 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Childhood Influenza Vaccination Coverage --- United States, 2004--05 Influenza SeasonPlease note: An erratum has been published for this article. To view the erratum, please click here. Children aged <2 years are at increased risk for influenza-related hospitalizations, and children aged 24--59 months are more likely than older children to visit a clinic, hospital, or emergency department with influenza-associated illness (1). In 2002, the Advisory Committee on Immunization Practices (ACIP) encouraged annual influenza vaccinations for children aged 6--23 months (and for household contacts of and out-of-home caregivers for children aged <2 years) (2). For the 2004--05 influenza season, ACIP strengthened its encouragement to a full recommendation (3). For the upcoming 2006--07 influenza season, ACIP has further extended its recommendation to include all children aged 6--59 months (and their household contacts and out-of-home caregivers) (1). Others recommended to receive influenza vaccination include children aged 6--18 years who have certain high-risk medical conditions, are on chronic aspirin therapy, or who are household contacts of persons at high risk for influenza complications (1). This report provides an assessment of influenza vaccination coverage among children aged 6--23 months during the 2004--05 influenza season. The findings demonstrate that vaccination coverage in that age group approximately doubled from the 2003--04 influenza season, with substantial variability among states and urban areas. However, the percentage of fully vaccinated children remained low, underscoring the need for increased measures to improve pediatric vaccination coverage and ongoing monitoring of coverage among young children and their close contacts. The findings in this report are based on data from the 2005 National Immunization Survey (NIS), which provides estimates of vaccination coverage among noninstitutionalized children aged 19--35 months at the time of household interview.* For the 2005 reporting period, NIS included children born during February 2002--July 2004 with adequate provider data. The survey was conducted in all 50 states and selected urban areas† (4,5) (Table). Complete influenza vaccination histories were obtained from children's vaccination providers. Two measures of childhood influenza vaccination coverage for the 2004--05 season are reported: 1) receipt of 1 or more doses of influenza vaccine during September--December 2004 and 2) full vaccination (based on ACIP recommendations for 2 doses of influenza vaccine for children who had not received vaccine for a previous influenza season and 1 dose for children who had received influenza vaccine for a previous season) (1). Children were considered fully vaccinated if they had 1) received no doses of influenza vaccine before September 1, 2004, but then received 2 doses from September 1 through the date of interview or January 31, 2005 (whichever came earlier), or 2) received 1 or more doses of influenza vaccine before September 1 and then received 1 or more doses during September--December 2004. Analyses for both measures included only those children who were aged 6--23 months during the entire span of September--December 2004. Data were weighted to adjust for households with multiple telephone lines, household nonresponse, nonassessment of households without telephones, and known population-control estimates. During the 2005 NIS, the household survey response rate was 65.1%; health-care provider vaccination records were obtained for 17,563 children (63.6%) aged 19--35 months for whom household interviews were completed. Of those children, 12,056 (68.6%) (unweighted sample size) met the age criteria for this assessment. Of these, 33.4% (95% confidence interval [CI] = +1.4) had received 1 or more doses of influenza vaccine, and 17.8% (CI = +1.1) were fully vaccinated (Table); consequently, 46.8% of those receiving at least 1 dose during the 2004--05 season needed, but did not receive, a second dose. In comparison, coverage estimates for the 2003--04 season were 17.5% for 1 or more doses of influenza vaccine and 8.4% for fully vaccinated. Substantial variability in influenza vaccination coverage was observed among states and surveyed urban areas. Percentages of children receiving 1 or more doses of influenza vaccine ranged from 9.1% (CI = +5.2) in Clark County, Nevada, to 59.3% (CI = +9.1) in Massachusetts (Table). Percentages of children who were fully vaccinated ranged from 3.3% (CI = +3.4) in Detroit, Michigan, to 35.5% (CI = +8.9) in Massachusetts (Table). Reported by: TA Santibanez, PhD, JA Singleton, MS, KM Shaw, MS, JM Santoli, MD, GL Euler, DrPH, CB Bridges, MD, National Center for Immunization and Respiratory Diseases (proposed), CDC. Editorial Note:The findings in this report indicate that, during the first season in which ACIP recommended routine annual influenza vaccination for children aged 6--23 months, coverage approximately doubled from the previous year. This increase in vaccination coverage from the 2003--04 to the 2004--05 influenza season likely was influenced by the change from an encouragement to a full recommendation. The 2004--05 influenza season was marked by a shortfall of influenza vaccine, resulting from one vaccine manufacturer's unexpected decrease in available supply for distribution in the United States (6). In response to the shortfall, ACIP issued recommendations that vaccine be targeted to persons in eight priority groups, including children aged 6--23 months, and that providers defer vaccination of persons not in the priority groups (6). Because the affected manufacturer's vaccine was not licensed for use in children aged <4 years, the supply of influenza vaccine for children aged 6--23 months for the 2004--05 influenza season was not directly affected by the shortfall. Current projections for the 2006--07 influenza season indicate that approximately 100--115 million doses of influenza vaccine likely will be available. The substantial variability in influenza vaccination coverage for children aged 6--23 months by state and urban area is similar to that observed for other routinely recommended childhood vaccines and is likely attributable to several factors. First, varying degrees of programmatic and provider implementation are observed in the first year after a new ACIP recommendation. Correspondingly, parental awareness, attitudes, and access to influenza vaccination services for children also likely varied. In addition, the influenza vaccine shortage that occurred during the 2004--05 season affected communities differently, with some having greater mismatches between supply and demand. The findings in this report reveal that during the first year of the recommendation, the percentage of children aged 6--23 months who were fully vaccinated for influenza remained low. The importance of 2 doses of influenza vaccine for previously unvaccinated children aged <9 years was highlighted in a recent study (7). During the 2003--04 influenza season, vaccine effectiveness§ in preventing medically attended influenza-like illness (ILI) or pneumonia and influenza (P&I) in fully vaccinated children aged 6--23 months was determined to be 25% and 49%, respectively. In contrast, for children aged 6--23 months receiving 1 dose of influenza vaccine, no statistically significant reduction in ILI or P&I was determined (7). The maximum benefit from influenza vaccination is obtained when all recommended doses are administered before the onset of influenza activity in the community, which might be particularly difficult to achieve among children requiring 2 doses because of the minimum interval of 4 weeks required between doses (8). However, providers should routinely offer influenza vaccine throughout the influenza season, even after influenza activity has been documented in the community (1). The influenza vaccine coverage estimates in this study differ from estimates from the Behavioral Risk Factor Surveillance System (BRFSS), which reported coverage of 48.4% for children aged 6--23 months who received at least 1 dose of influenza vaccine during the 2004--05 influenza season (9). At least three different factors might have contributed to the difference in estimates. First, different birth cohorts were included in the two surveys. BRFSS included children aged 6--23 months at the time of interview in February 2005, whereas NIS included children aged 6--23 months during the entire period of September--December 2004; these differences might have produced greater or lesser estimates, depending upon the population size and vaccination rates of groups excluded from either survey. Second, the vaccination periods differed. BRFSS estimates included vaccinations administered during September 2004--January 2005, whereas NIS estimates for 1 or more doses included vaccinations administered during September--December 2004. Third, BRFSS estimates are based on parental report, which might result in overestimates, whereas NIS estimates are confirmed by provider-reported data. A recent study reported that among children aged 6--23 months whose parents reported they had received influenza vaccination, only 65.8% actually had been vaccinated, according to medical records (10). The findings in this report are subject to at least four limitations. First, NIS is a telephone survey; although statistical adjustments compensate for nonresponse and households without telephones, some bias might remain. Second, NIS relies on provider-verified vaccination histories; incomplete record-keeping or incomplete reporting by providers might result in underestimates of vaccination coverage. Third, the estimates in this report count influenza vaccinations administered during the primary vaccination period and thus underestimate entire season coverage to the extent that vaccination late in the season occurred, particularly for fully vaccinated coverage. The estimates are for children who were aged 6--23 months during the entire September--December 2004 period and thus might overestimate coverage among all children recommended to receive influenza vaccination, to the extent that excluded children had lower coverage (i.e., those who became eligible for influenza vaccination at age 6 months after September 1, 2004, and those who reached 2 years of age before January 2005). Finally, because of sampling uncertainty and wide confidence intervals for many state and urban area estimates from NIS, these estimates should be interpreted with caution. This report underscores the need to continue monitoring annual influenza vaccination coverage among young children, including the newly recommended group aged 6--59 months. In addition, because protection of young children is enhanced by vaccination of household contacts and out-of-home caregivers, monitoring vaccination coverage among these persons also is important. Currently, NHIS is used to monitor vaccination coverage among older children and household contacts of persons aged <5 years; plans for assessing influenza vaccination among out-of-home caregivers are under consideration. Complete recommendations for the 2006--07 influenza season have been published (1), and updates on the influenza season and vaccine supply are available at http://www.cdc.gov/flu. References
* NIS is an ongoing, random-digit--dialed telephone survey of households, followed by a mail survey of all of the children's vaccination providers to obtain vaccination data. † Five new areas were separately sampled by the NIS in 2005: Alameda and San Bernardino counties, California; the Denver, Colorado, area consisting of Adams, Arapahoe, Denver, and Douglas counties; St. Louis County and city, Missouri; and Clark County, Nevada. Six urban areas separately sampled by the NIS in previous years were not separately sampled in 2005 but are included in statewide estimates: San Diego and Santa Clara counties, California; Miami-Dade County, Florida; Orleans Parish, Louisiana; Boston, Massachusetts; and Marion County, Indiana. Although Orleans Parish, Louisiana, was initially oversampled in 2005, estimates are not available because of interruptions in telephone service, movement of the population, and difficulty locating providers in the aftermath of Hurricane Katrina. § For this study, vaccine effectiveness (%) was defined as (1 -- hazard ratio) ´ 100, where the hazard ratio compared the rate of influenza-like illness or pneumonia and influenza outcomes in vaccinated children to the rate in unvaccinated children.
Table 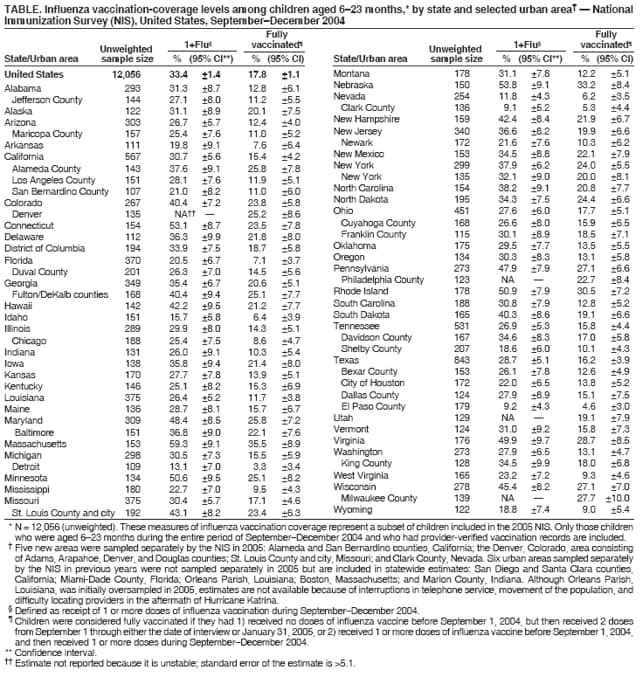 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Date last reviewed: 10/4/2006 |
|||||||||
|