 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Screening Tests To Detect Chlamydia trachomatis and Neisseria gonorrhoeae Infections --- 2002Prepared by The material in this report originated in the National Center for HIV, STD, and TB Prevention, Harold W. Jaffe, M.D., Acting Director, and the Division of Sexually Transmitted Diseases Prevention, Harold W. Jaffe, M.D., Acting Director; and the National Center for Infectious Diseases, James M. Hughes, M.D., Director, and the Division of AIDS, STD, and TB Laboratory Research, Jonathan E. Kaplan, M.D., Acting Director. SummarySince publication of CDC's 1993 guidelines (CDC. Recommendations for the prevention and management of Chlamydia trachomatis infections, 1993. MMWR 1993;42[No. RR-12]:1--39), nucleic acid amplification tests (NAATs) have been introduced as critical new tools to diagnose and treat C. trachomatis and Neisseria gonorrhoeae infections. NAATs for C. trachomatis are substantially more sensitive than previous tests. When using a NAAT, any sacrifice in performance when urine is substituted for a traditional swab specimen is limited, thus reducing dependence on invasive procedures and expanding the venues where specimens can be obtained. NAATs can also detect both C. trachomatis and N. gonorrhoeae organisms in the same specimen. However, NAATs are usually more expensive than previous tests, making test performance from an economic perspective a key consideration. This report updates the 1993 guidelines for selecting laboratory tests for C. trachomatis with an emphasis on screening men and women in the United States. (In this report, screening refers to testing persons in the absence of symptoms or signs indicating C. trachomatis or N. gonorrhoeae infection.) In addition, these guidelines consider tests from an economic perspective and expand the previous guidelines to address detection of N. gonorrhoeae as well as C. trachomatis infections. Because of the increased cost of NAATs, certain laboratories are modifying manufacturers' procedures to improve test sensitivity without incurring the full cost associated with screening with a NAAT. Such approaches addressed in these guidelines are pooling of specimens before testing with a NAAT and additional testing of specimens whose non-NAAT test result is within a gray zone. This report also addresses the need for additional testing after a positive screening test to improve the specificity of a final diagnosis. To prepare these guidelines, CDC staff identified pertinent concerns, compiled the related literature published during 1990 or later, prepared tables of evidence, and drafted recommendations. Consultants, selected for their expertise or disciplinary and organizational affiliations, reviewed the draft recommendations. These final guidelines are the recommendations of CDC staff who considered contributions from scientific consultants. These guidelines are intended for laboratorians, clinicians, and managers who must choose among the multiple available tests, establish standard operating procedures for collecting and processing specimens, interpret test results for laboratory reporting, and counsel and treat patients. IntroductionAn estimated 3 million Chlamydia trachomatis infections occur annually among sexually active adolescents and young adults in the United States (1). The majority of persons with C. trachomatis infection are not aware of their infection because they do not have symptoms that would prompt them to seek medical care (2). Consequently, screening is necessary to identify and treat this infection. Untreated, C. trachomatis infections can lead to serious complications. In certain studies, <40% of women with untreated C. trachomatis infections experience pelvic inflammatory disease (PID) (3,4). Of these, the majority have symptoms that are too mild or nonspecific for them to seek medical treatment. Regardless of symptom severity, the consequences of PID are severe. Of those with PID, 20% will become infertile; 18% will experience debilitating, chronic pelvic pain; and 9% will have a life-threatening tubal pregnancy (5). C. trachomatis infection during pregnancy leads to infant conjunctivitis and pneumonia and maternal postpartum endometritis. Among men, urethritis is the most common illness resulting from C. trachomatis infection. Complications (e.g., epididymitis) affect a minority of infected men and rarely result in sequelae. Among men who engage in receptive anal intercourse, the rectum is a common site of C. trachomatis infection. Rectal infections are usually asymptomatic, but can cause symptoms of proctitis or proctocolitis. C. trachomatis can cause conjunctivitis among adults and is a cause of sexually acquired reactive arthritis.* Estimated tangible costs of C. trachomatis illness in the United States exceed $2.4 billion annually (6). Also critical are the intangible costs, including the psychological and emotional injury caused by infertility and ectopic pregnancy. As of December 2000, all 50 states and the District of Columbia had enacted laws requiring the reporting of C. trachomatis cases. In 2001, C. trachomatis infections were the most commonly reported communicable infections, with a total of 783,242 reports to CDC (7). Rates of C. trachomatis infection for women are highest for adolescents (2,536/100,000 among women aged 15--19 years) and young adults (2,447/100,000 among women aged 20--24 years). These age groups had the highest rates of infection for men as well, although the peak rate occurred among men aged 20--24 years (605/100,000). Reported rates of C. trachomatis infections have risen significantly during 1987--2001 (51--278 cases/100,000 persons) (7). This increase is probably caused by a combination of factors, including an increased awareness of the need to screen women for C. trachomatis infection, resulting in the initiation of screening programs in both public and private health-care settings, improvement in the sensitivity of diagnostic tests, improved surveillance and reporting systems, and continued high infection rates. Introduction of large-scale screening programs (e.g., one initiated in the Department of Health and Human Services Region X [Alaska, Idaho, Oregon, and Washington] family planning clinics in 1988) have been followed by a reduction in C. trachomatis positivity rates by <60% (7--9). C. trachomatis screening programs have been initiated throughout the United States that are based on such demonstration projects. In 2001, Neisseria gonorrhoeae was second in frequency only to C. trachomatis among reported communicable infections in the United States, with 361,705 reported cases (7). The age distribution of N. gonorrhoeae infections is similar to that for C. trachomatis infections. Also similar to C. trachomatis, uncomplicated N. gonorrhoeae infection is usually confined to the mucosa of the cervix, urethra, rectum, and throat; N. gonorrhoeae infection is often asymptomatic among females; and, if untreated, N. gonorrhoeae infection can lead to PID, tubal infertility, ectopic pregnancy, and chronic pelvic pain (10). N. gonorrhoeae usually causes symptomatic urethritis among males, and occasionally results in epididymitis. Rarely, local infection disseminates to cause an acute dermatitis tenosynovitis syndrome, which can be complicated by arthritis, meningitis, or endocarditis (10). Also, similar to C. trachomatis, N. gonorrhoeae can be acquired at birth. N. gonorrhoeae neonatal infection can cause severe conjunctivitis, which can result in blindness if untreated and, rarely, sepsis with associated meningitis, endocarditis, or arthritis. After the introduction of a national control program in the mid-1970s, the overall rate of reported N. gonorrhoeae infection had declined by 74% during 1975--1997 (7,11). However, the rate increased by 8.1% during 1997--1999, followed by a limited decline in 2000--2001. Culture testing for C. trachomatis and N. gonorrhoeae has been the reference standard against which all other tests have been compared. However, other tests have been needed because culture methods for C. trachomatis are difficult to standardize, technically demanding, and expensive. Culture for either agent is associated with problems in maintaining the viability of organisms during transport and storage in the diverse settings in which testing is indicated. Thus, diagnostic test manufacturers have developed nonculture tests that do not require viable organisms, including tests that can be automated. The first nonculture screening tests for C. trachomatis and N. gonorrhoeae included enzyme immunoassays (EIAs), which detect specific chlamydial or gonococcal antigens, and direct fluorescent antibody (DFA) tests for C. trachomatis, which use fluorescein-conjugated monoclonal antibodies that bind specifically to bacterial antigen in smears. These antigen-detection tests were followed by nucleic acid hybridization tests, which detect C. trachomatis-specific or N. gonorrhoeae-specific deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) sequences. With the availability of these nonculture tests, screening programs for C. trachomatis were initiated, and screening programs for N. gonorrhoeae began to change from culture to using the more convenient and, in remote settings, more reliable nonculture methods. The primary drawback of these tests, chiefly for C. trachomatis, is that they fail to detect a substantial proportion of infections (12--23). Consequently, a new generation of nonculture tests, called nucleic acid amplification tests (NAATs), were developed that amplify and detect C. trachomatis-specific or N. gonorrhoeae-specific DNA or RNA sequences. These tests are substantially more sensitive than the first generation nonculture tests (12--23). These guidelines are intended to assist laboratorians, clinicians, and managers 1) select screening tests for C. trachomatis or N. gonorrhoeae from the complex array of tests available; 2) establish standard operating procedures for collecting, processing, and analyzing specimens; and 3) interpret test results for laboratory reporting, counseling, and treating patients. The guidelines were developed through literature reviews and extended consultation with non-CDC sexually transmitted disease (STD) specialists. Testing TechnologiesThe following discussion is a review of the complex array of technologies now available for laboratory diagnosis of C. trachomatis and N. gonorrhoeae infections. For this report, technologies are subdivided into those that are designed for 1) batch testing in a laboratory or 2) point-of-care testing as single tests or a limited number of tests performed while patients await results. Laboratory-based tests include culture, NAATs, nucleic acid hybridization and transformation tests, EIAs, and DFA tests. Point-of-care tests have long included the Gram-stained smear for N. gonorrhoeae. Point-of-care tests for C. trachomatis include solid-phase EIAs and a solid-phase optical immunoassay. The leukocyte esterase test (LET) is a dipstick test that is applied to urine specimens to screen for urinary tract inflammation (see Methods To Enhance Performance or Reduce Costs). Personnel, quality assurance, and quality control requirements relating to the use of all tests for medical care are published in the Clinical Laboratory Improvement Amendments of 1988 (CLIA) regulations (24) and are linked to testing complexity. Laboratory-Based TestsCulture Tests C. trachomatis Culture. Cell culture for C. trachomatis involves inoculating a confluent monolayer of susceptible cells with an appropriately collected and transported specimen. After 48--72 hours of growth, infected cells develop characteristic intracytoplasmic inclusions that contain substantial numbers of C. trachomatis elementary and reticulate bodies. These unique inclusions are detected by staining with a fluorescein-conjugated monoclonal antibody that is specific for the major outer membrane protein (MOMP) of C. trachomatis. Cell culture methods vary among laboratories, leading to probable substantial interlaboratory variation in performance (25). For example, because of a larger inoculum and reduced risk of cross-contamination, the shell vial method of culture is more sensitive and specific than the 96-well microtiter plate method (26,27). In certain laboratories, higher sensitivities are obtained by performing a blind pass in which an inoculated cell monolayer is allowed to incubate for 48--72 hours, after which the monolayer is disrupted and used to inoculate a fresh monolayer that is stained after another cycle of growth (28). Tissue culture detection of C. trachomatis is highly specific if a C. trachomatis-MOMP-specific stain is used, because stained C. trachomatis inclusions have a unique appearance. Less specific inclusion-detection methods using EIA, iodine, and Giemsa are not recommended (29,30). Certain CDC consultants believe that commercial stains employing monoclonal antibodies directed against lipopolysaccharide (LPS), which are genus-specific rather than species-specific, are more sensitive and more economical than species-specific monoclonal antibody stains directed against MOMP. Such stains might be suitable for routine use, but a species-specific stain would be preferable in situations requiring increased specificity. The high specificity of cell culture and ability to retain the isolate make cell culture the first choice when the results will be used as evidence in legal investigations. In addition, cell culture is the only method by which a clinical isolate can be obtained for antimicrobial susceptibility testing. The relatively low sensitivity, long turnaround time, difficulties in standardization, labor intensity, technical complexity, stringent transport requirements, and relatively high cost are the primary disadvantages of cell culture isolation of C. trachomatis. Additional information regarding cell culture for C. trachomatis is available elsewhere (14,28,31). N. gonorrhoeae Culture. Methods of gonococcal culture have been well-described elsewhere (12,32). Specimens are streaked on a selective (e.g.,Thayer-Martin or Martin-Lewis) or nonselective (e.g., chocolate agar) medium if specimens are from nonsterile or sterile sites, respectively. Inoculated media are incubated at 35ºC--36.5ºC in an atmosphere supplemented with 5% CO2 and examined at 24-hour intervals for <72 hours. Culture media for N. gonorrhoeae isolation include a base medium supplemented with chocolatized equine or bovine blood to support the growth of the gonococcus; selective media differ from routine culture media in that they contain antimicrobial agents (i.e., vancomycin, colistin, and an antifungal agent), which inhibit the growth of other bacteria and fungi. Supplemental CO2 can be supplied by a CO2 incubator, candle-extinction jar, or CO2-generating tablets. A presumptive identification of N. gonorrhoeae isolates recovered from a genital specimen on selective medium can be made with a Gram stain and oxidase test. A presumptive identification indicates only that a Gram-negative, oxidase-positive diplococcus (e.g., a Neisseria species or Branhamella [Moraxella] catarrhalis) has been isolated from a specimen. Certain coccobacilli, including Kingella denitrificans, might appear to be Gram-negative diplococci in Gram-stained smears. A confirmed laboratory diagnosis of N. gonorrhoeae cannot be made on the basis of these tests alone. A presumptive test result is sufficient to initiate antimicrobial therapy, but additional tests must be performed to confirm the identity of an isolate as N. gonorrhoeae. Culture isolation is also suitable for nongenital tract specimens. Using selective media is necessary if the anatomic source of the specimen normally contains other bacterial species. The sensitivity of culture can be monitored (i.e., quality-controlled) by evaluating results among males with urethral discharge. Culture results are compared with results obtained by using Gram-stained smear or by using nonselective medium. Vancomycin-sensitive strains, which were relatively common in certain areas in the past, now appear to be an uncommon cause of false-negative cultures (10,12,33,34). The advantages of culture are high sensitivity and specificity, low cost, suitability for use with different types of specimens, and the ability to retain the isolate for additional testing. Retention of the isolate for additional testing might be indicated for medicolegal purposes, antimicrobial susceptibility determination, and subtyping of isolates. The major disadvantage of culture for N. gonorrhoeae is that specimens must be transported under conditions adequate to maintain the viability of organisms. Another disadvantage is that a minimum of 24--72 hours is required from specimen collection to the report of a presumptive culture result. NAATs The common characteristic among NAATs is that they are designed to amplify nucleic acid sequences that are specific for the organism being detected. Similar to other nonculture tests, NAATs do not require viable organisms. The increased sensitivity of NAATs is attributable to their ability to produce a positive signal from as little as a single copy of the target DNA or RNA. Commercial tests differ in their amplification methods and their target nucleic acid sequences. The Roche Amplicor® (manufactured by Roche Diagnostics Corporation, Basel, Switzerland) test uses polymerase chain reaction (PCR); the Abbott LCx® (Abbott Laboratories, Abbott Park, Illinois) test uses ligase chain reaction (LCR); and the Becton Dickinson BDProbeTec™ ET (Becton, Dickinson and Company, Franklin Lakes, New Jersey) test uses strand displacement amplification to amplify C. trachomatis DNA sequences in the cryptic plasmid that is found in >99% of strains of C. trachomatis. The Gen-Probe APTIMA™ (Gen-Probe, Incorporated, San Diego, California) assay for C. trachomatis uses transcription-mediated amplification (TMA) to detect a specific 23S ribosomal RNA target. These nucleic acid amplification methods are also used to detect N. gonorrhoeae. The target for the Roche Amplicor test for N. gonorrhoeae is a 201 base pair sequence within the cytosine methyltransferase gene M:Ngo P11. The Abbott LCx test for N. gonorrhoeae detects a 48 base-pair sequence in the Opa genes, <11 copies of which occur per cell, whereas the BDProbeTec ET for N. gonorrhoeae detects a DNA sequence that is within the multicopy pilin gene-inverting protein homologue. The Gen-Probe APTIMA Combo 2 version of TMA detects the 16S ribosomal RNA (rRNA) of N. gonorrhoeae. Additional overviews of the various amplification methods can be found in other published reports (16,35,36) and in materials available from the manufacturers. The majority of commercial NAATs have been cleared† by the Food and Drug Administration (FDA) to detect C. trachomatis and N. gonorrhoeae in endocervical swabs from women, urethral swabs from men, and urine from both men and women. In addition, other specimens (e.g., those from the vagina [37--50] and eye [51--53]) have been used with satisfactory performance, although these applications have not been cleared by FDA. Testing of rectal and oropharyngeal specimens with NAATs has had limited evaluation and is not recommended. The ability of NAATs to detect C. trachomatis and N. gonorrhoeae without a pelvic examination or intraurethral swab specimen (for males) (e.g., by testing urine) is a key advantage of NAATs, and this ability facilitates screening males and females in other than traditional screening venues (e.g., STD and family planning clinics). A disadvantage of NAATs is that specimens can contain amplification inhibitors that result in false-negative results. Certain manufacturers provide amplification controls to detect inhibition. Selected NAATs might be substantially less sensitive than non-NAATs when performed on urine than when performed on endocervical specimens (54--56) or male urethral swabs (54,56). Although not documented by published head- to-head studies, the majority of CDC consultants believe that non-NAATs are substantially less sensitive than NAATs when used on urine specimens. The nucleic acid primers employed by commercial NAATs for C. trachomatis are not known to cross-react with DNA from other bacteria found in humans. However, the primers employed by certain NAATs for N. gonorrhoeae might cross-react with nongonococcal Neisseria species (54,56,57). NAATs are also more susceptible than non-NAATs to false-positive results because of contamination if strict quality control procedures are not applied. Nucleic Acid Hybridization (Nucleic Acid Probe) Tests Two nucleic acid hybridization assays are FDA-cleared to detect C. trachomatis or N. gonorrhoeae: the Gen-Probe PACE® 2 and the Digene Hybrid Capture® II assays. Both the PACE and Hybrid Capture assays can detect C. trachomatis or N. gonorrhoeae in a single specimen. The PACE 2C test and the Hybrid Capture II CT/GC versions of these tests do not differentiate between the two organisms and, when positive, should be followed by tests for each organism to obtain an organism-specific result. In the Gen-Probe hybridization assays, a DNA probe that is complementary to a specific sequence of C. trachomatis or N. gonorrhoeae rRNA hybridizes with any complementary rRNA that is present in the specimen (58). A competitive probe version of the PACE 2 assay is commercially available to augment specificity. In this version, the test is repeated on initially positive specimens with and without adding an unlabeled probe. The unlabeled probe competitively inhibits binding of the labeled probe; a reduction in signal when the assay is performed with the unlabeled probe is interpreted as verification of the initial positive test result. RNA hybridization probes in the Digene assay are specific for DNA sequences of C. trachomatis and N. gonorrhoeae, including both genomic DNA and cryptic plasmid DNA (59). Technical requirements and expertise necessary for performing nucleic acid hybridization tests are similar to those for the EIAs, which are described in this report. One of the advantages of the nucleic acid hybridization tests is the ability to store and transport specimens for <7 days without refrigeration before receipt and testing by the laboratory. Nucleic Acid Genetic Transformation Tests The Gonostat® test (Sierra Diagnostics, Incorporated, Sonora, California) uses a gonococcal mutant that grows when transformed by DNA extracted from a swab specimen containing N. gonorrhoeae. N. meningitidis causes false-positive results (60). The test has received limited evaluation in published studies (61--64), which include an evaluation of its use with mailed specimens (62). A genetic transformation test is not available for detection of C. trachomatis infection. EIA Tests A substantial number of EIA tests have been marketed for detecting C. trachomatis infection. By contrast, the performance and cost characteristics of EIA tests for N. gonorrhoeae infection have not made them competitive with culture (32). C. trachomatis EIA tests detect chlamydial LPS with a monoclonal or polyclonal antibody that has been labeled with an enzyme. The enzyme converts a colorless substrate into a colored product, which is detected by a spectrophotometer. Specimens can be stored and transported without refrigeration and should be processed within the time indicated by the manufacturer. One disadvantage of the EIA methods that detect LPS is the potential for false-positive results caused by cross-reaction with LPS of other microorganisms, including other Chlamydia species (28,30,65,66). Manufacturers have developed blocking assays that verify positive EIA test results. The test is repeated on positive specimens with the addition of a monoclonal antibody specific for chlamydia LPS. The monoclonal antibody competitively inhibits chlamydia-specific binding by the enzyme-labeled antibody; a negative test result when using the blocking antibody is interpreted as verification of the initial positive test result. EIA tests should not be used with rectal specimens because of cross-reactions with fecal bacteria. DFA Tests Depending on the commercial product used, the antigen that is detected by the antibody in the C. trachomatis DFA procedure is either the MOMP or LPS molecule. Specimen material is obtained with a swab or endocervical brush, which is then rolled over the specimen well of a slide. After the slide has dried and the fixative applied, the slide can be stored or shipped at ambient temperature. The slide should be processed by the laboratory in <7 days after the specimen has been obtained. Staining consists of covering the smear with fluorescein-labeled monoclonal antibody that binds to C. trachomatis elementary bodies. Stained elementary bodies are then identified by fluorescence microscopy. Only C. trachomatis organisms will stain with the anti-MOMP antibodies used in commercial kits. The anti-LPS monoclonal antibodies used in certain commercial kits can cross-react with nonchlamydial bacterial species, as well as with LPS of C. pneumoniae and C. psittaci. DFA with a C. trachomatis-specific anti-MOMP monoclonal antibody is considered to be highly specific, when performed by an experienced microscopist. An additional advantage of DFA is that the quality of endocervical smears can be assessed by checking for the presence of columnar cells (see Collecting and Transporting Specimens for Screening). DFA requires that the laboratorian be competent in fluorescent microscopy and adequately trained in identifying fluorescein-stained C. trachomatis elementary bodies. DFA is best suited for laboratories that test a limited number of specimens, because the procedure is fatiguing and time-consuming. DFA tests have not been established as an initial test for the direct detection of N. gonorrhoeae in clinical specimens. Serology Tests Serology has limited value in testing for uncomplicated genital C. trachomatis infection and should not be used for screening because previous chlamydial infection frequently elicits long-lasting antibodies that cannot be easily distinguished from the antibodies produced in a current infection. More specific information regarding serologic assays for C. trachomatis antibody has been reported elsewhere (14). A serologic screening or diagnostic assay is not available for N. gonorrhoeae. Point-of-Care TestsC. trachomatis Point-of-Care Tests Tests for C. trachomatis have been developed that can be performed within 30 minutes, do not require expensive or sophisticated equipment, and are packaged as single units. The results are read qualitatively. These so-called rapid or stat tests can offer advantages in physicians' offices, small clinics and hospitals, detention centers, and other settings where results are needed immediately (e.g., when decisions need to be made regarding additional testing or treatment while the patient is still present). These tests are classified under CLIA as tests of moderate complexity (24). Accordingly, personnel standards, quality control, quality assurance, and proficiency testing requirements apply when performing FDA-cleared C. trachomatis tests that are rapid enough to qualify as point-of-care tests. In addition, these tests are usually less sensitive and more expensive than laboratory-based C. trachomatis tests that require longer to perform. Similar to EIAs, these tests use antibodies against LPS that detect all three Chlamydia species that infect humans and are subject to the same potential for false-positive results caused by cross-reactions with other microorganisms. A point-of-care test should not be selected if it is performed in a laboratory after the patient's visit. Such use of point-of-care tests is inappropriate because sensitivity and specificity are typically less, controls less rigorous, and costs higher than for tests designed for laboratory use (67). Gram Stain for N. gonorrhoeae Gram stain is a key tool for the diagnosis of gonococcal urethritis in men, but its application to screening is limited because of the requirement for an intraurethral swab specimen if discharge is not present at the urethral meatus. A Gram stain for the presumptive diagnosis of N. gonorrhoeae infection is performed on thin smears of urethral exudate from men and is presumptively positive if the smear contains typical Gram-negative diplococci within polymorphonuclear (PMN) leukocytes. Unfortunately, other Neisseria species have a similar appearance. Although commensal Neisseria species are not normal flora of anogenital sites, isolates of Neisseria meningitidis and nonpathogenic Neisseria species have been reported occasionally from anogenital sites among both men and women (12). As a point-of-care test, Gram stain is most reliable for the presumptive identification of N. gonorrhoeae in urethral exudates from men. The sensitivity and specificity of a Gram stain for males with symptomatic urethritis are comparable to culture isolation followed by oxidase testing and Gram staining of the isolate (32,68,69). Gram-negative extracellular diplococci without any intracellular diplococci might be observed in smears from men with early symptomatic infections. The sensitivity of Gram stain for males with asymptomatic urethral infection has not been determined. Gram-negative intracellular diplococci might be observed with certain infections caused by nongonococcal Neisseria species (e.g., N. cinerea). The sensitivity of Gram stains of endocervical specimens is lower than for urethral specimens from men with symptomatic gonorrhea (10,32,69), and adequate specificity requires a skilled microscopist. For these reasons, Gram stain is not recommended for testing for N. gonorrhoeae infection among women. Gram stains of pharyngeal specimens are not recommended because N. meningitidis and commensal Neisseria species colonize the pharynx (12). As with point-of-care tests for C. trachomatis, the Gram-stained smear is classified under CLIA as a moderate complexity test for urethral and endocervical specimens (24). Gram stains from all other body sites are categorized as high complexity. A trained laboratorian is required for preparing and reading high-complexity Gram-stained smears. C. trachomatis and N. gonorrhoeae Test Performance When Used for ScreeningNumerous evaluations of C. trachomatis and N. gonorrhoeae tests when used for screening have been published. NAAT sensitivities have consistently exceeded the sensitivities of non-NAATs. However, the majority of published estimates of test performance are uncertain because of probable bias from 1) misclassification of study subjects' infection status by reference standard tests; 2) using reference standard tests that are more suitable for determining whether a study subject is infected at one anatomic site (e.g., the endocervix) versus determining whether the study subject is infected at any anatomic site; and 3) predominance of studies of female and symptomatic male patients from STD clinics who might not be representative of populations who would be targeted for screening. Estimates of the differences in the performance of NAATs and non-NAATs are also uncertain because only a limited number of evaluations have been conducted that evaluated the two sets of tests by using the same set of study subjects (i.e., a head-to-head study). Identifying a suitable standard for classifying study subjects with respect to true infection status has been difficult. Customary reference standards (e.g., culture) are highly specific but lack sensitivity. When such standards are used, evaluated test specificity is probably underestimated. Truly infected subjects who are misclassified as uninfected by the reference standard are more likely to be determined positive (and thus, inappropriately considered falsely positive) by the evaluated test than are the uninfected subjects. Misclassification of evaluated test results as false-positives occurs more frequently if the evaluated test (e.g., a NAAT) is more sensitive than the reference test (e.g., culture). The majority of evaluators of C. trachomatis and N. gonorrhoeae tests have attempted to address this problem by using a discrepant analysis procedure. This procedure has been strongly contested by statisticians and clinical epidemiologists because reference tests are applied dependent upon evaluated test results. This dependency contributes a positive bias to estimates of sensitivity and does not eliminate ambiguity regarding specificity estimates (70--75). Identifying a reference standard that accurately classifies study subjects who can be infected at multiple anatomic sites is also problematic, chiefly for women. The majority of test evaluations for women use an endocervical reference standard only. Estimates of evaluated test sensitivity are probably higher and specificity lower, when the reference standard for infection is based on a positive result from a single anatomic site (e.g., an endocervical swab) rather than from multiple potentially infected sites (e.g., an endocervical swab or urine specimens) (54,76,77). The former standard, which produces estimates by using a specimen from one anatomic site, can be considered a specimen standard and addresses the concern of test performance regarding identification of infection at that site. The latter standard can be considered a patient standard. The following sections summarize C. trachomatis and N. gonorrhoeae test performance on the basis of recent test evaluation studies, conducted without discrepant analysis, and consultation with specialists in test evaluation. C. trachomatis Tests for Screening Women and MenSensitivity Reviews of screening tests for C. trachomatis conclude that sensitivities of commercial NAATs exceed those of non-NAATs (14--23). A substantial proportion of the published evaluations of NAATs cited in these reviews have relied on discrepant analysis. However, certain studies permit the calculation and comparison of NAAT and non-NAAT sensitivities by using culture as an independent reference standard. A five-center study reported sensitivities of NAATs for endocervical specimens that exceeded the sensitivity of a nonamplified nucleic acid hybridization test by 19.7% (95% confidence interval [CI] = 12.9%--26.6%) for LCR and 12.4% ( 95% CI = 2.1%--22.7%) for PCR (78). The sensitivities of LCR and PCR were slightly lower when performed on urine specimens than on endocervical specimens (83.4% versus 91.4% and 79.5% versus 84.0% , respectively). The sensitivity of the nonamplified hybridization test (71.6%) was similar to that reported in an earlier study (75.3%) that compared the hybridization test, three EIAs, and a DFA performed on endocervical swab specimens by using culture as the reference standard (79). Sensitivities of the tests evaluated in that study were 61.9%--75.3%. Culture, a NAAT (LCR), and the nonamplified hybridization test have also been compared by using an independent reference standard (78). For this evaluation, the reference standard was a positive PCR performed on an endocervical or urine specimen. For endocervical swab specimens, the sensitivity of LCR (85.5%) exceeded that of culture (74.7%) by 10.8% and that of the hybridization test (61.9%) by 23.6%. For the urine specimen, the sensitivity of LCR was 80.8%. A limited number of studies have used an independent reference standard to compare the sensitivities of tests for detection of C. trachomatis infection in asymptomatic men. One five-center study compared LCR and PCR performed on urine from asymptomatic males by using culture of intraurethral swab specimens as the independent reference standard (80). The sensitivities of LCR (84.4%) and PCR (85.4%) were similar. The majority of C. trachomatis test evaluation specialists consulted for these guidelines believe that the sensitivities of the point-of-care tests for C. trachomatis are substantially less than the sensitivities of tests with longer processing times. However, published studies do not provide a basis for specifying the magnitude of the difference. Specificity and Positive Predictive Value At the prevalences of C. trachomatis infection typical of populations who are screened (e.g., 2%--10%), the specificity of the screening test and the infection prevalence are key variables because they strongly influence the proportion of positive test results that reflect infection. The term used for this proportion is the positive predictive value (PPV) (81,82).§ At lower prevalences, a positive screening test might need to be followed by an additional test to ensure an adequate PPV. Published evaluations of NAATs that have used alternative target NAATs¶ to perform discrepant analysis have reported increased specificities (e.g., exceeding 99.0%--99.5%); such reports have addressed NAATs for detection of C. trachomatis in endocervical specimens, male urethral specimens, and male or female urine. However, statisticians and clinical epidemiologists have criticized these estimation procedures also. Published studies or studies described in package inserts that have used DFA to perform discrepant analysis or have used culture as the standard without performing discrepant analysis have reported specificities of 94.1%--99.5% (75). In 1993, CDC reported a similar range of specificities for nonamplified nucleic acid probe and EIA tests to detect C. trachomatis (31). This report includes a graph of PPVs associated with a test with a sensitivity of 85% across a range of test specificities and prevalences typical of those reported for C. trachomatis screening applications (Figure). All positive tests should be considered presumptive evidence of infection. Regardless of screening setting, screening location, patient population, and patient characteristics, a false-positive test result for C. trachomatis can have adverse medical, social, and psychological impacts for a patient. In such a case, consideration should be given to increasing specificity by performing an additional test after a positive screening test and requiring that both the screening test and additional test be positive to make a diagnosis of C. trachomatis infection (83). PPV of the diagnosis will be increased unless the screening and additional tests are falsely positive for the same reasons (e.g., both are mislabeled or both cross-react with a nonchlamydial organism). However, an additional test does not fully resolve all concerns because it might provide a false-negative result. Because therapy for C. trachomatis is safe and should not be delayed, therapy can be offered while awaiting additional test results or even if the additional test is negative. Patients with positive screening test results require counseling regarding both the risks of delaying therapy and the possibility of a false-positive test result. Consideration should be given to routine additional testing for persons with positive C. trachomatis screening tests when risk factor information or actual surveys indicate that the prevalence is low, resulting in an inadequate PPV (e.g., <90%) (see Additional Considerations in Selecting a Screening Test and also, Methods To Enhance Performance or Reduce Costs). N. gonorrhoeae Tests for Screening Women and MenSensitivity As for C. trachomatis, a substantial proportion of published evaluations of NAATs for N. gonorrhoeae have relied on discrepant analysis for calculation of test sensitivities, which might have positively biased the estimates. However, a meta-analysis of evaluations of N. gonorrhoeae summarizes sensitivity estimates for those studies for which sensitivity estimates could be based on culture as the independent reference standard (13). By using a culture standard, sensitivities were similar for an endocervical swab nucleic acid hybridization test (92.1%) and an FDA-cleared NAAT (96.7%). Package inserts for NAATs recently cleared by FDA now include results of studies conducted by using independent reference standards without employing discrepant analysis. Two such package inserts include the results of culture, a previously FDA-cleared NAAT, and the candidate NAAT (84,85). When the independent reference standard for infection was a previously FDA-cleared NAAT positive on either endocervical swab or urine specimens, the sensitivities of endocervical swab culture were less than for the Becton Dickinson BDProbeTec (83.7% versus 90.2%) and Gen-Probe APTIMA (89.3% versus 100.0%) tests; the sensitivities of the NAATs were lower when performed on urine than on endocervical swabs (78.2% versus 90.2% and 91.7% versus 100.0%, respectively). Compared with tests for C. trachomatis, differences in sensitivities among NAATs, nucleic acid hybridization tests without nucleic acid amplification, and culture are reduced when using endocervical swabs. The exception is that culture sensitivity can decline when transport or storage conditions compromise organism viability. Gram-stain sensitivity is not presented for men because Gram stain of urethral swab smears is seldom used for screening men outside STD clinic settings, and its performance for that purpose has not been well-evaluated. Gram stain is also not widely used for screening women because of decreased sensitivity and variable specificity. Specificity and PPV As with C. trachomatis, test specificities and prevalences among populations screened for N. gonorrhoeae are key determinants of PPV of positive screening test results. Reported specificities of N. gonorrhoeae screening tests are similar to those for C. trachomatis. Unlike C. trachomatis tests, cross-reactivity between N. gonorrhoeae and pathogenic and nonpathogenic Neisseria has been demonstrated for certain NAATs (54,56,57). This cross-reactivity has not been reported for the Abbott LCx and Gen-Probe APTIMA and PACE 2 tests. However, nongonococcal Neisseria are infrequently recovered from the genitourinary tract. As with C. trachomatis tests, all positive screening tests should be considered presumptive evidence of infection, and routine additional testing after a positive N. gonorrhoeae screening test result would usually be indicated when screening among a low-prevalence population. Prevalences of N. gonorrhoeae are usually lower than for C. trachomatis, possibly resulting in lower PPVs. The decision to screen and conduct additional tests after a positive screening test should be made separately for the two organisms (see Additional Considerations in Selecting a Screening Test and also, Methods To Enhance Performance or Reduce Costs). Selecting Screening TestsMultiple considerations affect the selection of a screening test. Test sensitivity is emphasized to minimize occurrence of false-negative tests, which can result in complications of untreated infection and ongoing transmission. However, additional considerations might lead to the selection of a different test (see Additional Considerations in Selecting a Screening Test). For example, a goal of maximizing test sensitivity to avoid missing the opportunity to treat infected persons might warrant tolerating a limited number of false-positive diagnoses. However, consideration must also be given to reducing the rate and consequences of false-positive tests and to cost (see Methods To Enhance Performance or Reduce Costs). This report focuses on screening applications of tests for C. trachomatis and N. gonorrhoeae infections. This report also contains a listing of additional indications for C. trachomatis testing and recommendations for choice of test and type of specimen (Appendix A); similar information for N. gonorrhoeae is also included (Appendix B). Performance Perspective for Selecting Screening TestsOn the basis of sensitivity, ease of specimen collection, and ability to assess antimicrobial susceptibility (N. gonorrhoeae), recommendations for screening women and men for C. trachomatis and N. gonorrhoeae genitourinary tract infections are outlined in this section. Recommended screening tests will, compared with less sensitive tests, minimize the risk for disease sequelae and continued transmission of infections as a result of false-negative screening tests. Clinicians should be aware of the potential for adverse consequences caused by a false-positive test result (e.g., substantial psychosocial or legal consequences); patients with positive results should be counseled regarding the potential for false-positive results, and additional testing should be considered. Because such a result might itself be falsely negative and therapies for C. trachomatis and N. gonorrhoeae are safe and effective, treatment might be offered while awaiting results from additional testing or even if an additional test is negative. At lower prevalences, consideration should be given to routine additional testing after a positive screening test. Testing strategies have been proposed that can increase specificity and constrain costs by combining use of NAATs and non-NAATs. A particularly promising strategy called gray-zone testing involves screening with a non-NAAT and using a lower cutoff value than that established by the manufacturer as a criterion for a positive result. A NAAT is then performed as an additional test when the non-NAAT screening test results are in a zone above the new cutoff value. This strategy could achieve greater sensitivity than would be provided by using a non-NAAT by itself and greater specificity than would be provided by the separate use of either a non-NAAT or a NAAT, and cost less than using a NAAT by itself. This strategy might be useful for screening among lower prevalence populations for which both PPV and cost of detecting an infection are of increased concern. This strategy warrants further evaluation (see Additional Considerations in Selecting a Screening Test and also, Methods To Enhance Performance or Reduce Costs). Screening Women for C. trachomatis and N. gonorrhoeae Genitourinary Tract Infections The following recommendations are based on sensitivity, ease of specimen collection, and ability to assess antimicrobial susceptibility (N. gonorrhoeae) (Box 1). Additional concerns, including cost and PPV, are addressed in subsequent sections (see Additional Considerations in Selecting a Screening Test and also, Methods to Enhance Performance or Reduce Costs). C. trachomatis.
No tests have been cleared by FDA for use with vaginal specimens. However, studies have assessed using vaginal specimens for screening by NAATs (37--49), including vaginal specimens collected by the patient (37,38,40,42,44,47--49). The results of these studies are promising. Unless manufacturers obtain FDA clearance for this intended use, individual laboratories testing vaginal specimens will need to conduct a study to establish, rather than just to verify, test performance characteristics to satisfy CLIA requirements (Appendix C). The sensitivity of non-NAATs with urine or vaginal swab specimens is suboptimal. In the case of EIAs, specificity with vaginal swab and urine specimens is also lower than with endocervical swab specimens (65). Such uses of non-NAATs are not recommended. N. gonorrhoeae.
As with C. trachomatis, no tests are FDA-cleared for use with vaginal specimens, although a study that assessed using patient-obtained vaginal specimens for screening by NAATs yielded promising results (50). Unless manufacturers obtain FDA clearance for this intended use, laboratories testing vaginal specimens will need to conduct a study to establish, rather than just to verify, test performance characteristics to satisfy CLIA requirements (Appendix C). The sensitivity of non-NAAT tests to detect N. gonorrhoeae with urine or vaginal swab specimens is suboptimal. Such uses of non-NAATs are not recommended. Screening Men for C. trachomatis and N. gonorrhoeae Urethral Infections This section includes recommendations for tests for screening men for C. trachomatis and N. gonorrhoeae urethral infections. These recommendations are based on sensitivity, ease of specimen collection, and ability to assess antimicrobial susceptibility (N. gonorrhoeae) (Box 2). Additional concerns, including cost and PPV, are addressed in subsequent sections (see Additional Considerations in Selecting a Screening Test and Methods to Enhance Performance or Reduce Costs). C. trachomatis.
N. gonorrhoeae.
Screening Women or Men with Possible Rectal or Pharyngeal Exposure to C. trachomatis or N. gonorrhoeae Infection Selecting tests for screening for C. trachomatis and N. gonorrhoeae rectal or pharyngeal infections is difficult because of limited experience with nonculture tests and increased potential for cross-reactivity with other organisms (Box 3). C. trachomatis.
N. gonorrhoeae.
If the foregoing methods are not available, screening for C. trachomatis and N. gonorrhoeae in rectal or pharyngeal specimens is not recommended. Only limited evaluations have been published of nonculture tests to detect C. trachomatis or N. gonorrhoeae oropharyngeal or rectal infections (32,94,95). Additional Considerations in Selecting a Screening TestIn addition to test sensitivity, ease of specimen collection, and assessment for antimicrobial susceptibility (N. gonorrhoeae), other considerations in choosing a screening test include 1) the relatively high cost of NAATs (i.e., economic considerations); 2) laboratory environmental changes necessary to implement NAATs; 3) the need for additional testing to support C. trachomatis or N. gonorrhoeae diagnoses; and 4) the likelihood of screening-test--positive persons returning for treatment. The ability of some tests to detect C. trachomatis and N. gonorrhoeae in the same specimen might also affect the choice of test (see Methods To Enhance Performance or Reduce Costs). Recommendations for transporting and storing specimens must also be considered (Appendix D). Economic Analysis Economic analyses can assist in selecting the optimal tests and strategy for C. trachomatis and N. gonorrhoeae screening. Moreover, economic analyses can be relevant for STD screening because, in the absence of symptoms or signs, the cost of screening tests, including the costs of labor and other nonkit costs, is often not reimbursed by insurance plans or other payers. Thus, knowing the costs and benefits of a screening program that might not be fully reimbursed can be critical to decision makers (Box 4). Laboratory Environment The laboratory must consider how candidate tests match the laboratory environment. For example, laboratories must satisfy the requirements for physical space, separation of work areas (e.g., sample processing and amplification), and use of any special equipment (e.g., biosafety cabinets) as recommended by the test kit manufacturer. Differences in throughput among different NAATs must be considered. Additional considerations include technician time, turnaround time, technical difficulty, equipment costs, and time required to maintain equipment. Laboratories are encouraged to investigate these concerns and their impact on cost-effectiveness when choosing among the available testing methods. Need for Additional Testing To Support C. trachomatis or N. gonorrhoeae Diagnoses Efforts to maximize test sensitivity to avoid missing the opportunity to identify and treat infected persons might warrant tolerating a certain number of false-positive diagnoses. However, consideration must also be given to reducing the rate and consequences of false-positive tests. All tests, including culture for C. trachomatis, occasionally generate false-positive results. For these reasons, all positive tests are considered to be presumptive evidence of infection (Box 5). Regardless of health-care provider setting, patient population, and patient characteristics, a false-positive test result for C. trachomatis or N. gonorrhoeae can have adverse medical, social, and psychological impacts for a patient. In such cases, consideration should be given to performing an additional test to verify a positive screening test. The potential for false-positive test results complicates the interpretation of positive tests among patients in populations with a low prevalence of infection. This occurs because the proportion of total positive tests that are truly positive (i.e., PPV) is lower among such a population. Consideration should be given to routine additional testing for persons with positive C. trachomatis or N. gonorrhoeae screening tests when risk-factor information or actual surveys indicate that the prevalence is low, resulting in a lower PPV (e.g., <90%) (see C. trachomatis and N. gonorrhoeae Test Performance When Used for Screening). Economic analysis of additional testing to increase the specificity of a screening test is problematic because the primary benefit of such additional testing is in averting costs that are intangible. These include psychosocial costs that can result from a false-positive test result. These costs vary according to setting and are not well-researched. The benefits of reducing these intangible costs must be weighed against the tangible direct costs of performing the confirmatory tests. Fortunately, these costs apply only to patients who initially test positive and frequently constitute only a limited proportion of total costs per positive test, particularly in low prevalence settings where the additional testing is typically needed (see Methods To Enhance Performance or Reduce Costs). Consideration of Point-of-Care Testing Point-of-care tests for C. trachomatis screening are less sensitive than laboratory-based tests but should be considered in situations where screening-test--positive persons might fail to return for treatment or return after substantial delays. Point-of-care tests are not a cost-effective option if they are processed after the patient visit because they are relatively insensitive and require labor-intensive processing. Each health-care provider needs to compare the sensitivities, costs, and treatment rates for point-of-care and laboratory-based tests. Providers need to determine whether the opportunity to provide treatment to certain patients who would otherwise go untreated warrants the additional cost and less favorable sensitivity of point-of-care testing. FDA-cleared C. trachomatis and N. gonorrhoeae tests that can be performed rapidly enough to qualify as point-of-care tests must be performed in a CLIA-certified laboratory because they are classified under CLIA as moderate complexity tests (24). Methods To Enhance Performance or Reduce CostsDifferent approaches have been used to increase the efficiency of standard screening methods. Although selective screening is not a laboratory method, using selective screening criteria is included in the following discussion because the predictive values and cost to detect an infection are strongly influenced by infection prevalence. Another approach is to use a NAAT to test specimens that yield results from an EIA or unamplified nucleic acid probe test that fall in a zone around the cutoff (i.e., gray zone). This technique warrants further evaluation as a method to decrease the gap in sensitivity between NAATs and other tests without incurring the full additional cost of testing all specimens with a NAAT. Interest in pooling specimens for testing by a NAAT is similarly motivated. Augmenting screening tests with additional testing to improve test specificity is of increasing importance because C. trachomatis and N. gonorrhoeae prevalences have declined after the introduction of screening programs and because C. trachomatis screening has expanded into lower prevalence populations. Using test formats (e.g., nucleic acid probe tests or NAATs) that permit testing for both C. trachomatis and N. gonorrhoeae might reduce costs. Finally, the urine leukocyte esterase test, which has a low sensitivity but is inexpensive, has been used to select specimens for testing with a specific C. trachomatis or N. gonorrhoeae test when use of a more sensitive initial test was not feasible. Selective Screening To Increase the Percentage of Positive Tests Selecting persons for testing who are at high risk can increase the prevalence of infection among tested persons, thereby reducing screening costs to detect persons with C. trachomatis infection. Symptoms or signs are not included as screening criteria because they warrant immediate diagnostic testing. Evaluations of screening algorithms for C. trachomatis, including an algorithm recommended by CDC in 1993 (31), have been published (96--104). The third U.S. Preventive Services Task Force (USPSTF) reviewed this information and published C. trachomatis screening recommendations (105) (available at http://www.ahrq.gov/clinic/uspstfix.htm) (Appendix E). USPSTF also concluded that satisfactory urine screening tests for men had been introduced too recently for sufficient evidence to have been developed to make screening recommendations. Using criteria to select women or men to screen for N. gonorrhoeae infection has received only limited evaluation, although the prevalence of N. gonorrhoeae is usually lower than the prevalence of C. trachomatis. Thus, concerns regarding the cost to detect an infection and PPV might be greater for N. gonorrhoeae than for C. trachomatis (106). Gray-Zone Testing To Improve Test Performance To establish the positive-negative cutoff value for a diagnostic test, a manufacturer attempts to achieve the highest sensitivity while keeping the specificity at or near 100%. This is done by setting the cutoff at the lowest level possible while minimizing false-positive results. Certain test readings from truly infected persons will fall below this cutoff value (false-negatives). Often, a substantial proportion of the readings for persons with false-negative results cluster just below the cutoff, whereas the readings for persons with truly negative results are clustered farther below the cutoff. Consequently, a relatively narrow zone below the cutoff might exist within which the proportion of readings that are from truly infected persons is substantially higher than the proportion of readings farther below the cutoff. The ratio of true positives to true negatives in this zone is affected by the prevalence of infection as well as by test performance among the tested population. Similarly, false-positive results are clustered just above the cutoff, whereas the readings for persons with truly positive results are clustered farther above the cutoff. A relatively narrow zone above the cutoff might exist within which the proportion of readings that are from truly uninfected persons is substantially higher than the proportion of readings farther above the cutoff. When screening test results are in these zones, commonly called the negative and positive gray zones, respectively, a laboratory has the option of retesting specimens with another test. By using a NAAT to retest specimens with gray-zone results from less sensitive and less expensive screening tests, the sensitivity and specificity of the screening test might be improved at less cost than switching entirely to the NAAT. However, such a restricted use of a NAAT might be less sensitive than testing the entire population with a NAAT. A NAAT is also theoretically preferable to a competition or blocking antibody assay for additional testing of specimens from persons with positive non-NAAT screening tests (see Additional Testing To Improve Test Specificity). Testing strategies that combine use of a NAAT by itself in higher prevalence populations and as an additional test after gray-zone non-NAAT results among lower prevalence populations warrant further evaluation. Laboratories should establish feasibility, cost-effectiveness, and gray-zone limits before implementation of the strategy. Although gray-zone testing with a NAAT has been evaluated in multiple studies (107--113), these studies have not employed independent reference standards. In certain cases, the second test was performed on the specimen that was tested by the screening test. If the second test has not been cleared by FDA for use with that type of specimen, CLIA requires that the laboratory conduct a study to establish the performance of the second test when conducted on the screening test specimen (Appendix C). This regulatory requirement is critical because NAATs might generate more false-positive and false-negative results when performed on specimens collected for tests designed with less stringent protection against contamination and amplification enzyme inhibitors. Gray-zone testing must be evaluated in appropriately designed research studies by using an independent reference standard before a recommendation is made regarding its utility in routine practice. Pooling Specimens To Reduce Costs Because of the high sensitivity of NAATs, pooling specimens before testing for C. trachomatis and N. gonorrhoeae with LCR and PCR has been proposed as a method of reducing costs (114--120). Samples of individual specimens are first combined into a pool, which is then tested by a NAAT. If the pool is negative, all specimens forming the pool are reported as negative. If the pool is positive, a second aliquot of each specimen that contributed to the pool is tested individually. The potential cost-savings with pooling increases with decreasing prevalence of infection, because more specimens can be included in a pool at lower prevalences without increasing the probability of a pool testing positive. Available evidence indicates that pooling might be a cost-effective alternative to testing individual specimens with minimal if any loss of sensitivity or specificity (114--120). However, insufficient data exist from published, peer-reviewed studies that address the implementation or performance concerns raised by pooling to make a recommendation concerning testing of pooled specimens. Theoretically, pooling could either increase or decrease the sensitivity and specificity of a NAAT compared with processing individual specimens. To be reported as positive, pooled specimens must be positive according to the pooled result and according to the individual test. This requirement should contribute an increase in specificity and a decrease in sensitivity compared with individual testing. In addition, pooling could reduce NAAT sensitivity because the majority of pooling protocols result in a reduced amount of an individual specimen being tested. Moreover, pooling can permit inhibitors present in a single specimen to cause specimens that would have been truly positive when tested individually to be falsely negative when tested in a pool. Such inhibition will go undetected if an internal control for inhibition is not included when testing pooled specimens. Conversely, dilution of specimens by pooling can decrease the effect of inhibitors. Pooling adds testing and data-recording steps that can result in errors and reporting of additional false-positive or false-negative results. In addition, pooling results in a higher number of specimens in a run being positive. This increases the chances for sub-optimal technique (e.g., by pipetting) to result in cross-contamination and false-positive results. Published studies of pooling using PCR and LCR tests for C. trachomatis and N. gonorrhoeae are still limited, and the majority of such studies have been conducted without an independent reference standard. Nevertheless, published studies indicate that pooling 4--10 urine or endocervical specimens before testing can substantially reduce costs and improve throughput while maintaining the high performance characteristics associated with NAATs (114--120). Savings from reduced reagent costs have ranged from 40% to 60%. Because of the increased complexity of the pooling protocol, savings in personnel time are proportionately less than the savings in reagent costs. In addition to these performance concerns, the following concerns should be addressed when considering use of pooling:
Because pooling is a modification of FDA-cleared procedures, laboratories that implement pooling of patient specimens must satisfy CLIA requirements by establishing performance specifications (Appendix C). Additional Testing To Improve Test Specificity An additional test might be indicated for a person with a positive screening test result, if a false-positive result would have a serious adverse consequence (Box 5). Because treatments for C. trachomatis and N. gonorrhoeae are safe and relatively inexpensive, the person might wish to receive and complete treatment while additional testing is being done, or even if the additional test is negative. Routine additional testing to improve the predictive value of a positive screening test should be considered when the prevalence of either C. trachomatis or N. gonorrhoeae infection is low, resulting in a low PPV (e.g., <90%) (see C. trachomatis and N. gonorrhoeae Test Performance When Used for Screening and also, Additional Considerations in Selecting a Screening Test). False-positive results might occur for multiple reasons, including the following:
Approaches to detect false-positive results by applying an additional test can be ordered by preference on the basis of theoretical considerations (Box 6). Theoretically, testing a second specimen with a different type of test is least likely to confirm a false-positive result, whereas repeating the original test on the original specimen is most likely to do so. Using a NAAT as an additional test after a positive non-NAAT test might be an effective and highly economical approach that deserves additional evaluation. However, except when culture is used to obtain an isolate, a non-NAAT should not be used as an additional test after a NAAT because of the lower sensitivity of non-NAATs. Such additional testing could be extended to persons with non-NAAT screening test results in the gray zone (see Gray-Zone Testing To Improve Test Performance). An advantage of isolating the organism by culture is flexibility in the choice of multiple additional testing procedures, and compared with NAATs, a reduced risk of error caused by contamination during initial or subsequent testing. Using an FDA-cleared test as an additional test to verify the positive results of a screening test does not by itself introduce additional requirements under CLIA (24). However, if the additional test is performed on the original screening test specimen and the additional test has not been cleared by FDA for use with that type of specimen, CLIA requirements for establishing performance characteristics (24) need to be satisfied (Appendix C). Additional Testing After a Positive C. trachomatis Screening Test. Selection of an additional test to verify a positive C. trachomatis screening test is straightforward when a second specimen can be collected, as when a patient returns for screening test results or when the need for additional testing can be anticipated when the screening specimen is collected (Box 7). Using culture with a C. trachomatis-specific anti-MOMP stain as the additional test has the advantage of high specificity and flexibility in choice of additional testing, if warranted. However, culture lacks sensitivity and necessitates obtaining and maintaining a suitable sample either at the initial or a subsequent visit. This fact has made culture less desirable. In addition, finding a laboratory to perform culture can be problematic. If obtaining a separate specimen for additional testing is difficult, the additional test must perform adequately with the transport medium used for the screening test. Using a blocking antibody format to verify a positive EIA screening test and using a competitive probe format to verify a positive nucleic acid probe screening test have been the additional tests most widely used. The blocking antibody or competitive probe additional test is usually performed on the original screening specimen. These formats have been widely used for additional testing, despite not being the theoretically preferred approaches. This is because approaches preferred on theoretical grounds introduce the logistical and economic challenges of establishing a format for the additional test that differs from that of the screening test. Collecting a separate specimen for the additional test also presents logistical difficulties and increases cost. Studies have not been conducted to compare application of such different approaches. Because of the greater sensitivity of NAATs, a NAAT is the only recommended additional test to verify a result from another NAAT and is, potentially, a superior additional test to verify a non-NAAT positive C. trachomatis screening test. Except for using culture to obtain an isolate, a non-NAAT should not be used as an additional test after a NAAT because of the lower sensitivity of the non-NAAT. Using a NAAT as an additional test has received limited evaluation. In particular, determining that NAAT specificity and sensitivity are not reduced is critical if a NAAT is performed on specimens collected, transported, and possibly processed by using non-NAAT procedures that might be less stringent with respect to preventing contamination and inhibition of amplification enzymes. Manufacturers of NAATs for C. trachomatis infection have developed alternate target (e.g., MOMP targets) versions of their commercial tests that the manufacturers have employed for resolving discrepant results in evaluation studies. Using the same format for the additional test as for the screening test offers logistical and economic advantages analogous to those offered by the blocking antibody and competitive probe approaches for non-NAAT tests. Such assays are not offered commercially by NAAT manufacturers but might be in the future. Additional Testing After a Positive N. gonorrhoeae Screening Test. Methods to confirm that Gram-negative, oxidase-positive, bacteria isolated on a selective culture medium are N. gonorrhoeae have been reviewed (Box 8) (12,32). Acid production from carbohydrates and the nucleic acid probe culture confirmation test (AccuProbe,® manufactured by Gen-Probe, San Diego, California) are the most sensitive and specific methods. PACE 2 is also FDA-cleared for identifying N. gonorrhoeae. Pure growth on subculture is required for acid production tests, but not for the AccuProbe test. AccuProbe is not known to cross-react with other organisms found in humans; however, variant strains of certain pathogenic and commensal Neisseria species can provide false-positive acid production results, and certain strains of N. gonorrhoeae might provide false-negative acid production results. Additional tests will be required to differentiate between species that might produce acid from glucose but not from maltose, sucrose, or lactose (Appendix F) or to identify variant gonococcal isolates that fail to produce acid from glucose. Although nongonococcal pathogenic and commensal Neisseria species are commonly found in the oropharynx, they are unusual in genitourinary specimens. Either the acid production or the nucleic acid probe methods could be used for routine additional testing after presumptively positive screening cultures. Requiring that both types of tests be positive should guarantee an increased level of specificity. A detailed discussion of problems in differentiating N. gonorrhoeae from other Neisseria and related species is located at http://www.cdc.gov/ncidod/dastlr/gcdir/NeIdent/Index.html. If an isolate cannot be conclusively identified as N. gonorrhoeae at a local laboratory, the isolate should be sent to a reference laboratory (e.g., a city, county, or state public health laboratory) for confirmation, chiefly in cases of alleged sexual assault or rape. Antibody tests (i.e., tests that detect gonococcal antigens) are not recommended for detection of N. gonorrhoeae. Coagglutination tests, which might cross-react with nongonococcal strains, are less sensitive or specific than acid production tests or the AccuProbe or PACE 2 tests. The monoclonal fluorescent antibody test for N. gonorrhoeae does not detect all gonococcal isolates and is not recommended as the primary test for detecting N. gonorrhoeae. Additional testing after positive nonculture screening tests for N. gonorrhoeae involves the same approaches that are described in the previous section for C. trachomatis. Because verification of positive nonculture tests for N. gonorrhoeae has received only limited evaluation and certain NAATs might cross-react with nongonococcal Neisseria species (54,56,57), substantial effort is warranted to arrange for culture and the assistance of a reference laboratory if a false-positive diagnosis of N. gonorrhoeae would have serious adverse consequences, as in testing of children. Reporting Test Results. When additional testing has been performed, the laboratory should report the results of both the screening test and the additional tests, as well as the overall interpretation. The laboratory has the responsibility to educate clinicians regarding the importance of all laboratory results, including both screening and additional test results. In particular, clinicians need to be aware of the limitations of the additional tests, including the possibility that they yield false-negative results when the screening test is positive. Because serious side effects from therapies for C. trachomatis and N. gonorrhoeae are uncommon, clinicians might recommend treatment after a positive screening test for a person at risk for infection, pending additional testing or even when a positive screening test is not verified by additional testing. Cotesting for C. trachomatis and N. gonorrhoeae To Reduce Costs Multiple tests permit testing for both organisms by using the same specimen. The prevalence of N. gonorrhoeae is less than C. trachomatis in the majority of areas of the United States; however, the prevalence of each varies widely, even within such limited areas as cities or counties. Usually, screening for N. gonorrhoeae will not be justified unless screening for C. trachomatis is also warranted. Decisions regarding screening for either or both organisms should not be made without a careful evaluation of the local epidemiology of N. gonorrhoeae and C. trachomatis. Cotesting for C. trachomatis and N. gonorrhoeae by using tests specially designed for such cotesting should be considered, if transport conditions would reduce the sensitivity of N. gonorrhoeae culture or if using such tests reduces the cost. However, provision should be made to perform an additional test to improve test specificity whenever indicated (see Additional Testing To Improve Test Specificity) and to obtain isolates for antimicrobial susceptibility testing in the case of a repeated treatment failure (see Test of Cure, Treatment Failure, and Antimicrobial Resistance). Using LET to Select for C. trachomatis or N. gonorrhoeae Tests LET, which has a low sensitivity but is inexpensive, has been used to select specimens for testing with a specific C. trachomatis or N. gonorrhoeae test when universal testing with a more sensitive initial test is not feasible. Studies have demonstrated that using a positive LET to select patients for screening with a more sensitive and specific test can be cost-effective. Published studies have focused primarily on asymptomatic men and have reported that this approach is more cost-effective when the prevalence is low (89,91,121--124). One study (121) determined that the negative predictive value of LET among asymptomatic men aged >30 exceeded 98%, thereby providing support for no additional C. trachomatis testing among that population. Among men aged <30 years, whether a negative result is a valid basis on which to exclude that population from testing with more sensitive tests is uncertain. Because the test does not detect either C. trachomatis or N. gonorrhoeae, but rather nonspecific inflammatory enzymes, a positive LET should be followed by specific tests for C. trachomatis and N. gonorrhoeae. The importance of a positive LET that is followed by negative tests for C. trachomatis and N. gonorrhoeae is unknown, but could indicate infection with other organisms (e.g., Trichomonas vaginalis, Ureaplasma urealyticum, or Mycoplasma genitalium). Collecting, Transporting, and Storing SpecimensCorrect specimen collection and handling techniques are critical for all methods used to identify C. trachomatis and N. gonorrhoeae. Even diagnostic tests with the highest performance characteristics cannot produce accurate results when specimens submitted to the laboratory are incorrectly collected. Recommendations for transporting and storing specimens are summarized in this report (Appendix D). Clinicians require training and periodic assessment to maintain correct technique for specimen collection (125--127). The presence of columnar epithelial cells has been associated with increased sensitivity in the majority of studies that evaluated collection of endocervical specimens for C. trachomatis screening with NAATs as well as other types of tests (125--132). How these results apply to other types of specimens is unclear. Obtaining columnar cells is less critical for detecting N. gonorrhoeae than for detecting C. trachomatis. Collecting and Transporting Specimens for ScreeningSpecimen Collection Recommendations Applicable to Culture and Nonculture Tests Although the requirement for columnar endocervical cells applies less to N. gonorrhoeae than to C. trachomatis, guidelines included in this report are appropriate for both organisms (Box 9). Specific Requirements for C. trachomatis Culture Swabs with plastic or wire shafts can be used to obtain specimens for cell culture (133--135). Swab tips can be made of cotton, rayon, or Dacron,® but should not be made of calcium alginate (133--136). Swabs with wooden shafts should not be used because the wood might contain substances that are toxic to C. trachomatis or tissue culture cells (133--135). As part of routine quality control, samples of each lot of swabs that are used to collect specimens for C. trachomatis isolation should be screened for possible inhibition of C. trachomatis growth and toxicity to tissue culture cells. The substitution of an endocervical brush for a swab might increase the sensitivity of culture for endocervical specimens from nonpregnant women (137). However, using an endocervical brush might induce bleeding. Although such bleeding does not interfere with the isolation of C. trachomatis, patients should be advised regarding possible spotting. When culture isolation of C. trachomatis from women is to be performed, processing a specimen from the urethra as well as the endocervix can increase sensitivity by 23% (138). Placing the two specimens in the same transport container is acceptable. The viability of C. trachomatis organisms must be maintained during transport to the laboratory (28).
Specific Requirements for N. gonorrhoeae Culture Swabs with plastic or wire shafts can be used to obtain specimens for culture. Swab tips can be made of rayon, Dacron, or calcium alginate. Obtaining a second cervical specimen for N. gonorrhoeae culture is associated with an increase in sensitivity (139). Inoculating the two specimens on the same culture plate is acceptable. The viability of N. gonorrhoeae organisms must be maintained during transport to the laboratory; therefore, the following recommendations are made for transporting specimens:
Adequacy of Endocervical Specimens Without endocervical specimen quality assurance, >10% of specimens collected for C. trachomatis testing are probably unsatisfactory because they contain secretions or exudate, but lack endocervical cells (126,128,145). A substantially reduced likelihood exists of detecting C. trachomatis in inadequate specimens by all tests, including NAATs (125--132). Assessing Endocervical Specimen Quality. Health-care providers and laboratorians are encouraged to evaluate the effect on endocervical C. trachomatis test positivity rates of monitoring the endocervical columnar cell content of specimens and then providing feedback and, as indicated, training to clinicians. Until proven unnecessary, routine or periodic assessment of endocervical specimen quality is recommended for all types of C. trachomatis tests. A report presenting alternative methods of measuring specimen quality has been developed by the National Chlamydia Laboratory Committee for the CDC-supported C. trachomatis screening program and distributed to constituent screening programs (additional information is available at http://www.aphl.org/chlamydia_lab.cfm). The majority of published studies have categorized specimens dichotomously as either containing the appropriate endocervical cells or not. In one study, a dose-response association between quantity of the appropriate endocervical cells and the C. trachomatis positivity rate was demonstrated by using a multiple-category scale for quantity of appropriate cells (132). This study also prescribed a systematic process for reviewing slides. Diff-Quick stain has been described as efficient and inexpensive (125). The principal purpose of assessing the quality of endocervical specimens is to determine whether the sensitivity of C. trachomatis tests would be enhanced by providing feedback and training to clinicians. No evaluations have been published of the cost-effectiveness of alternative approaches to measuring the quality of endocervical cell specimens and improving specimen-collection practices, and no studies have correlated measures of specimen adequacy with test positivity for specimens collected for N. gonorrhoeae testing. Reporting and Follow-Up of Inadequate Specimens. An inadequate specimen should be reported as inadequate. Whenever possible, a second specimen should be collected for repeat testing. Collecting Specimens for Indications Other Than ScreeningAnatomic site-specific recommendations for application of C. trachomatis and N. gonorrhoeae tests, including for medicolegal applications, are summarized in this report (Appendices A and B). Multiple sources provide directions for collecting specimens for N. gonorrhoeae testing (12,143,144). Laboratory Implementation of NAATSNAATs require more attention to procedural details and development and maintenance of quality control systems than other nonculture screening tests (e.g., EIAs and nonamplified nucleic acid hybridization assays). An increase in such requirements results from the susceptibility of NAAT amplification enzymes to inhibition and the potential of NAATs to generate cross-contaminating target and to detect limited quantities of target present as a contaminant. Despite close attention to quality control, concerns regarding consistency of test performance and reproducibility persist, as indicated by reports of heterogeneity of results in clinical trials (78,80) and varying rates of reproducing positive results (146--151). As laboratories and health-care providers transition to amplification tests, certain critical concerns should be addressed, including
Test of Cure, Treatment Failure, and Antimicrobial ResistanceTest-of-cure is not recommended as a routine procedure after therapy for C. trachomatis or N. gonorrhoeae infection with first-line CDC-recommended treatment regimens, except after C. trachomatis therapy during pregnancy (152). Nonculture tests that are performed <3 weeks after completion of antimicrobial therapy might be falsely positive because of the presence of nonviable organisms; this applies in particular to NAATs (153--160). CDC recommends that clinicians contact their local or state health department for guidance and to arrange for antimicrobial susceptibility testing of isolates from patients apparently failing CDC-recommended therapy for C. trachomatis infection or CDC-recommended or FDA-approved therapy for N. gonorrhoeae infection. For this purpose, a patient's infection is considered to have failed therapy if the patient is laboratory-test--positive for C. trachomatis or N. gonorrhoeae after treatment and the patient provides a history of having complied with the prescribed therapy and denies posttreatment sexual exposure to an untreated or new sex partner. Because knowledge is limited regarding the ability of C. trachomatis to develop antimicrobial resistance, CDC encourages health departments to inform CDC of treatment failures and, if possible, arrange for shipment of a swab specimen suitable for tissue culture for test confirmation. To report apparent C. trachomatis treatment failure, contact the following: Surveillance and Special Studies Section, Mail Stop E-02 To submit specimens for C. trachomatis testing, contact the following: Chlamydia Laboratory CDC encourages health departments to inform CDC of N. gonorrhoeae treatment failures and, if possible, arrange for N. gonorrhoeae culture and testing of any isolate for susceptibility to CDC-recommended regimens used to treat patients. If susceptibility testing cannot be performed at a local or state laboratory, isolates should be submitted to CDC as should any isolate that is resistant to a CDC-recommended therapy. Antimicrobial susceptibility testing should be performed according to recommendations of the National Committee for Clinical Laboratory Standards (NCCLS), and results should be interpreted according to criteria designated by NCCLS. If NCCLS has not provided criteria for resistance for a CDC-recommended therapy (e.g., cephalosporins), an isolate is considered to be resistant if the isolate fails to meet the NCCLS criteria for susceptibility. NCCLS might not have designated criteria for definition of a susceptible category for antimicrobial agents that are used for gonorrhea treatment. When no NCCLS criterion is available, consult http://www.cdc.gov/ncidod/dastlr/gcdir/Resist/diskdif.html for additional information or contact the Neisseria Reference Laboratory for further guidance. To report apparent N. gonorrhoeae treatment failure, contact the following: Surveillance and Special Studies Section, Mail Stop E-02 To submit specimens for N. gonorrhoeae testing, contact the following: Neisseria Reference Laboratory, Unit 31 In addition, statewide programs should be maintained to routinely isolate gonococcal strains and monitor antimicrobial susceptibilities to CDC-recommended therapies and to other FDA-cleared therapies with established usage in the state. Sexual Assault and Sexual AbuseDetailed information concerning evaluation and treatment of suspected victims of sexual assault or abuse can be obtained from the 2002 STD treatment guidelines (152). Presented here are general guidelines pertaining only to C. trachomatis and N. gonorrhoeae infections (Box 10). Examination of victims is required for two purposes: 1) to determine if an infection is present so that it can be successfully treated and 2) to acquire evidence for potential use in a legal investigation. Testing to satisfy the first purpose requires a method that is highly sensitive, whereas satisfying the second purpose requires a method that is highly specific. Because of the health and legal implications of test results, the additional time, labor, and cost of performing tests that are sensitive and highly specific are justified. Using highly specific tests is critical with preadolescent children for whom the diagnosis of a sexually transmitted infection might lead to initiation of an investigation for child abuse. Local legal requirements and guidance should be sought for maintaining and documenting a chain of custody for specimens and results that might be used in a legal investigation. References
* These guidelines do not address trachoma and lymphogranuloma venereum, which rarely occur in the United States. † The term cleared is used by the Food and Drug Administration (FDA) to describe the process they use to review applications to market the class of diagnostic tests that includes C. trachomatis and N. gonorrhoeae tests discussed in these guidelines. The term approved is used by FDA to describe a more rigorous process they use to review applications to market classes of diagnostic tests that involve, for example, higher levels of risk if the test result is erroneous than is the case for C. trachomatis or N. gonorrhoeae. § For example, when a test with a specificity of 99% and a sensitivity of 85% is used to screen a population of 10,000 patients with a C. trachomatis prevalence of 10% (i.e., 1,000 patients have an infection), an average of 940 test results will be positive: 850 patients with a positive result will actually be infected, and 90 will not be infected (i.e., false-positives). PPV is 850/940 = 0.904. When this same test is used to screen 10,000 patients with a chlamydia prevalence of only 2% (i.e., 200 patients have an infection), an average of 268 test results will be positive: 170 patients will be infected and 98 will not. The PPV is 170/268 = 0.634. ¶ Published evaluations of commercial NAATs have augmented reference tests with an alternative target NAAT performed by the manufacturer that employs the same amplification method as the commercial NAAT, except for substitution of a different set of primers. This report is also available at http://www.cdc.gov/std/labguidelines. A limited number of copies can be ordered at that website.Box 1 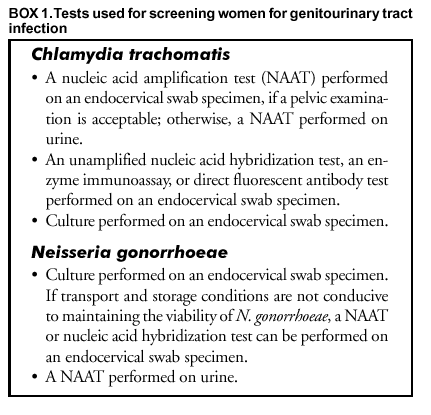 Return to top. Box 2 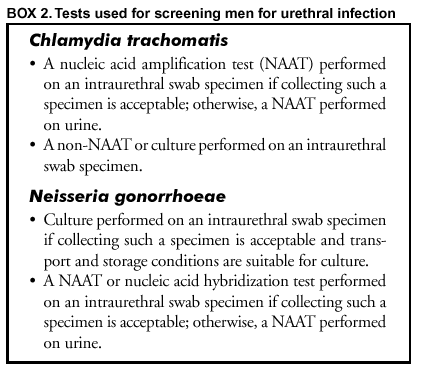 Return to top. Box 3 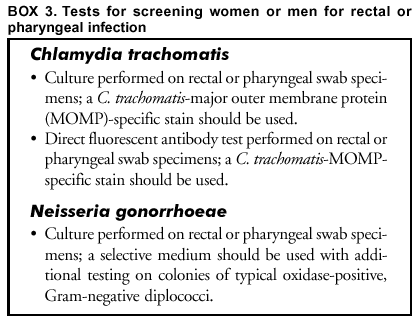 Return to top. Box 4  Return to top. Box 5 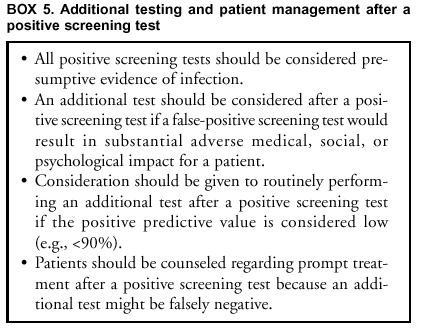 Return to top. Box 6 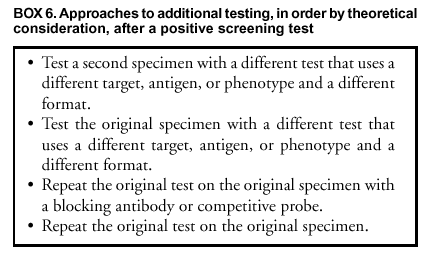 Return to top. Box 7 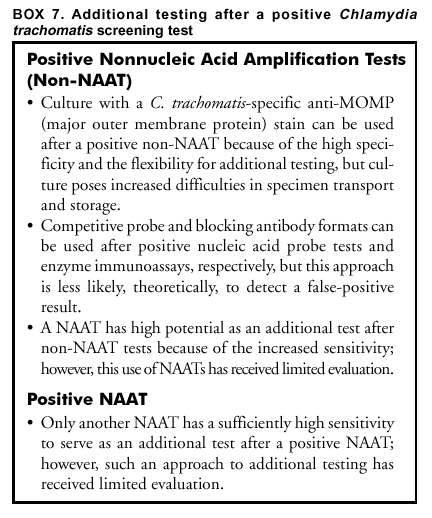 Return to top. Box 8 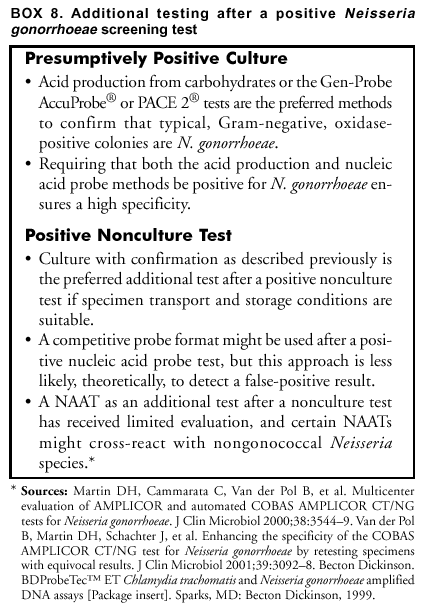 Return to top. Box 9 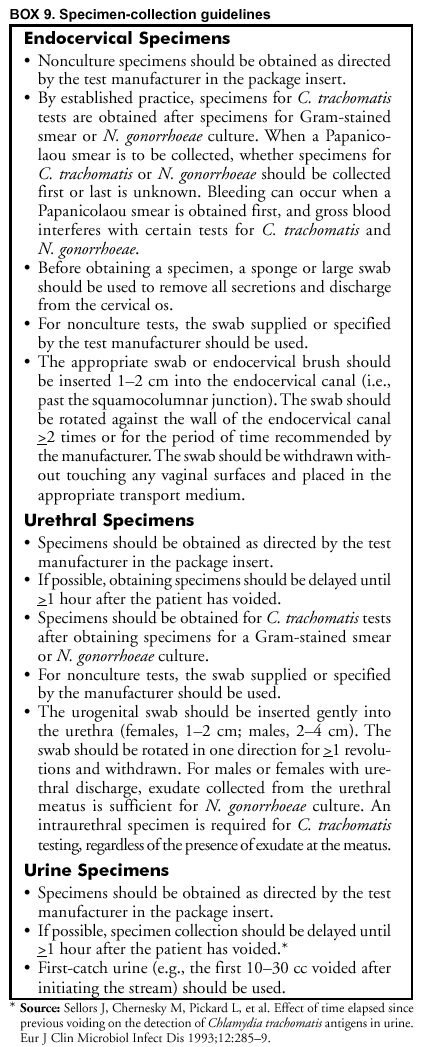 Return to top. Box 10  Return to top. Figure 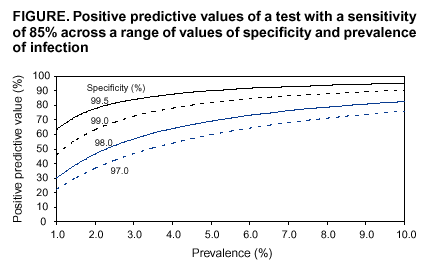 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 10/2/2002 |
|||||||||
This page last reviewed 10/2/2002
|