 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Pneumococcal Vaccination for Cochlear Implant Candidates and Recipients: Updated Recommendations of the Advisory Committee on Immunization PracticesOn July 31, this report was posted on the MMWR website (http://www.cdc.gov/mmwr). In October 2002, CDC recommended that all persons with cochlear implants receive age-appropriate pneumococcal vaccination with 7-valent pneumococcal conjugate vaccine (PCV7) (Prevnar®), 23-valent pneumococcal polysaccharide vaccine (PPV23) (Pneumovax®), or both according to the Advisory Committee on Immunization Practices (ACIP) schedules for persons at high risk (1). CDC issued these recommendations on the basis of preliminary data suggesting an increased risk for pneumococcal meningitis in persons with cochlear implants. Findings of a recent investigation by CDC, the Food and Drug Administration (FDA), and state health departments support this recommendation. Children aged <6 years with a cochlear implant had a substantially greater risk for having pneumococcal meningitis, compared with children in the general U.S. population of the same age (2). Some children who are candidates for cochlear implants have pre-existing anatomic factors that might contribute to an increased risk for meningitis; however, the recent study was not designed to assess this association (2). Because the rate for pneumococcal meningitis is higher in children with cochlear implants and Streptococcus pneumoniae is the most common pathogen causing bacterial meningitis in cochlear implant recipients of all ages with meningitis of known etiology (2,3), ACIP recommends the following for persons who have or are scheduled to receive a cochlear implant (Table): Health-care providers should review vaccination records of their patients who are cochlear implant recipients or candidates to ensure that they have received pneumococcal vaccinations based on the age-appropriate schedules for persons at high risk. In addition, all cases of meningitis should be reported to state health departments according to state requirements. Because information about Streptococcus pneumoniae serotypes causing pneumococcal meningitis in persons with cochlear implants is limited, providers are encouraged to send isolates to their state health department, which can forward isolates to CDC, where serotyping can be performed to determine whether the type is included in the vaccines. To send an isolate, contact CDC's National Center for Infectious Diseases, telephone 404-639-2215. Providers also are encouraged to report cases of meningitis in cochlear implant recipients to FDA's MedWatch. Reports can be submitted online at http://www.accessdata.fda.gov/scripts/medwatch; by telephone, 800-332-1088; by fax, 800-332-0178; or by mail, MedWatch, Food and Drug Administration, HF-2, 5600 Fishers Lane, Rockville, Maryland 20857. Cases also can be reported directly to the device manufacturer. References
Table 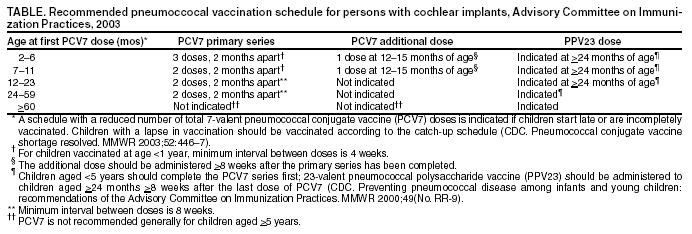 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 8/7/2003 |
|||||||||
This page last reviewed 8/7/2003
|