 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
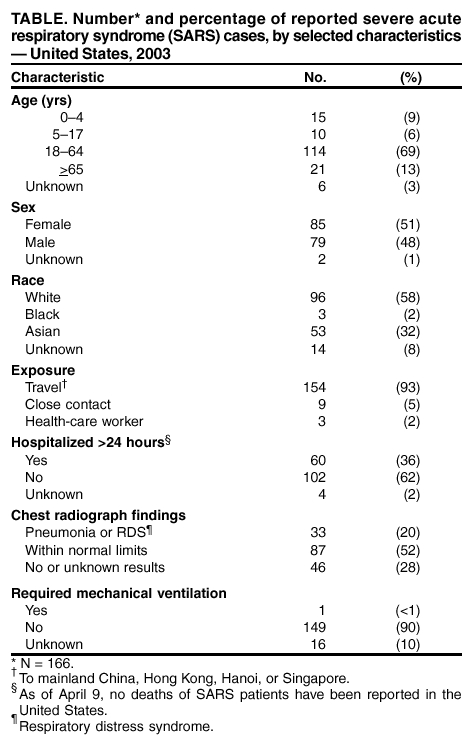
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Severe Acute Respiratory Syndrome (SARS) and Coronavirus Testing --- United States, 2003Please note: An erratum has been published for this article. To view the erratum, please click here. CDC and the World Health Organization (WHO) are continuing to investigate the multicountry outbreak of severe acute respiratory syndrome (SARS). Infection with a novel coronavirus has been implicated as a possible cause of SARS (1). This report updates information on U.S. residents with SARS and summarizes the clinical histories of the five U.S. residents identified as of April 9, 2003, who have both suspected SARS and laboratory evidence of infection with a novel coronavirus. Epidemiologic and laboratory investigations of SARS are ongoing. CDC's interim suspected SARS case definition (available at http://www.cdc.gov/ncidod/sars/casedefinition.htm) continues to be based on clinical criteria and epidemiologic linkage to other SARS cases or areas with community transmission of SARS; abnormal radiographic findings are not required for suspected cases. The WHO case definition for probable SARS includes radiographic evidence of infiltrates consistent with pneumonia or respiratory distress syndrome (RDS) on chest radiograph (2). Cases reported to WHO outside the United States are probable SARS cases; the United States reports all suspected cases. As of April 9, a total of 2,722 SARS cases have been reported to WHO from 16 countries, including the United States; 106 deaths (case-fatality proportion: 3.9%) have been reported to WHO (3). As of April 9, CDC had received 166 reports from 30 states of suspected SARS cases among U.S. residents (Figure); 135 (81%) cases occurred among adults (Table). Of the 166 persons with suspected SARS, 154 (93%) had traveled within the 10 days before illness onset to one or more of the areas listed in the case definition, nine (5%) had household contact with a person with suspected SARS, and three (2%) were health-care workers (HCWs) who had provided medical care to a patient with suspected SARS. The majority of U.S. patients had normal chest radiographs (Table). As of April 9, a total of 33 (20%) patients were reported to have pneumonia or RDS. Of the 60 (36%) patients who were hospitalized for >24 hours, four (7%) remained hospitalized as of April 9, and no deaths were reported. Travel AdvisoriesTravel advisories from WHO and CDC remain in effect. CDC has issued a travel advisory (available at http://www.cdc.gov/travel/other/acute_resp_syn_multi.htm) recommending that persons planning nonessential or elective travel to mainland China, Hong Kong, Hanoi, or Singapore consider postponing such travel until further notice. Persons who have traveled recently to these locations are urged to seek medical care if they develop fever of >100.4º F (38.0º C), cough, or difficulty breathing within 10 days of travel and to inform their health-care providers about recent travel to regions where SARS cases have been reported. Infection-Control GuidelinesInterim infection-control guidelines for health-care, household, and community settings will be updated and revised as new information becomes available. Infection-control practitioners, clinicians providing medical care for patients with suspected SARS, and persons who might have contact with persons with suspected SARS should consult these guidelines frequently to keep current with recommendations (available at http://www.cdc.gov/ncidod/sars/index.htm). Diagnostic TestingLaboratory diagnostic tests used at CDC to test clinical specimens for evidence of this novel coronavirus are still in development and are not available outside a research setting. Serologic testing for coronavirus antibody consists of indirect fluorescent antibody testing and enzyme-linked immunosorbent assays that are specific for antibody produced after infection. Although some patients have detectable coronavirus antibody within 14 days of illness onset, definitive interpretation of negative coronavirus antibody tests is possible only for specimens obtained >21 days after onset of fever. For other suspected SARS cases in the United States, a second serum specimen collected >21 days after fever onset will be necessary to determine whether infection with the novel coronavirus can be documented. A reverse transcriptase-polymerase chain reaction (RT-PCR) test specific for RNA from the novel coronavirus has been positive within the first 10 days after fever onset in specimens from some SARS patients, but the duration of detectable viremia or viral shedding is unknown, and RT-PCR tests on samples collected during convalescence might be negative. Viral culture followed by RT-PCR also has been used to detect the novel coronavirus in some specimens. Case HistoriesOn April 3, CDC reported to the respective health departments positive coronavirus test results for five persons with SARS. All five had pneumonia requiring hospitalization and had traveled recently to a country in which community transmission had occurred. The five patients did not travel together or at the same time. Although two patients had a common hotel exposure in Hong Kong, no evidence of a single common exposure for all five patients has been found. Specimens from these five patients were among the first tested; patients were selected on the basis of their clinical and exposure histories. A description of the exposure and brief clinical history for each of these five SARS patients follows. Case 1. Patient I is a pregnant woman aged 36 years with a history of intermittent chronic cough; as of April 9, she was in her 26th week of pregnancy. During February 19--March 2, she traveled to Hong Kong and Guangdong province in China to visit her family. While in Hong Kong, she stayed at Hotel M during February 19--22 and again during February 24--March 2. The first stay was on the same floor and during the same time as Patient A (the index case in a large cluster of persons with suspected SARS described previously) (1). On February 8, Patient I's intermittent cough resumed. On February 24, she had onset of fever, chills, and headache. During the next 3 days, her cough progressed, and she had shortness of breath, myalgia, and blood-streaked sputum. She sought medical care in Hong Kong and received an antibiotic. Her symptoms worsened, and on return to the United States on March 2, she was hospitalized with a diagnosis of pneumonia. On admission, her temperature was 100.5º F (38.1º C), and rales were noted on chest examination. A chest radiograph showed bilateral lower lobe infiltrates, and her oxygen saturation was 93%. Laboratory studies on admission included a white blood cell count (WBC) of 3,300/mm3 (12% lymphocytes), platelets of 103,000/mm3, and alanine aminotransferase (ALT) of 42 U/L. During the next 3 days, despite treatment with broad-spectrum antibiotics, she worsened clinically with persistent fever and progressive pulmonary infiltrates. On March 5, she had respiratory failure and required mechanical ventilation, and oseltamivir was added to her treatment. She improved gradually during the next week and was extubated on March 12. On March 17, she was discharged and was recovering as of April 9. Serologic testing of a serum specimen collected 12 days after illness onset was positive for coronavirus antibody. RT-PCR testing for human metapneumovirus is pending. Case 2. Patient L is a man aged 39 years with a medical history of sleep apnea and hypothyroidism. He traveled on vacation to Thailand on February 23 and then to Hong Kong on March 1. During March 1--6, he stayed at Hotel M, at the same time as three other suspected SARS patients who were ill during their hotel stays (1). On March 6, he returned to the United States. On March 13, he had fever, myalgia, and a mild cough. During the next 3 days, he had diarrhea, vomiting, diaphoresis, and shortness of breath. On March 17, he was hospitalized with pneumonia and a right upper lobe infiltrate on a chest radiograph. Laboratory studies included a WBC of 6,600/mm3 (50% neutrophils and 30% lymphocytes) and platelets of 439,000/mm3. Maximum temperature during hospitalization was 102.4º F (39.1º C). He received broad-spectrum antibiotics but no antiviral therapy and was discharged on March 25. Serologic testing of a blood specimen collected 6 days after symptom onset was positive for coronavirus antibody. RT-PCR testing for human metapneumonia virus was negative. On March 19, his wife, who had traveled with him to Hong Kong, developed suspected SARS, including pneumonia requiring hospitalization. Her illness onset occurred 13 days after return from Hong Kong and resulted presumably from close contact with patient L. Case 3. Patient X is a woman aged 49 years with a medical history of chronic sinusitis. She traveled to Hong Kong on business on March 2 and returned to the United States on March 8. The same day, she had fever, cough, and shortness of breath for which she sought medical care. She was given an oral antibiotic. Her symptoms persisted; on March 20, she was hospitalized with shortness of breath, chest pain, and rigors. On admission, she had a temperature of 101.4º F (38.6º C), a chest radiograph showed interstitial infiltrates, and oxygen saturation on room air was 92%. Laboratory studies on admission included a WBC of 5,100/mm3 (68% neutrophils and 28% lymphocytes), platelets of 156,000/mm3, and ALT of 25 U/L. During her hospitalization, she received broad-spectrum antibiotics and corticosteroids but no antiviral therapy. On March 28, she was discharged in stable condition. An RT-PCR assay detected the novel coronavirus on a sputum specimen collected 14 days after illness onset. RT-PCR testing for human metapneumovirus was negative. Case 4. Patient Y is a man aged 22 years with no notable medical history. He traveled to Hong Kong on vacation on March 3 and returned to the United States on March 6. On March 12, he had onset of fever, chills, myalgia, headache, and shortness of breath. On March 13, he had a cough and chest pain and was treated with oral antibiotics. The following day, he reported to an emergency department (ED) with persistent fever and cough. A chest radiograph demonstrated a right perihilar infiltrate. He received intravenous antibiotics in the ED and was discharged the same day on an oral antibiotic. On March 16, he had worsening shortness of breath and respiratory distress, and was admitted to a hospital intensive-care unit. On admission, his temperature was 102.9º F (39.4º C), with an oxygen saturation of 81% on room air. Chest radiograph demonstrated bilateral infiltrates with pleural effusion. Laboratory studies on admission included a WBC of 5,300/mm3 (82% neutrophils and 14% lymphocytes), platelets of 197,000 mm3/mL, and ALT of 74 U/L. He received broad-spectrum antibiotics and oseltamavir. A direct fluorescent antibody assay for influenza type A and influenza type B was negative. By March 20, his condition stabilized, and he was discharged on March 22. Serologic tests of specimens obtained 4, 6, and 13 days after illness onset were positive for antibody to coronavirus. Case 5. Patient Z is a woman aged 53 years with no notable medical history. She traveled to Singapore on February 27 and returned to the United States on March 13. While in Singapore, she visited hospitals that were providing care for patients with pneumonia and had close contact with several persons with probable SARS. She did not use a surgical mask or any respiratory precautions while in the Singapore hospitals. On March 9, she had a headache. During March 12--15, she had fever, chills, and myalgia. On March 15, she was hospitalized with a temperature of 102.7º F (39.3º C). On admission, a chest radiograph indicated bilateral basilar atelectasis. Laboratory studies on admission included a WBC of 6,500/mm3 (68% neutrophils and 19% lymphocytes), platelets of 216,000/mm3, and ALT of 56 U/L. She received broad-spectrum intravenous antibiotics but no antiviral therapy. Her condition stabilized by March 21, and she was discharged on March 26. Although serologic testing of a specimen obtained 3 days after fever onset was negative, a second specimen collected 26 days after onset was positive for antibody to coronavirus. RT-PCR testing for human metapneumovirus is pending. Reported by: CDC SARS Investigative Team; M Charles, DO, EIS Officer, CDC. Editorial Note:Evidence of infection with a novel coronavirus has been identified in patients with suspected SARS in several countries (4--6), including the five patients in the United States described in this report. These patients were among those selected for priority coronavirus testing because of their specific clinical presentations and exposure histories. All had fever and respiratory symptoms (e.g., nonproductive cough, shortness of breath, and radiographic evidence of pneumonia). No consistent abnormal laboratory findings were noted, and the majority were within the normal ranges. Some laboratory tests that have been reported to be elevated in SARS patients, such as lactate dehydrogenase and creatine phosphokinase (4,6--7), were not measured for any of these patients. All five patients received broad-spectrum antibiotics appropriate for coverage of typical and atypical respiratory pathogens. Patients I and Y received oseltamivir, and Patient X received corticosteroids; no patients received ribavirin. These clinical histories are similar to those reported from Canada and Hong Kong, but, as of April 9, no initial characteristic signs or symptoms that clearly distinguish SARS from pneumonia caused by other pathogens have been described (4,6--8). However, if this novel coronavirus is the cause of SARS in these patients, the clinical symptoms described in this report most likely do not represent the full spectrum of illness related to coronavirus infection. Viruses that cause respiratory illness typically are capable of causing a range of clinical manifestations, and asymptomatic infections are possible. State and local health departments are coordinating collection of follow-up serum specimens from SARS patients whose initial serum specimen might have been collected too early to indicate serologic evidence of infection. These results and investigations among well household and other well contacts of SARS patients (including travelers who were on airline flights with persons with SARS symptoms) will provide additional information about the spectrum of illness among patients with SARS and coronavirus infection. The majority of U.S. residents with SARS, including the five persons described in this report who had evidence of coronavirus infection, have recovered or stabilized clinically without specific antiviral therapy. The efficacy of available antiviral therapies against coronavirus infection is unknown. Ribavirin is a known teratogen, and clinicians who use it should be aware of all potential adverse events, including severe hemolytic anemia (9). Preliminary results from in vitro testing indicate that ribavirin concentrations that inhibit ribavirin-sensitive viruses do not inhibit replication or cell-to-cell spread of the novel coronavirus (JW Huggins, U.S. Army Medical Research Institute of Infectious Diseases, personal communication, 2003). However, further in vitro testing of antiviral drugs on other coronavirus isolates, and more information on the clinical outcomes of patients treated with ribavirin or other antiviral drugs in controlled trials is needed. In several countries, widespread community transmission, as well as transmission among HCWs, has been observed. As of April 9, no U.S. HCWs who provided care for the five patients with coronavirus infection described in this report had suspected SARS. Among the close contacts of these five SARS patients, only one (the wife of Patient L) has suspected SARS. The different transmission patterns observed probably are not attributable to differences in infection-control practices alone. The inability to predict which patients are more capable of transmitting the virus that causes SARS underscores the need to adhere strictly to infection-control recommendations in both health-care and household settings. Similarly, close contacts of SARS patients should be vigilant to detect fever or respiratory symptoms, and persons who develop fever or respiratory symptoms should seek health-care evaluation. On April 4, 2003, the president of the United States signed an executive order adding SARS to the list of quarantinable communicable diseases (http://www.whitehouse.gov/news/releases/2003/04/iraq/20030404-8.html). This act provides CDC, through its Division of Global Migration and Quarantine, with the legal authority to implement isolation and quarantine measures as part of transmissible disease-control measures, if necessary. Isolation refers to the practice of keeping a patient with a communicable disease separate from other persons, usually within a health-care facility or at home. Isolation is used routinely in hospital and health-care settings to reduce the transmission of infections to uninfected patients. Quarantine refers to any situation in which a person or group of persons who have been exposed to a communicable disease and might be infected, but who are not yet ill, are kept apart from others to prevent disease spread. States generally have authority to invoke and enforce quarantine within their jurisdictions although quarantine laws vary among states. Quarantine is an effective public health tool. Quarantine in the United States is used primarily to restrict patients with pulmonary tuberculosis who remain infectious but are unable or unwilling to remain in settings where they are less likely to transmit illness. During the previous month, health officials in Singapore, Hong Kong, and Canada have implemented quarantine and isolation measures to limit the spread of SARS. Although evidence is accumulating that a novel coronavirus is the primary causative agent of SARS, more laboratory and epidemiologic data are needed before this link is established fully. Once definitive identification of the cause of SARS has been achieved, an intensive focus on development of effective treatment regimens might reduce morbidity and mortality of patients with SARS. However, specific measures to prevent transmission (e.g., vaccination programs, prophylactic drugs, or hyperimmune globulin) might require more time to develop and implement. In the interim, strengthening traditional public health functions such as collection and rapid analysis of surveillance and epidemiologic data, and implementing essential infection-control measures for suspected SARS patients and their contacts, will be the mainstay of SARS control. A sustained and cooperative global public health response will be necessary to limit further dissemination of SARS and to prepare for emerging global microbial threats. References
 Return to top. Figure 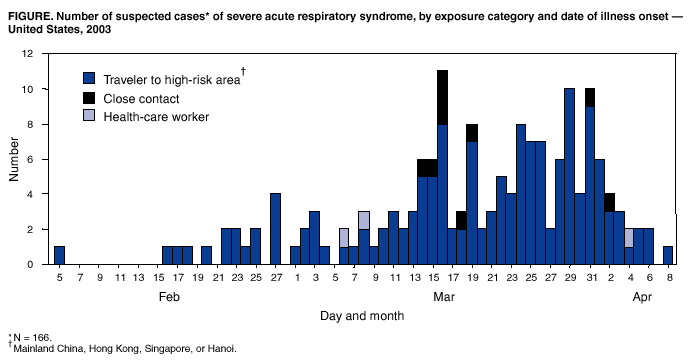 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 4/10/2003 |
|||||||||
This page last reviewed 4/10/2003
|