Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Influenza Vaccination Coverage Among Children Aged 6--23 Months --- United States, 2007--08 Influenza Season
Infants and children aged <2 years often require medical care for influenza and have higher rates of influenza-related hospitalization than any other age group except persons aged ≥65 years (1). Since 2004, the Advisory Committee on Immunization Practices (ACIP) has recommended seasonal influenza vaccination for all children aged 6--23 months (2). Full vaccination for these children requires receipt of 2 doses in the current influenza season if they have not been vaccinated previously or received a single dose during the preceding season. To assess influenza vaccination coverage among children aged 6--23 months during September--December of the 2007--08 influenza season, CDC analyzed data from the 2008 National Immunization Survey (NIS). The results of those analyses indicated that, during the 4 months, 40.7% of children aged 6--23 months received ≥1 doses of influenza vaccine, and 23.4% were fully vaccinated. Substantial variability was observed among the 50 states and participating local areas; the percentage of children with full vaccination ranged from 6.4% to 40.9% among states and local areas. Nationally, the percentage of children aged 6--23 months receiving ≥1 doses of influenza vaccine increased from 31.8% in 2006--07 (3) to 40.7% in 2007--08, and the percentage with full vaccination increased from 21.3% to 23.4%; however, influenza vaccination coverage among children remains low. Further study is needed to identify barriers to influenza vaccination and to implement strategies that can increase vaccination coverage with emphasis on attaining full vaccination in this population at greater risk for complications from influenza.
NIS is an ongoing, random-digit--dialed telephone survey of households with children who are aged 19--35 months at the time of interview, followed by a mail survey of all of the children's vaccination providers (nominated by the household respondent) to obtain vaccination data (4). The 2008 NIS* interviews were conducted during January 3, 2008--February 4, 2009, in all 50 states and in 17 local areas.† Histories of influenza vaccination since birth were obtained through a mail survey of children's vaccination providers.
Two measures of influenza vaccination coverage were reported for children aged 6--23 months: 1) receipt of ≥1 doses of influenza vaccine during September--December 2007, and 2) full vaccination. Full vaccination was defined as 1) receipt of 2 doses from September 1, 2007, through the date of interview or January 31, 2008 (whichever was earlier), among previously unvaccinated children and children who received 1 dose for the first time in the previous influenza season, or 2) receipt of 1 dose of influenza vaccine during September 1, 2007--December 31, 2007, among all other children. The definition of full vaccination reflects a change in ACIP recommendations in 2007; the previous ACIP recommendation considered children fully vaccinated if they received 1 dose during the current season and had only 1 dose their first season (5). Vaccination later in the season was not assessed because data collection began in January 2008. NIS methodology, including the weighting procedure, has been described previously (4). Season-to-season comparisons of influenza vaccination coverage estimates were conducted using t-tests to determine statistical significance at p<0.05.
During the 2008 NIS, the household survey response rate was 63.2%§; provider-reported vaccination records were obtained for 18,430 children (71.0%) aged 19--35 months for whom household interviews were completed (4). Among those with provider-reported records, 11,964 met the 6--23 month age criteria for this assessment. Of these 11,964 children, 40.7% received ≥1 or more doses of influenza vaccine, and 23.4% received full vaccination; substantial variability in influenza vaccination coverage was observed among participating states and local areas (Table). The percentages of children receiving full influenza vaccination was >35% in four states (Rhode Island, 40.7%; Massachusetts, 39.8%; Wisconsin, 38.4%; and Connecticut, 35.8%) and one local area (Santa Clara County, California, 40.9%). Full vaccination coverage was <10% in two states (Arkansas, 8.1%, and Mississippi, 7.1%) and three local areas (northern California counties, 9.6%; El Paso County, Texas, 9.6%; and Miami-Dade County, Florida, 6.4%).
Overall in the United States, the percentage of children aged 6--23 months receiving ≥1 doses of influenza vaccine increased 28.0%, from 31.8% in 2006--07 to 40.7% in 2007--08, and the percentage with full vaccination increased 9.9%, from 21.3% to 23.4% (Figure 1) (5). A total of 42.4% of participating children who received at least 1 dose during the 2007--08 required a second dose but did not receive one by January 31, 2008 (or date of interview, if interviewed in January).
First-dose (or only dose) influenza vaccinations most often were administered during epidemiology weeks 42--45 (October 21--November 17, 2007) with a drop in doses administered during week 46 (Figure 2). Among children requiring 2 doses, the second dose was most often administered during weeks 47--50 (November 26--December 22).
Reported by: TA Santibanez, PhD, A Fiore, MD, JA Singleton, MS, Immunization Svcs Div, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note:
This report of influenza vaccination coverage during the 2007--08 influenza season, the fourth season since ACIP recommended routine vaccination for all children aged 6--23 months, indicates the percentage of children receiving ≥1 dose increased 28.0% and the percentage fully vaccinated increased 9.9%, compared with the 2006--07 season. However, despite these increases, the percentage of children fully vaccinated remains low (23.4%). Similarly suboptimal influenza vaccination coverage during the 2007--08 season has been reported for other groups, using data from the National Health Interview Survey: 40.3% among children aged 2--4 years, 38.4% among persons aged 50--64 years, 30.4% among persons with high-risk conditions aged 18--49 years, and 24.2% among pregnant women (6).
Strategies that have been successful at improving influenza vaccination coverage among children include standing orders, vaccination-only visits for children requiring only immunization services, and reminder/recall systems (6). Severity of the influenza season and the amount of corresponding media attention also have been found to affect parental perceptions and acceptance of vaccine for their children (7).
In 2007, ACIP recommended that children aged <9 years who received only 1 dose in their first year of vaccination receive 2 doses the following year, with single annual doses in subsequent years (5). This change in recommendation was based on a study indicating that children aged <9 years who received only 1 dose during their initial year of vaccination and then received 2 doses the following season had better protection against influenza than children who received only 1 dose in each of their first two seasons (8). Although this change in recommendation increased by 19% the number of children in the NIS sample who were recommended to receive 2 doses, a 9.9% increase in the percentage of children receiving full vaccination was observed from 2006--07 to 2007--08.
The findings in this report are subject to at least two limitations. First, because NIS interviews were conducted during the influenza season and some children received influenza vaccinations after the interview, coverage estimates likely are underestimated. Second, coverage estimates might be greater among children in this analysis, compared with all children aged 6--23 months, at some point during September--December. Children who became eligible for influenza vaccination at age 6 months after September 1, 2007 (and thus were excluded from the analysis), might have been less likely to have been vaccinated because of a shorter duration of time they were eligible during the vaccination period. Other limitations of vaccination coverage data obtained through the NIS have been described previously (4,9).
During the 2009--10 influenza season, children aged 6--23 months are recommended to receive both the seasonal influenza vaccine and influenza A (H1N1) 2009 monovalent vaccine; many children in this age group might require 2 doses of each vaccine (10). Vaccination providers are encouraged to begin offering doses as soon as vaccine becomes available and to continue vaccination efforts throughout the influenza season. These recommendations are especially important for children who require 2 doses. When possible, providers should use strategies shown to improve vaccination coverage such as reminder/recall systems and standing orders.
References
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2002;51(No. RR-3).
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2004;53(No. RR-6).
- CDC. Influenza vaccination coverage among children aged 6--23 months---United States, 2006--07 influenza season. MMWR 2008;57:1039--43.
- Smith PJ, Hoaglin DC, Battaglia MP, Khare M, Barker LE. Statistical methodology of the National Immunization Survey, 1994--2002. Vital Health Stat 2 2005(138).
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR 2007;56(No. RR-6).
- CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009 MMWR 2009;58(No. RR-8).
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006;117:e268--77.
- Allison MA, Daley MF, Crane LA, et al. Influenza vaccine effectiveness in healthy 6- to 21-month-old children during the 2003--2004 season. J Pediatr 2006;149:755--62.
- CDC. National, state, and local area vaccination coverage among children aged 19-35 months--United States, 2008. MMWR 2009;58:921--6.
- CDC. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-10).
* Eligible participants were born during January 4, 2005--July 4, 2007.
† The 17 local areas sampled separately for the 2008 NIS included six areas that receive federal immunization grant funds and have been included in the NIS every year since its inception in 1994 (District of Columbia; Chicago, Illinois; New York, New York; Philadelphia County, Pennsylvania; Bexar County, Texas; and Houston, Texas). Also included were eight areas chosen by state grantees based on local need that had been included during 1996--2007 (Los Angeles County, California; northern California counties; Santa Clara County, California; Miami-Dade County, Florida; Baltimore, Maryland; Dallas County, Texas; El Paso County, Texas; and eastern/western Washington counties). Also included were three areas sampled for the first time (Madison and St. Clair counties, Illinois; Minneapolis/St. Paul, Minnesota; and Orange County, Florida).
§ The Council of American Survey Research Organizations (CASRO) household response rate is the product of the resolution rate (82.3%), the screening completion rate (90.3%), and the interview completion rate (85.1%).
|
TABLE. (Continued) Percentage of children aged 6--23 months who received influenza vaccination during September--December 2007, by vaccination status and state/area --- National Immunization Survey (NIS), United States, 2007--08 influenza season |
|||||
|---|---|---|---|---|---|
|
State/Area |
Unweighted sample size |
≥1 doses |
Full vaccination† |
||
|
% |
(95% CI§) |
% |
(95% CI) |
||
|
Tennessee |
213 |
36.1 |
(28.3--44.6) |
21.1 |
(15.1--28.6) |
|
Texas |
901 |
35.6 |
(29.6--42.0) |
18.5 |
(14.2--23.8) |
|
Bexar County |
194 |
33.3 |
(25.8--41.8) |
13.5 |
(9.1--19.5) |
|
City of Houston |
186 |
41.6 |
(33.6--50.1) |
21.9 |
(16.1--29.0) |
|
Dallas County |
178 |
41.2 |
(32.9--49.9) |
19.7 |
(13.9--27.3) |
|
El Paso County |
179 |
23.0 |
(17.3--30.0) |
9.6 |
(6.1--14.7) |
|
Rest of state |
164 |
34.5 |
(26.0--44.0) |
18.7 |
(12.7--26.7) |
|
Utah |
157 |
42.5 |
(33.0--52.6) |
20.6 |
(14.2--28.9) |
|
Vermont |
149 |
46.5 |
(37.7--55.4) |
34.6 |
(26.6--43.6) |
|
Virginia |
142 |
43.0¶ |
(32.8--53.9) |
24.7 |
(16.6--35.0) |
|
Washington |
278 |
40.5 |
(32.9--48.6) |
23.6 |
(17.9--30.5) |
|
Eastern/western counties |
151 |
32.2 |
(24.5--41.0) |
18.6 |
(12.7--26.4) |
|
Rest of state |
127 |
44.0¶ |
(33.7--54.8) |
25.7 |
(18.1--35.1) |
|
West Virginia |
184 |
40.7 |
(33.1--48.7) |
24.3 |
(18.1--31.8) |
|
Wisconsin |
140 |
55.5 |
(45.9--64.7) |
38.4 |
(29.6--47.9) |
|
Wyoming |
165 |
36.6 |
(29.2--44.7) |
23.3 |
(17.2--30.6) |
|
* Only those children aged 6--23 months during the entire period of September--December 2007 and who had provider-reported immunization records are included. † Full vaccination: 1) receipt of 2 doses from September 1, 2007, through the date of interview or January 31, 2008 (whichever was earlier), among influenza vaccine naïve children and children who received 1 dose for the first time in the previous influenza season; or 2) receipt of 1 dose of influenza vaccine during September 1, 2007--December 31, 2007, among all other children. § Confidence interval. ¶ Estimate might not be reliable; confidence interval width >20.0. |
|||||
FIGURE 1. Percentage of children aged 6--23 months who received influenza vaccination during September--December, by influenza season and vaccination status --- National Immunization Survey, United States, 2002--03 through 2007--08 influenza seasons
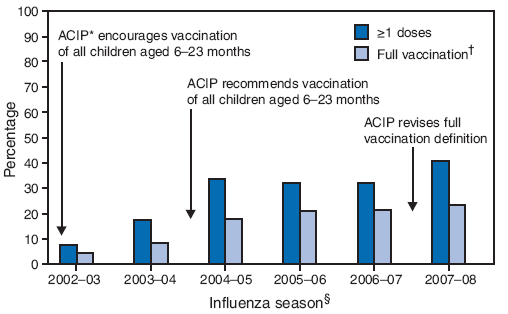
* Advisory Committee on Immunizaton Practices.
† Full vaccination: 1) receipt of 2 doses from September 1, 2007, through the date of interview or January 31, 2008 (whichever was earlier), among influenza vaccine naïve children and children who received 1 dose for the first time in the previous influenza season; or 2) receipt of 1 dose of influenza vaccine during September 1, 2007--December 31, 2007, among all other children.
§ Number of children: 2002--03 (n = 13,831), 2003--04 (n = 13,881), 2004--05 (n = 12,056), 2005--06 (n = 13,546), 2006--07 (n = 9,710), and 2007--08 (n = 11,964).
Alternative Text: The figure above shows the percentage of children aged 6-23 months who received influenza vaccination during September-December, by influenza season and vaccination status, from the National Immunization Survey for the 2002-03 through 2007-08 influenza seasons. According to the figure, in the United States, the percentage of children aged 6-23 months receiving >1 doses of influenza vaccine increased 28.0%, from 31.8% in 2006-07 to 40.7% in 2007-08, and the percentage with full vaccination increased 9.9%, from 21.3% to 23.4%.
FIGURE 2. Percentage of children aged 6--23 months receiving influenza vaccination September--December 2007, by week of vaccination and dose received --- National Immunization Survey, United States, 2007--08 influenza season
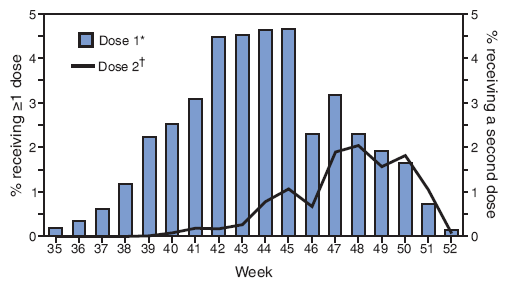
* Among all age-eligible children (n = 11,964).
† Among those age-eligible children who met the Advisory Committee on Immunization Practices recommendation to receive 2 doses during the current influenza season (i.e., had received no influenza dose before September 1, 2007) or who had received only 1 dose for the first time during the 2006--07 influenza season (n = 9,889).
Alternative Text: The figure above shows the percentage of children aged 6-23 months receiving influenza vaccination from September-December 2007, by week of vaccination and dose received, from the National Immunization Survey for the 2007-08 influenza season. According to the figure, first-dose (or only dose) influenza vaccinations most often were administered during epidemiology weeks 42-45 (October 21-November 17, 2007), with a dip occurring in number of doses administered during week 46. Among children requiring 2 doses, the second dose was most often administered during weeks 47-50 (November 26-December 22).
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to [email protected].Date last reviewed: 10/1/2009


