At a glance
Introduction
Accurate and timely medical diagnoses support public health and patient care. In a 2015 report, "Improving Diagnosis in Healthcare," the National Academies of Medicine identified diagnostic error as a major public health challenge.1 Annually, approximately 800,000 Americans die or become disabled due to diagnostic errors associated with serious conditions that include cancer, cardiovascular, and infectious diseases.12 These diagnostic errors contribute to about one-third of all medical errors.
The Diagnostic Excellence Initiative within the CDC Division of Laboratory Systems was established in 2022 to advance the inclusion of laboratory expertise and capabilities in healthcare delivery to reduce diagnostic errors and improve accurate, timely, and actionable diagnoses.3 Many of our projects and activities are described below.
The Total Testing Process
The Total Testing Process provides a framework for engaging with partners to advance diagnostic excellence and support the Diagnostic Excellence Initiative projects and activities described below.4
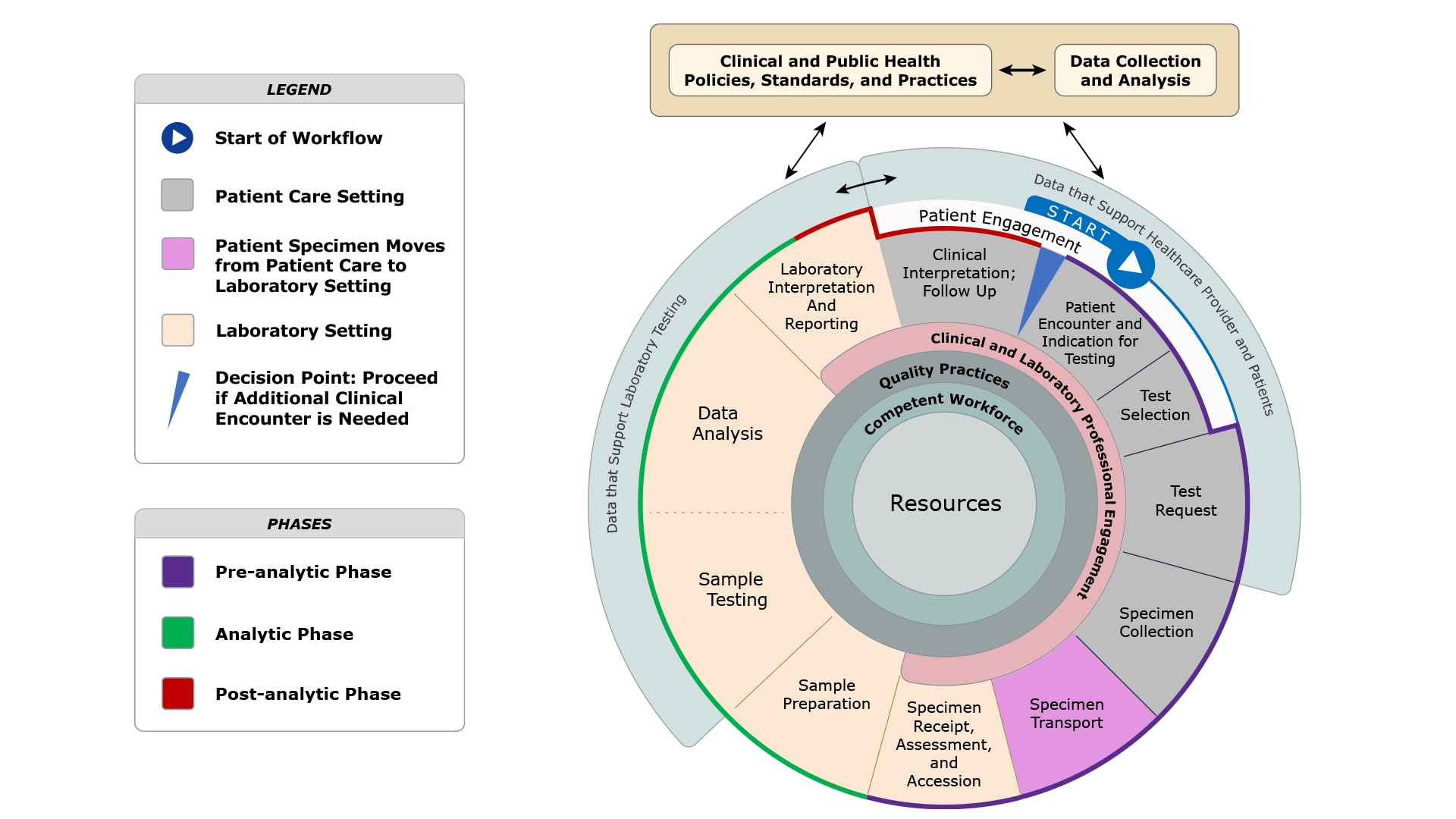
- Pre-analytic Phase: Steps that occur prior to testing of the patient sample that begins with an indication for testing and concludes with specimen receipt, assessment, and accession.
- Analytic Phase: Steps that occur during testing of the patient sample that includes sample preparation, sample testing, and subsequent data analysis, laboratory interpretation and reporting.
- Post-analytic Phase: Steps that occur after testing of the patient sample that includes clinical interpretation and follow-up.
Projects and activities
Learn more about our projects and activities that support diagnostic excellence:
Improve diagnosis and follow-up for severe hypercholesterolemia in a medically underserved community.
Elements of the Total Testing Process Involved
- Laboratory Interpretation and Reporting
- Clinical Interpretation and Follow-up
- Data that Support Healthcare Providers and Patients
- Clinical and Laboratory Professional Engagement
- Quality Practices
- Competent Workforce
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
Purpose or Problem Statement
Severe hypercholesterolemia, defined as having an LDL cholesterol ≥ 190 mg/dL, has an estimated prevalence of 1 in 48 Americans. Among those having severe hypercholesterolemia, 1 in 4 are unaware and untreated.5 Federally qualified health centers serve medically underserved populations, that have additional healthcare delivery challenges. This project is designed to develop a prototype for communicating the importance of statin prescribing to patients and clinicians when clinical laboratory test results are indicative of severe hypercholesterolemia.
Outcomes Sought
Increase the number of patients within a federally qualified health center receiving guideline-recommended diagnoses and follow-up for severe hypercholesterolemia.
Collaborators
- CDC Division of Heart Disease and Stroke Prevention
- National Association for Community Health Centers (NACHC)
- Million Hearts Initiative
- Zufall Health
- Health Efficient
Resources
- About the Health Center Program
- NACHC Million Hearts Initiative
- The Scoop on Statins: What Do You Need to Know?
- CDC Division of Heart Disease and Stroke Prevention
- Family Heart Foundation
Project Period
FY 2021-2024
Identify disproportionately affected populations not receiving guideline-recommended diagnoses, based on test results, and follow-up for severe hypercholesterolemia.
Elements of the Total Testing Process Involved
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
Purpose or Problem Statement
Severe hypercholesterolemia is underdiagnosed and undertreated in the United States.
Outcomes Sought
Use health data to identify disproportionately affected populations potentially amenable to a clinical laboratory-involved intervention to improve diagnoses and follow-up.
Collaborator
CDC Division of Heart Disease and Stroke Prevention
Project Period
FY 2024-2025
Advance the adoption of the National Blood Culture Contamination (BCC) Patient Safety Measure.
Elements of the Total Testing Process Involved
- Specimen Collection
- Quality Practices
- Laboratory Interpretation and Reporting
- Clinical Interpretation and Follow-up
- Clinical and Public Health Policies, Standards, and Practices
- Data Collection and Analysis
- Resources
Purpose or Problem Statement
False negative blood culture results due to inadequate volumes of blood can result in misdiagnosis, delay therapy, and put patients at heightened risk of morbidity and mortality from bacteremia. 67The presence of commonly occurring bacteria or fungi on human skin (i.e., commensal organisms) can increase the risk of blood culture contamination, possibly causing false positives. This can compromise care by leading to unnecessary antibiotic therapy and prolonged hospitalization.
Outcomes Sought
- Increased adoption of the blood culture contamination (BCC) measure as a quality monitor among laboratory accreditation organizations.
- Improved data collection of laboratory quality indicators for BCC.
- Appropriate collection of blood cultures among hospitals and healthcare systems.
- Improved communication, follow-up, and training among clinical laboratories and clinical care teams in collaboration with antibiotic and diagnostic stewardship teams.
Collaborators
- CDC Division of Healthcare Quality Promotion
- National Healthcare Safety Network
Project Period
- December 2022: Initial Measure Endorsement
- FY 2025: Measure maintenance/renewal application due Spring 2026 cycle
Resources
- National Healthcare Safety Network
- Preventing Adult Blood Culture Contamination: A Quality Tool for Clinical Laboratory Professionals
- Blood Culture Contamination: An Overview for Infection Control and Antibiotic Stewardship Programs Working with the Clinical Laboratory
- Adult Blood Culture Contamination Rate; A national measure and standard for clinical laboratories and antibiotic stewardship programs
Diagnostic Stewardship Toolkit
Elements of the Total Testing Process Involved
- Resources
Purpose or Problem Statement
A toolkit that supports diagnostic stewardship. Diagnostic stewardship supports ordering the right test for the right patient at the right time. This process leads to the right diagnostic actions to improve patient outcomes.
Outcomes Sought
Diagnostic stewardship uses a multidisciplinary team approach to optimize clinical testing. Testing helps guide patient care. Inappropriate testing or not ordering appropriate tests may lead to patient harm. Diagnostic stewardship focuses on reducing patient harm by informing clinical decisions. Process changes aim to reduce diagnostic errors by improving the use and interpretation of tests.
Developer
Release Date
December 2024
Toolkit Link
Resources
Determine pharmacogenetic allele frequencies in a representative population-based sample (NHANES 2009-2010 and 2011-2012) from the United States.
Elements of the Total Testing Process Involved
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
- Resources
Purpose or Problem Statement
Limited understanding of the prevalence of pharmacogenomic variants commonly tested because affected populations aren’t included in most assessments.
Outcomes Sought
Findings are anticipated to improve the use and interpretation of pharmacogenetic tests among the diverse U.S. population. For many diagnoses, pharmacogenetic test results supports the selection of appropriate follow-up therapy.
Collaborators
- CDC Office of Genomics and Precision Public Health
- CDC Office of Advanced Molecular Detection
- PRPD Diagnostics
Project Period
FY 2024-2027
Assess the use of estimated glomerular filtration rate (eGFR) to identify early kidney failure.
Elements of the Total Testing Process Involved
- Laboratory Interpretation and Reporting
- Clinical Interpretation and Follow-up
- Data that Support Healthcare Providers and Patients
- Clinical and Laboratory Professional Engagement
- Quality Practices
- Competent Workforce
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
Purpose or Problem Statement
Many individuals with early chronic kidney disease (CKD) are not identified in time for treatment because best practices are not followed. The new race-neutral CKD-EPI 2021 algorithm is appropriate for estimating glomerular filtration rate (eGFR) in all populations, but not all facilities have adopted it. Disparities likely exist that cause some populations to be more affected.
Outcomes Sought
Use large electronic health record (EHR) datasets to identify gaps, inform interventions, and track progress by assessment of:
- Presence of eGFRs in the patient record when blood creatinine levels are recorded.
- Use of the CKD-EPI 2021 equation to calculate eGFRs.
- Low eGFRs are followed up with a repeat test to identify CKD.
- Indication of CKD for patients having chronic low eGFRs.
- Use of urine albumin to creatine ratio to detect proteinuria for low eGFRs.
Collaborators
- CDC Division of Diabetes Translation
- National Kidney Foundation
Project Period
FY 2024–2025
Explore the use of point-of-care testing in disproportionately affected populations to expedite diagnoses and follow-up.
Elements of the Total Testing Process Involved
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
Purpose or Problem Statement
Understanding the extent to which point-of-care testing (POCT) is used in disproportionately affected populations may help identify shortcomings and opportunities for improving the utilization of these tests for earlier diagnosis and intervention.
Outcomes Sought
Literature review and report on the use of POCT in disproportionately affected populations.
Project Period
FY 2024-2025
HLA-B*57:01 Genotyping to Inform Abacavir Prescribing.
Elements of the Total Testing Process Involved
- Test Selection
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
- Resources
Purpose or Problem Statement
Abacavir is a commonly used antiretroviral therapy for HIV. Approximately 5-8% of the population carries the HLA-B*57:01 allele, putting them at risk for a severe hypersensitivity reaction. Current guidelines recommend HLA-B*57:01 screening for all patients at the time of abacavir initiation. This study evaluated the fraction of patients that received this screen before and after initiation of abacavir.
Outcomes Sought
The data collected may be useful to inform efforts to increase utilization of HLA-B*57:01 testing and promote awareness of the importance of timely pharmacogenetic testing post-diagnosis when abacavir initiation is a therapeutic option.
Collaborator
CDC National Center for HIV, Viral Hepatitis, STD, and TB Prevention
Project Period
FY 2023-2024
Recommendations from the Clinical Laboratory Improvement Advisory Committee (CLIAC) relevant to advancing diagnostic excellence.
Elements of the Total Testing Process Involved
- Clinical and Public Health Policies, Practices, and Standards
Purpose or Problem Statement
Explore the role of clinical laboratory practice in advancing diagnostic excellence and the reduction of diagnostic errors.
Outcomes Sought
Federal advisory committee and associated workgroup discussion summaries and recommendations.
Collaborators
CLIAC's recommendations are primarily directed at the Department of Health and Human Services, the Centers for Disease Control and Prevention, the Food and Drug Administration, and the Centers for Medicare and Medicaid Services.
Project Period
Ongoing
Resource
- Clinical Laboratory Improvement Advisory Committee (CLIAC) Recommendations Table (Oct 28-29, 2018; Nov 7-8, 2018; Nov 1-2, 2017; Nov 2-3, 2016)
Educational resources for patients to increase their understanding of clinical testing and the total testing process, using chronic kidney disease as the primary example.
Elements of the Total Testing Process Involved
- Patient Engagement
- Resources
Purpose or Problem Statement
A lack of understanding of clinical testing can compromise patients' opportunities to interact effectively with their healthcare providers and compromise informed decision-making.
Outcomes Sought
An educational video, infographic(s), and a listing of resources to improve patients’ understanding of clinical testing in the diagnostic process using a workup for chronic kidney disease as an example.
Project Period
FY 2024-2025
Resources
- CDC Chronic Kidney Disease
- NIH Chronic Kidney Disease
- National Kidney Foundation
- Improving Your Laboratory Testing Process | Agency for Healthcare Research and Quality
- Implementing a safer and more reliable system to monitor test results at a teaching university-affiliated facility in a family medicine group: a quality improvement process report | BMJ Open Quality
- Improving Diagnosis in Healthcare
- Burden of Serious Harms from Diagnostic Error in the USA
- Bringing the Clinical Laboratory into the Strategy to Advance Diagnostic Excellence
- The Clinical Laboratory Is an Integral Component to Health Care Delivery: An Expanded Representation of the Total Testing Process
- Prevalence, Awareness, and Treatment of Elevated LDL Cholesterol in US Adults
- Advancing Diagnostic Stewardship for Healthcare-Associated Infectious, Antibiotic Resistance, and Sepsis
- Practical Guidance for Clinical Microbiology Laboratories: A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem
- National Kidney Foundation Laboratory Engagement Working Group Recommendations for Implementing the CKD-EPI 2021 Race-Free Equations for Estimated Glomerular Filtration Rate: Practical Guidance for Clinical Laboratories
- Reported Awareness and Adoption of 2021 Estimated Glomerular Filtration Rate Equations Among US Clinical Laboratories
