 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Recommended Childhood Immunization Schedule --- United States, 2001Each year, CDC's Advisory Committee on Immunization Practices (ACIP) reviews the recommended childhood immunization schedule to ensure that it remains current with changes in manufacturers' vaccine formulations, revisions in recommendations for the use of licensed vaccines, and recommendations for newly licensed vaccines. This report presents the recommended childhood immunization schedule for 2001 (Figure 1) and documents the changes that have occurred since the January 2000 publication (4). For 2001, ACIP, the American Academy of Family Physicians, and the American Academy of Pediatrics have added pneumococcal conjugate vaccine to the schedule (2) and have extended the recommendation for the use of hepatitis A vaccine to include persons through age 18 years in selected geographic areas and in certain high-risk groups (3). Detailed recommendations for using vaccines are available from the manufacturers' package inserts, ACIP statements on specific vaccines, and the 2000 Red Book (5). ACIP statements for each recommended childhood vaccine can be viewed, downloaded, and printed from CDC's National Immunization Program World-Wide Web site, http://www.cdc.gov/nip/publications/ACIP-list.htm. Pneumococcal Conjugate VaccineIn February 2000, the Food and Drug Administration licensed a heptavalent pneumococcal polysaccharide-protein conjugate vaccine (PCV) (Prevnar™,* Wyeth Lederle Vaccines and Pediatrics, Philadelphia, Pennsylvania) for use among infants and young children. All children aged 2--23 months should receive four doses of PCV intramuscularly at ages 2, 4, 6, and 12--15 months. ACIP also recommends the vaccine for children aged 24--59 months who are at increased risk for pneumococcal disease (e.g., children with sickle cell hemoglobinopathies, human immunodeficiency virus infection, and other immunocompromising or chronic medical conditions). For these children, ACIP recommends two doses of PCV administered 2 months apart followed by one dose of a 23-valent pneumococcal polysaccharide vaccine (PPV 23) administered two or more months after the second dose of PCV. ACIP also recommends that PCV be considered for all other children aged 24--59 months, with priority given to children aged 24--35 months, American Indian/Alaska Native and black children, and children who attend child-care centers. ACIP recommends one dose of PCV for children in these groups. Additional information on the use of PCV can be found in the ACIP statement (2). Hepatitis A Vaccination RecommendationACIP continues to recommend hepatitis A vaccine (Hep A) for routine use in some states and regions. For 2001, the recommendation has been extended to include adolescents through age 18 years and for persons in certain high-risk groups (i.e., persons traveling to countries where hepatitis A is moderately or highly endemic, men who have sex with men, users of injectable and noninjectable drugs, persons who have clotting-factor disorders, persons working with nonhuman primates, and persons with chronic liver disease). The hepatitis A vaccine label is shaded on the 2001 Immunization Schedule to indicate its use in selected states and regions, and for certain high-risk groups. Providers can contact their local public health authority for the current recommendations for hepatitis A vaccination in their community. Additional information on the use of Hep A can be found in the ACIP statement (3). Vaccine Information StatementsThe National Childhood Vaccine Injury Act requires that all health-care providers give to parents or patients copies of Vaccine Information Statements before administering each dose of the vaccines listed in this schedule. Vaccine Information Statements, developed by CDC, can be obtained from state health departments and CDC's World-Wide Web site, http://www.cdc.gov/nip/publications/VIS. Instructions on use of the Vaccine Information Statements are available at http://www.cdc.gov/nip/publications/VIS/vis-Instructions.pdf. References
* Use of trade names and commercial sources is for identification only and does not constitute endorsement by CDC or the U.S. Department of Health and Human Services. Figure 1 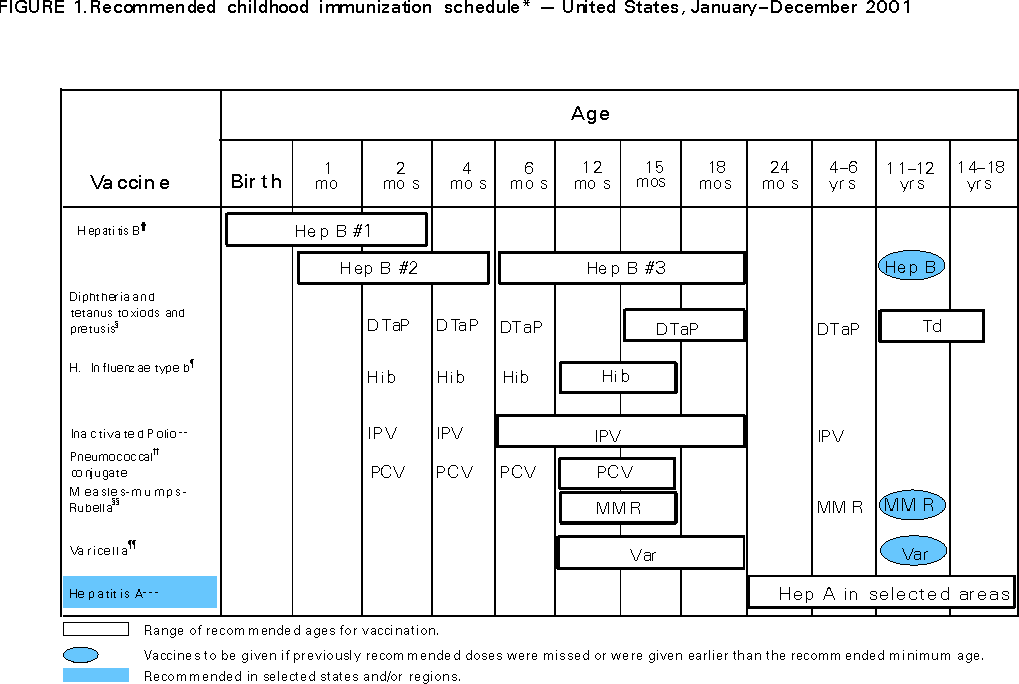  Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 1/10/2001 |
|||||||||
This page last reviewed 5/2/01
|