine, Phoenix, Arizona; Tamera Coyne-Beasley, MD, University of North Carolina, Chapel Hill, North Carolina; Jeffrey Duchin, MD, University of Washington, Seattle, Washington; Kristen Ehresmann, MPH, Minnesota Department of Health, St. Paul, Minnesota; Renée Jenkins, MD, Howard University School of Medicine, District of Columbia; Wendy Keitel, MD, Baylor College of Medicine, Houston, Texas; Michael Marcy, MD, UCLA Center for Vaccine Research, Torrance, California; Cody Meissner, MD, Tufts Medical Center, Boston, Massachusetts; Sara Rosenbaum, JD, Georgetown University, District of Columbia; Mark Sawyer, MD, University of California at San Diego, California; Jonathan Temte, MD, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin; Marietta Vázquez, MD, Yale University School of Medicine, New Haven, Connecticut.
Ex Officio Members: Geoffrey S. Evans, MD, Health Resources and Services Administration, Rockville, Maryland; Jesse Geibe, MD, Department of Defense, CDC, Atlanta, Georgia; Bruce Gellin, MD, National Vaccine Program Office, District of Columba; Richard Gorman, MD, National Institutes of Health, Bethesda, Maryland; Amy Groom, MPH, Indian Health Service, Albuquerque, New Mexico; Mary Beth Hance, Centers for Medicare and Medicaid Services, Baltimore, Maryland; Linda Kinsinger, MD, Department of Veterans Affairs, Durham, North Carolina; Wellington Sun, MD, Food and Drug Administration, Bethesda, Maryland.
Liaison Representatives: American Academy of Family Physicians, Jamie Loehr, MD, Ithaca, New York; American Academy of Pediatrics, Michael Brady, MD, Columbus, Ohio, David Kimberlin, MD, Birmingham, Alabama; American Academy of Physician Assistants, Marie-Michèle Léger, MPH, Alexandria, Virgina; American College Health Association, James C. Turner, MD, Charlottesville, Virginia; American College of Obstetricians and Gynecologists, Laura Riley, MD, Boston, Massachusetts; American College of Physicians, Gregory Poland, MD, Rochester, Minnesota; American Geriatrics Society, Kenneth Schmader, MD, Durham, North Carolina; America's Health Insurance Plans, Mark Netoskie, MD, Houston, Texas; American Medical Association, Litjen Tan, PhD, Chicago, Illinois; American Nurses Association, Katie Brewer, MSN, Silver Springs, Maryland; American Osteopathic Association, Stanley Grogg, DO, Tulsa, Oklahoma; American Pharmacists Association, Stephan L. Foster, PharmD, Memphis, Tennessee; Association of Immunization Managers, Kelly Moore, MD, Nashville, Tennessee; Association for Prevention Teaching and Research, W. Paul McKinney, MD, Louisville, Kentucky; Association of State and Territorial Health Officials, José Montero, MD, Concord, New Hampshire; Biotechnology Industry Organization, Clement Lewin, PhD, Cambridge, Massachusetts; Canadian National Advisory Committee on Immunization, Bryna Warshawsky, MDCM, Ontario, Canada; Council of State and Territorial Epidemiologists, Christine Hahn, MD, Boise, Idaho; Department of Health, United Kingdom, David M. Salisbury, MD, London, United Kingdom; Healthcare Infection Control Practices Advisory Committee, Alexis Elward, MD, St. Louis, Missouri; Infectious Diseases Society of America, Kathleen Neuzil, MD, Seattle, Washington; National Association of County and City Health Officials, Matthew Zahn, MD, Louisville, Kentucky; National Association of Pediatric Nurse Practitioners, Patricia Stinchfield, MPH, St. Paul, Minnesota; National Foundation for Infectious Diseases, William Schaffner, MD, Nashville, Tennessee; National Immunization Council and Child Health Program, Mexico, Vesta Richardson, MD, Mexico City, Mexico; National Medical Association, Patricia Whitley-Williams, MD, New Brunswick, New Jersey; National Vaccine Advisory Committee, Walter Orenstein, MD, Atlanta, Georgia; Pharmaceutical Research and Manufacturers of America, Damian A. Braga, Swiftwater, Pennsylvania,; Society for Adolescent Health and Medicine, Amy Middleman, MD, Houston, Texas; Society for Healthcare Epidemiology of America, Harry Keyserling, MD, Atlanta, Georgia.
Meningococcal Vaccines Work Group
Chair: Lorry Rubin, MD, Steven and Alexandra Cohen Children's Medical Center of New York, New Hyde Park, New York.
Members: Carol Baker, MD, Baylor College of Medicine, Houston, Texas; Michael Brady, MD, Ohio State University, Columbus, Ohio; Douglas Campos-Outcalt, MD, University of Arizona College of Medicine, Phoenix, Arizona; Richard Clover, MD, University of Louisville School of Public Health, Lousiville, Kentucky; Kristen Ehresmann, MPH, Minnesota Department of Health, St. Paul, Minnesota; Lucia Lee, MD, Food and Drug Administration, Rockville, Maryland; Martin Luta, MD, Delaware Division of Public Health, Dover, Delaware; Michael Marcy, MD, UCLA Center for Vaccine Research, Torrence, California; W. Paul McKinney, MD, Association for Prevention Teaching and Research, Louisville, Kentucky; Cody Meissner, MD, Tufts University School of Medicine, Boston, Massachusetts; Amy Middleman, MD, Society for Adolescent Health and Medicine, Houston, Texas; Karen O'Brien, MD, US Army Training and Doctrine Command, Fort Monroe, Virginia; Paul Offit, MD, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania; Georges Peter, MD, Rhode Island Hospital, Providence, Rhode Island; William Schaffner, MD, National Foundation for Infectious Diseases, Nashville, Tennessee; David Stephens, MD, Emory University School of Medicine, Atlanta, Georgia; James C. Turner, MD, American College Health Association, Charlottesville, Virginia; Marietta Vázquez, MD, Yale University School of Medicine, New Haven, Connecticut.
Contributors: William Atkinson, MD; Elizabeth Briere, MD; Thomas Clark, MD; Jonathan Duffy, MD; Jessica MacNeil, MPH; Nancy E. Messonnier, MD; Ismael R. Ortega-Sanchez, PhD; Shannon Stokley, MPH, CDC, Atlanta, Georgia.
Secretariat (CDC): Amanda C. Cohn, MD, CDC, Atlanta, Georgia.
 ShareCompartir
ShareCompartir