 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
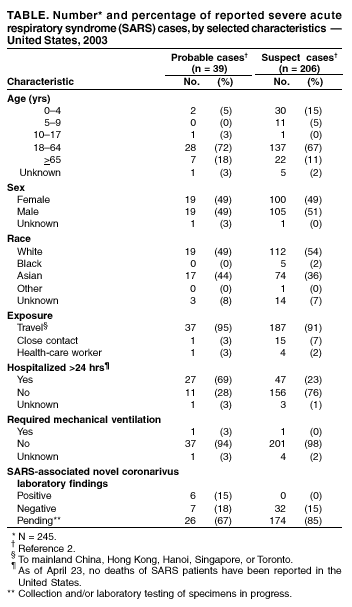
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Severe Acute Respiratory Syndrome --- United States, 2003CDC continues to work with the World Health Organization (WHO) and other partners to investigate cases of severe acute respiratory syndrome (SARS). This report updates information on reported SARS cases worldwide and among U.S. residents and summarizes information on one additional case with laboratory evidence of infection with the SARS-associated coronavirus (SARS-CoV). During November 1, 2002--April 23, 2003, a total of 4,288 SARS cases were reported to WHO from 25 countries, including the United States; 251 deaths (case-fatality proportion: 5.8%) have been reported (1). In the United States as of April 23, a total of 245 SARS cases were reported to CDC from 37 states (Figure). Of these, 39 (16%) had illnesses characterized by the presence of pneumonia or acute respiratory distress syndrome consistent with the interim U.S. surveillance case definition for probable SARS (2). The remaining 206 (84%) had fever and respiratory symptoms (Table). Of the 39 probable SARS patients, 37 (94%) had traveled to mainland China, Hong Kong, Singapore, Hanoi, or Toronto; one (3%) was a health-care worker (HCW) who provided care to a SARS patient, and one (3%) was a household contact of a SARS patient. Twenty-seven (69%) of the probable SARS patients were hospitalized, and one (3%) required mechanical ventilation. As of April 23, of the 245 reported SARS cases, 45 (18%) have diagnostic SARS-CoV laboratory findings (i.e., positive findings based on detection of antibody to SARS-CoV in serum or evidence of virus in respiratory specimens by reverse transcriptase polymerase chain reaction analysis, or negative findings based on absence of antibody to SARS-CoV in convalescent serum obtained >21 days after symptom onset). Thirty-nine reported cases (32 suspect and seven probable based on SARS case definition) tested negative for SARS-CoV; six have been identified with laboratory-confirmed SARS-CoV infection, all of which were classified as probable cases. Five of these six patients were described previously (3). Clinical information for the one additional patient and the related public health investigation and actions are summarized below. PennsylvaniaOn April 3, a man aged 52 years had onset of symptoms including fatigue, myalgia, headache, chills, and diaphoresis (sweating). The patient had diarrhea on April 5 and sought care at the emergency department (ED) of hospital A on April 6. A temperature of 100.7o F (38.2o C) was recorded, but diagnostic testing was not performed and he was discharged with a diagnosis of acute viral syndrome. By April 10, despite initiation of oral amoxicillin, his symptoms progressed to include a dry cough, prompting him to visit his primary-care provider. He had no fever or abnormal findings on physical examination. The patient had a chest radiograph at hospital B and phlebotomy at an outpatient laboratory. The chest radiograph was normal. On April 14, the patient went to the ED of hospital B with dehydration, cough, and severe shortness of breath. Bilateral interstitial infiltrates were present on chest radiograph. In the ED, he was identified as a suspect SARS patient approximately 2.5 hours after arrival. He was subsequently admitted to the hospital with a diagnosis of atypical pneumonia and possible SARS, and was placed in an isolation room with negative pressure. Serum samples collected on April 15 (day 12 of illness) demonstrated SARS-CoV antibodies. The patient received supportive care and antibiotic treatment (e.g., levofloxacin for pneumonia and metronidazole for diarrhea associated with laboratory-confirmed Clostridium difficile). By April 17, the patient's shortness of breath improved considerably, and he was discharged on April 21. The patient had traveled to Toronto, Canada, for a religious retreat during March 29--30; the event has been linked to subsequent SARS cases among the attendees (4). On April 17, a CDC team traveled to Pennsylvania to assist the Pennsylvania Department of Health in its investigation of this patient and his contacts. Twenty-three HCWs (from hospitals A and B, the physician's office, and the outpatient laboratory) who had contact with the patient before his placement in an isolation room in the hospital were evaluated for their types and durations of contact with the patient, their use of personal protective equipment, and their subsequent health status. Six HCWs with unprotected, close contact were furloughed for 10 days after exposure and advised to monitor their temperatures twice daily and to report fever and respiratory symptoms to the hospital's occupational health clinic. The six furloughed HCWs included three persons from hospital B exposed on April 10 and three persons from hospital B exposed on April 14. While furloughed, two HCWs had mild symptoms (sore throat, rhinorrhea, mild cough, or headache), which resolved without treatment. Two additional HCWs (one each from hospital B and the outpatient laboratory) who had mild respiratory symptoms subsequently were furloughed from work, although neither had fever >100.4o F (>38.0o C) or evidence of SARS on clinical evaluation. After the man was identified as a potential SARS patient, HCWs in hospital B used fit-tested N95 respirators and wore gowns and gloves but did not wear eye protection. The patient had close contact with four family members before SARS was diagnosed. Beginning April 9, the patient and his family members reported intermittently wearing surgical masks during close contact. One family member reported illness consistent with the case definition for suspect SARS; however, symptom onset occurred before contact with the index patient; this family member's illness has resolved and persons who had contact with this family member are being monitored. Among six additional nonfamily contacts, one reported new respiratory symptoms since exposure, but continues to be without fever or other symptoms of SARS. The investigation is ongoing and SARS-CoV testing of specimens from all contacts is under way. Reported by: State and local health departments. SARS Investigative Team; A Peck, MD, C Newbern, PhD, EIS officers, CDC. Editorial Note:The majority of suspect and probable cases of SARS in the United States continue to be travel associated, with only limited secondary spread to contacts such as family members and HCWs. Toronto has been added to the list of areas with suspected or documented community transmission of SARS included in the interim U.S. SARS case definition (2). SARS transmission in Toronto has been limited to a small number of hospitals, households, and specific community settings. In particular, cases of SARS have been documented among some members of a religious community who attended a large gathering in Toronto in late March; some of these persons infected members of their households and other close contacts (4). In response to these reports, CDC recommended that U.S. travelers to Toronto observe precautions to safeguard their health, including avoidance of places in which SARS is most likely to be transmitted (e.g., Toronto health-care facilities) (5). The Pennsylvania resident who attended this religious meeting is the only reported U.S. patient with SARS associated with travel to Toronto. The availability of diagnostic testing for SARS-CoV is critical to more precisely characterize the epidemiology and clinical spectrum of the SARS epidemic, both worldwide and in the United States. Many U.S. patients, particularly those with milder clinical illness, have tested negative for SARS-CoV, reflecting the low specificity of the current case definition, which captures persons with respiratory infections caused by other infectious agents, and underscoring the importance of obtaining convalescent serum samples to make a final determination about infection with SARS-CoV. CDC is planning to update its interim surveillance case definition for SARS to include laboratory criteria in addition to the clinical and epidemiologic criteria. Careful attention to infection-control precautions, both in home and health-care settings, remains critical to containment of SARS. Symptomatic persons should use infection-control precautions to minimize the potential for transmission and should seek health-care evaluation (6). Patients should inform health-care providers about the symptoms in advance so arrangements can be made, if necessary, to prevent potential transmission to others in the health-care setting. Patients in ambulatory settings should be screened promptly for fever, respiratory symptoms, recent travel, and close contact with SARS patients (7). The investigations summarized in this report suggest that, although both patients and health-care providers are aware of appropriate infection-control precautions, additional efforts are needed to ensure that recommended precautions are instituted immediately when SARS is suspected and that such precautions are used consistently and correctly thereafter. Acknowledgments This report is based on data contributed by A Weltman, MD, Pennsylvania Dept of Health; S Stites, MT Temarantz, Northeastern District, Pennsylvania Dept of Health. T Burger, L Rhoades, MD, Lehigh Valley Hospital, Allentown; T Le, MD, Bethlehem, Pennsylvania. References
 Return to top. Figure 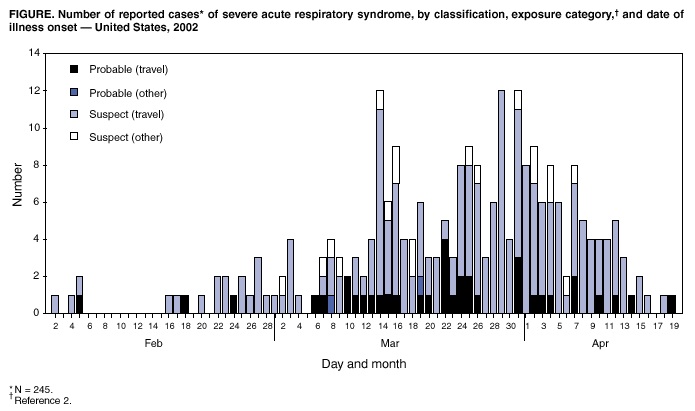 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 4/24/2003 |
|||||||||
This page last reviewed 4/24/2003
|