 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Treating Opportunistic Infections Among HIV-Exposed and Infected ChildrenRecommendations from CDC, the National Institutes of Health, and the Infectious Diseases Society of America Prepared by The material in this report originated in the Office of the Director, National Center for HIV, STD and TB Prevention, Janet L. Collins, M.D., Acting Director. Corresponding author: Lynne M. Mofenson, M.D., Pediatric, Adolescent and Maternal AIDS Branch, National Institute of Child Health and Human Development, National Institutes of Health, 6100 Executive Blvd., Room 4B11, Rockville, MD 20852. Telephone: 301-435-6870; Fax: 301-496-8678. SummaryIn 2001, CDC, the National Institutes of Health, and the Infectious Diseases Society of America convened a working group to develop guidelines for therapy of human immunodeficiency virus (HIV)-associated opportunistic infections to serve as a companion to the Guidelines for Prevention of Opportunistic Infections Among HIV-Infected Persons. In recognition of unique considerations related to HIV infection among infants, children, and adolescents, a separate pediatric working group was established. Because HIV-infected women coinfected with opportunistic pathogens might be more likely to transmit these infections to their infants than women without HIV infection, guidelines for treating opportunistic pathogens among children should consider treatment of congentially acquired infections among both HIV-exposed but uninfected children and those with HIV infection. In addition, the natural history of opportunistic infections among HIV-infected children might differ from that among adults. Compared with opportunistic infections among HIV-infected adults, which are often caused by reactivation of pathogens acquired before HIV infection when host immunity was intact, opportunistic infections among children often reflect primary acquisition of the pathogen and, among children with perinatal HIV infection, infection acquired after HIV infection has been established and begun to compromise an already immature immune system. Laboratory diagnosis of opportunistic infections can be more difficult with children. Finally, treatment recommendations should consider differences between adults and children in terms of drug pharmacokinetics, dosing, formulations, administration, and toxicities. This report focuses on treatment of opportunistic infections that are common in HIV-exposed and infected infants, children, and adolescents in the United States. IntroductionIn 1995, the U.S. Public Health Service (USPHS) and the Infectious Diseases Society of America (IDSA) developed guidelines for preventing opportunistic infections among adults, adolescents, and children infected with human immunodeficiency virus (HIV) (1). These evidence-based guidelines, developed for health-care providers and patients, were revised in 1997, 1999, and 2002 (2--4). Although individual guidelines for treatment of different opportunistic infections can be found in multiple sources, a compilation of recommendations for treatment and management of common HIV-associated opportunistic infections into a single document has not been available. As a result, in 2001, the National Institutes of Health (NIH), IDSA, and CDC convened a working group to develop guidelines for therapy of HIV-associated opportunistic infections, with a goal of providing evidence-based guidelines for health-care providers on treatment and prophylaxis. In recognition of unique considerations for HIV-infected infants, children, and adolescents, including differences between adults and children in mode of acquisition, natural history, diagnosis, and treatment of HIV-related opportunistic infections, a separate pediatric guidelines writing group was established. In 1998, the Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children, convened by the Francois-Xavier Bagnoud Center (FXBC), published guidelines for treatment of HIV and opportunistic infections among children in a special supplement to Pediatrics (5). However, since these guidelines were published, advances have been made in laboratory and clinical research related to individual opportunistic infections, and use of highly active antiretroviral therapy (HAART) has dramatically increased in HIV-infected children, changing the epidemiology and presentation of opportunistic infections among children and adults. Members of the pediatric guidelines writing group for this report, in consultation with members of the FXBC's Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children and other infectious disease specialists, have developed this document focused on treatment of HIV-associated opportunistic infections among infants and children. An important mode of acquisition of opportunistic infections and HIV infection among children is from an infected mother to her child. HIV-infected women co-infected with opportunistic pathogens might be more likely to transmit these infections to their infants than women without HIV infection. For example, greater rates of perinatal transmission of hepatitis C and cytomegalovirus have been reported from HIV-infected than uninfected women (6,7). In addition, HIV-infected women or HIV-infected family members co-infected with certain opportunistic pathogens might be more likely to transmit these infections horizontally to their children, resulting in an increased likelihood of primary acquisition of such infections in the young child. For example, Mycobacterium tuberculosis infection among children primarily reflects acquisition from family members with active tuberculosis (TB) disease, and increase in the incidence and prevalence of tuberculosis among HIV-infected persons is well documented. HIV-exposed or -infected children in the United States might have a higher risk for exposure to M. tuberculosis than comparably aged children in the general U.S. population because of residence in households with HIV-infected adults (8). Therefore, infections with opportunistic pathogens might affect not just infants who are themselves HIV-infected but also infants who are uninfected with HIV but who become infected by the pathogen because of transmission from HIV-infected mothers or family members with co-infections. Guidelines for treatment of opportunistic infections in children must also include consideration of the treatment of infections among uninfected and HIV-infected children. The natural history of opportunistic infections among children might differ from that observed among HIV-infected adults. Many opportunistic infections in adults are secondary to reactivation of previously acquired opportunistic pathogens, which were often acquired before HIV infection at a time when host immunity was intact. However, opportunistic infections among HIV-infected children more often reflect primary infection with the pathogen. In addition, among children with perinatal HIV infection, the primary infection with the opportunistic pathogen is occurring after HIV infection is established when the child's immune system might already be compromised. This can lead to different manifestations of disease associated with the pathogen among children than among adults. For example, young children with TB are more likely to have nonpulmonic and disseminated infection than adults, even without concurrent HIV infection. Multiple difficulties exist in making laboratory diagnosis of various infections in children. Diagnosis is often compounded by a child's inability to describe the symptoms of disease. For infections where the primary diagnostic modality is the presence of antibody (e.g., the hepatitis viruses and cytomegalovirus), the ability to make a diagnosis in young infants is complicated by transplacental transfer of maternal antibody that can persist in the infant for 12--15 months. Assays capable of directly detecting the pathogen are required to definitively diagnose such infections in infants. In addition, diagnosing the etiology of lung infections among children can be difficult because they do not generally produce sputum, and more invasive procedures might be needed. Data related to the efficacy of various therapies for opportunistic infections in adults can generally be extrapolated to children, but issues related to drug pharmacokinetics, formulation, ease of administration, and drug dosing and toxicity require special considerations among children. Young children in particular metabolize drugs differently from adults and older children. However, data on appropriate drug dosing recommendations for children aged <2 years often are lacking. The frequency of different opportunistic pathogens among HIV-infected children in the pre-HAART era varied by age, pathogen, previous opportunistic infection, and immunologic status (9). In the pre-HAART era, the most common opportunistic infections among children in the United States (event rates >1.0/100 child-years) were serious bacterial infections (with pneumonia, often presumptively diagnosed, and bacteremia being most common), herpes zoster, disseminated Mycobacterium avium complex (MAC), Pneumocystis jiroveci (formerly carinii) pneumonia (PCP), and candidiasis (esophageal and tracheobronchial disease). Less commonly observed opportunistic infections (event rate <1.0/100 child-years) included cytomegalovirus disease, cryptosporidiosis, tuberculosis, systemic fungal infections, and toxoplasmosis. History of a previous acquired immunodeficiency syndrome (AIDS)-defining opportunistic infection was a predictor of developing a new infection. Although the majority of infections occurred among children who were substantially immunocompromised, serious bacterial infections, herpes zoster, and TB occurred across the spectrum of immune status. Descriptions of opportunistic infections in the HAART era among children have been limited. As with HIV-infected adults, substantial decreases in mortality and morbidity, including opportunistic infections, have been observed among children receiving HAART (10). Although the number of opportunistic infections has decreased, the relative prevalence of AIDS-defining infections remains similar to that observed in the pre-HAART era (11). In comparison with recurrent serious bacterial infections, few of the protozoan, fungal, or viral opportunistic infections complicating HIV are curable with available treatments. Successful implementation and maintenance of HAART, resulting in improved immune status, has been established as the most important factor in control of opportunistic infections among both HIV-infected adults and children (12). For many opportunistic infections, following treatment of the initial infectious episode, secondary prophylaxis in the form of suppressive therapy is indicated to prevent recurrent clinical disease (4). These guidelines serve as a companion to the USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections Among HIV-Infected Persons and the Guidelines for the Treatment of Opportunistic Infections in Persons Infected with the Human Immunodeficiency Virus, which is focused on HIV-infected adults. Treatment of opportunistic infections is an evolving science, and availability of new agents or clinical data on existing agents might change therapeutic options and preferences. As a result, these recommendations will need to be periodically updated. During development of these guidelines, members of the pediatric treatment guidelines writing group and of the Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children reviewed published manuscripts and abstracts presented at professional meetings related to treatment of selected pathogens among children and adults. Because the guidelines are targeted to HIV-exposed and infected children in the United States, the opportunistic pathogens discussed are those common to U.S. children and do not include certain pathogens (e.g., Penicillium marneffei) that might be seen more frequently in resource-poor countries. The document is organized to provide information about the epidemiology, clinical presentation, diagnosis, and treatment for each pathogen. Each treatment recommendation is accompanied by a rating that includes a letter and a Roman number and is similar to the rating systems used in other USPHS/IDSA guidelines (13). The letter indicates the strength of the recommendation, which is based on the opinion of the Working Group, and the Roman numeral reflects the nature of the evidence supporting the recommendation (Box). Appendices at the end of this document summarize the treatment recommendations for each of the opportunistic pathogens (Appendix A), provide information on pediatric drug preparations and major toxicities (Appendix B), and provide information about clinically significant drug interactions for the drugs recommended for treatment of individual opportunistic infections among children (Appendix C). Treatment Recommendations by OrganismPneumocystis jiroveci (formerly carinii) PneumoniaEpidemiology Pneumocystis microbes are classified in the fungus kingdom on the basis of DNA analysis but also share biologic characteristics with protozoa. Additional DNA analyses have demonstrated that Pneumocystis organisms in different mammals are quite different, which has led to changes in taxonomy (14). The organism that infects humans and causes PCP is now named Pneumocystis jiroveci; P.carinii refers to the organism that is found only in rats. P. jiroveci is usually acquired in childhood; serum antibodies are found in >80% of children aged 2--4 years (15). Immunocompetent infants with P. jiroveci infection might have either mild respiratory symptoms or are asymptomatic. PCP remains the most common AIDS-indicator disease among HIV-infected children, accounting for 33% of AIDS cases overall. The highest incidence of PCP in HIV-infected children is the first year of life, with cases peaking at age 3--6 months (16,17). PCP accounts for 57% of AIDS-defining conditions among infants aged <1 year. The mortality rate among children with PCP is high. Among AIDS cases reported to CDC, before the availability of HAART therapy, 35% of children with PCP died within 2 months of diagnosis, and the estimated median survival time was 19 months (16,18). CD4+ cell counts are not a good indicator of risk for PCP in infants aged <1 year; many young infants with PCP have CD4+ cell counts >1,500/µL, and counts can drop rapidly shortly before PCP develops in infants (19--21). Since publication of the 1995 PCP prophylaxis guidelines recommending initiation of primary PCP prophylaxis in all infants born to HIV-infected women for the first year of life (or until lack of HIV infection is documented) (18,22), PCP has become unusual among HIV-infected infants born to women who know their HIV serostatus during or soon after pregnancy. However, PCP is still seen in infants born to women with unrecognized HIV infection (19,21). Clinical Manifestations Clinical features of PCP among HIV-infected children are similar to those in adults (i.e., fever, tachypnea, dyspnea, and cough), and the severity of these signs and symptoms might vary from child to child. Onset can be abrupt or insidious with nonspecific symptoms (e.g., mild cough, dyspnea, poor feeding, and weight loss). Certain patients might not be febrile, but almost all patients will have tachypnea by the time pneumonitis is observed on chest radiograph (23). Physical examination might show bibasilar rales with evidence of respiratory distress and hypoxia. Extrapulmonary pneumocystosis is seen in <2.5% of HIV-infected adults (24); it is uncommon among HIV-infected children (25,26). It can occur without concurrent PCP and can be located at multiple noncontiguous sites. Sites have included ear, eye, thyroid, spleen, gastrointestinal tract, peritoneum, stomach, duodenum, small intestine, transverse colon, liver, and pancreas and less frequently, adrenal glands, bone marrow, heart, kidney and ureter, lymph nodes, meninges and cerebral cortex, and muscle. Infants with dual infection with cytomegalovirus (CMV) and P. jiroveci might have more severe pneumonic disease and are more likely to require assisted ventilation, receive corticosteroids, or die than those with PCP alone (27). Whether corticosteroids for PCP among children with dual P. jiroveci/CMV infection increases the risk for CMV dissemination to the lung is unclear. Diagnosis The majority of children with PCP have substantial hypoxia with low arterial oxygen pressure and an alveolar-arterial oxygen gradient of >30 mm/Hg. Lactic dehydrogenase (LDH) is often increased but is not specific for PCP. Serum albumin might be depressed. Chest radiographs most commonly indicate bilateral diffuse parenchymal infiltrates with "ground-glass" or reticulogranular appearance, but they also might be normal or indicate only mild parenchymal infiltrates. The earliest infiltrates are perihilar, progressing peripherally before reaching the apical portions of the lung. Rarely, lobar, cavitary, nodular or miliary lesions, pneumothorax, or pneumomediastinum are observed (23). Definitive diagnosis of PCP requires demonstration of the organism in pulmonary tissues or fluids. Diagnostic procedures are the same as used for adults with suspected PCP, but selected procedures might be more difficult to perform in children. Induced sputum analysis, where the patient produces sputum after inhalation of nebulized 3% hypertonic saline, might be difficult among children aged <2 years because of small airways and poor ability to produce sputum. Complications can include nausea, vomiting, and bronchospasm. Sensitivity of induced sputum analysis ranges from 25%--90%; because negative predictive value is only 48%, following a negative induced sputum sample with bronchoalveolar lavage for definitive diagnosis might be necessary. Bronchoscopy with bronchoalveolar lavage is the diagnostic procedure of choice for infants. Sensitivity ranges from 55%--97% and might be positive for at least 72 hours after PCP treatment has been initiated; treatment should not be delayed while awaiting results. Complications included hemoptysis, pneumothorax, transient increase in hypoxemia, transient increase in pulmonary infiltrates at the lavage site, and postbronchoscopy fever. Fiberoptic bronchoscopy with transbronchial biopsy is not recommended unless bronchoalveolar lavage is negative or nondiagnostic despite the child having a clinical picture consistent with PCP. Sensitivity is 87%--95%, and cysts can be identified up to 10 days after initiation of treatment (up to 4--6 weeks in certain patients). Complications include pneumothorax and hemorrhage; this procedure is contraindicated among children with thrombocytopenia. Open-lung biopsy is the most sensitive diagnostic technique but because it requires thoracotomy and often chest tube drainage, is not recommended routinely. Histopathology shows alveoli filled with eosinophilic, acellular, proteinaceous material that contains cysts and trophozoites but few inflammatory cells. Complications include pneumothorax, pneumomediastinum, and hemorrhage. Three types of stains can be used to diagnose P. jiroveci organisms in specimens. Gomori's methenamine-silver stains the cyst wall brown or black. Toluidine blue stains the cyst wall blue or lavender and also stains fungal elements. Giemsa or Wright's stains stain the trophozoites and intracystic sporozoites pale blue with a punctate red nucleus but, unlike other stains, these stains do not stain the cyst wall. Monoclonal immunofluorescent antibodies that stain the cyst wall also can be used for diagnosis and have enhanced specificity compared with the other methods. Identification of P. jiroveci DNA sequences using polymerase chain reaction (PCR) assays in blood or serum, nasopharyngeal aspirates, and bronchoalveolar lavage specimens might be more sensitive in detecting infection in lavage specimens than standard cytologic stains among children (28). However, this is a research tool and is not available at some institutions. Coinfection with other organisms (e.g., CMV or pneumococcus) has been reported in HIV-infected children (27,29,30). Children with dual infections might have more severe disease. Although the presence of CMV in lung secretions of children with PCP might indicate colonization that does not require therapy, the presence of P. jiroveci is always an indication for treatment. Treatment Trimethoprim/sulfamethoxazole (TMP/SMX) is the recommended treatment for PCP (AI). The dose for HIV-infected children aged >2 months is 15--20 mg/kg body weight/day of the TMP component (75--100 mg/kg of SMX component) adminstered intravenously in 3--4 divided doses, with the dose infused over 1 hour for 21 days (AI). After the acute pneumonitis has resolved, children with mild to moderate disease who do not have malabsorption or diarrhea can be adminstered oral treatment with the same dose of TMP/SMX in 3--4 divided doses to complete a 21--day course. Adverse reactions to TMP/SMX reported in children include rash (including erythema multiforme and rarely Stevens Johnson syndrome), hematologic abnormalities (e.g., neutropenia, thrombocytopenia, megaloblastic, or aplastic anemia), gastrointestinal complaints (usually mild), hepatitis, and renal disorders (e.g., interstitial nephritis) (31,32). The overall frequency of adverse reactions appears to be lower among HIV-infected children than adults; approximately 15% of children have substantial adverse reactions to TMP/SMX (23). For mild or moderate skin rash, TMP/SMX can be temporarily discontinued and restarted when the rash has resolved. If an urticarial rash or Stevens-Johnson syndrome occurs, TMP/SMX should be discontinued and not readministered (EIII). Pentamidine isothionate (4 mg/kg/day once daily administered intravenously over 60--90 minutes) is recommended for patients intolerant of TMP/SMX or who demonstrate clinical treatment failure after 5--7 days of TMP/SMX therapy (AI). No evidence exists for synergistic or additive effects on efficacy of these agents; therefore, because of potential increased toxicity, their combined use is not recommended (DIII). Among patients with clinical improvement after 7--10 days of intravenous therapy with pentamidine, an oral regimen (e.g., atovaquone) might be considered to complete a 21--day course (BIII). The most common adverse drug reaction to pentamidine is renal toxicity, which usually occurs after 2 weeks of therapy and can be averted by adequate hydration and careful monitoring of renal function and electrolytes. Severe hypotension (particularly if infused rapidly), prolonged QT interval (torsades de pointes), and cardiac arrhythmias can occur. Hypoglycemia (usually after 5--7 days of therapy) or hyperglycemia, hypercalcemia, hyperkalemia, pancreatitis, and insulin-dependent diabetes mellitus have also been reported. A metallic or bitter taste might be experienced. Serious adverse reactions to pentamidine have been reported in approximately 17% of children receiving pentamidine (33). Care should be taken if administering this drug with other nephrotoxic agents (e.g., aminoglycosides, amphotericin B, cisplatin, or vancomycin) or if coadministered with agents associated with pancreatitis (e.g., didanosine). Atovaquone is an alternative for treatment of mild to moderately severe PCP in adults (BI). Data are limited for children; dosage is 30--40 mg/kg/day in 2 divided doses given orally with fatty foods. Food increases the bioavailability of atovaquone 1.4-fold over that achieved with the fasting state. Infants aged 3--24 months might require a higher dosage of 45 mg/kg/day (34) (AII). Atovaquone concentration is increased with coadminstration of fluconazole and prednisone and decreased by coadministration with acyclovir, opiates, cephalosporins, rifampin, and benzodiazepines. Most adverse reactions occur after the first week of therapy. Skin rashes (10%--15%), nausea, and diarrhea can occur. Elevated liver enzymes also might be observed. Clindamycin/primaquine has been used for treatment of mild to moderate PCP among adults (BI); data for children are not available (CIII). Primaquine is contraindicated among patients with glucose-6-dehydrogenase deficiency associated with the possibility of inducing hemolytic anemia. Dose information for treatment of PCP is available only for adults. For patients weighing <60 kg, clindamycin 600 mg intravenously every 6 hours for 10 days, then 300--450 mg orally every 6 hours to complete 21 days of treatment is recommended. Primaquine is adminstered as 30 mg of the base orally for 21 days. Dosing for children is based on use of these drugs for treatment of other infections: the usual pediatric dose of clindamycin for treatment of bacterial infections is 10 mg/kg every 6 hours, and the pediatric dose of primaquine equivalent to an adult dose of 30 mg base (when used for malaria) is 0.3 mg/kg of the base daily. Adverse reactions include skin rashes, nausea, and diarrhea. Trimetrexate glucuronate with leucovorin (folinic acid) has been used as initial therapy in severe PCP in adults (BI); data are limited for children (CIII). The dose is 45 mg/m2/day of trimetrexate glucuronate for 21 days. Leucovorin should be administered at 20 mg/m2 every 6 hours for 24 days. Dapsone/trimethoprim is effective in treatment of mild-to-moderate PCP among adults (BI); data on toxicity and efficacy among children are not available (CIII). The dose for adults of dapsone is 100 mg orally once daily and trimethoprim 15 mg/kg divided into 3 daily doses orally, administered for 21 days. Among children aged <13 years, a dapsone dose of 2 mg/kg/day is required to achieve therapeutic levels in children (35) (AII). The pediatric dose of trimethoprim is 15 mg/kg divided into 3 daily doses. The primary adverse reaction is reversible neutropenia; other reactions include skin rashes, elevated serum transaminases, anemia, and thrombocytopenia. On the basis of studies in adults, a short course of corticosteroids might be indicated in some cases of PCP of moderate or great severity, started within 72 hours of diagnosis (AI). Pediatric studies have indicated reduction in acute respiratory failure, decreased need for ventilation, and decrease in mortality with early use of corticosteriods in HIV-infected children with PCP (36--38). Indications for corticosteroid treatment include a PaO2 value of <70 mm Hg or an alveolar-arterial gradient of >35 mm Hg. Doses in children varied between studies. Alternative regimens include 1) prednisone on days 1--5, 40 mg twice daily; days 6--10, 40 mg once daily; days 11--21, 20 mg once daily; 2) prednisone (or methylprednisolone sodium) on days 1--5, 1 mg/kg twice daily; day 6--10, 0.5 mg/kg twice daily; days 11--21, 0.5 mg/kg once daily; or 3) methylprednisolone (intravenous) on days 1--7, 1 mg/kg every 6 hours; days 8--9, 1 mg/kg twice daily; days 10--11, 0.5 mg/kg twice daily; days 12--16, 1 mg/kg once daily. Some case reports about children have documented improved pulmonary function with use of surfactant in cases of severe disease (e.g., respiratory distress syndrome with established respiratory failure requiring ventilation) (39--41) (CIII). Among HIV-infected children, lifelong suppression is indicated following treatment for PCP to prevent recurrence; details on secondary prophylaxis (maintenance therapy) have been published (4). Safety of discontinuation of secondary prophylaxis after immune reconstitution with HAART in children has not been studied extensively. ToxoplasmosisEpidemiology The major mode of transmission of Toxoplasma gondii infection among infants and young children is congenital, occurring almost exclusively among neonates born to women who sustain primary Toxoplasma infection during pregnancy. The incidence of congenital toxoplasmosis in the United States is an estimated one case per 1,000--12,000 live-born infants (42,43) and is believed to have decreased substantially during the preceding 20 years. Older children, adolescents, and adults typically acquire Toxoplasma infection by eating poorly cooked meat that contains parasitic cysts or by unintentionally ingesting sporulated oocysts in soil or contaminated food or water. The overall risk for maternal-fetal transmission in women without HIV infection who acquire primary Toxoplasma infection during pregnancy is 29% (95% confience interval [CI] = 25%--33%) (44). The risk for congenital infection is low among infants born to women who become infected during the first trimester (range: 2%--6%) but increases sharply thereafter, with a risk as high as 81% for women acquiring infection during the last few weeks of pregnancy (44). Infection of the fetus in early gestation usually results in more severe involvement, compared with milder disease when infection occurs late in gestation. The prevalence of latent Toxoplasma infection among women with and without HIV infection in the United States was assessed in a cross-sectional study of 2,525 non-pregnant women enrolled in the Women's Interagency Health Study (45). The prevalence of Toxoplasma seropositivity was 15% and did not differ by HIV infection status. A few cases of mother-to-infant transmission of Toxoplasma in HIV-infected women have been reported (46--50). Perinatal transmission of Toxoplasma gondii from women without HIV infection who have chronic Toxoplasma infection acquired before pregnancy is uncommon (51). However, in the setting of HIV co-infection, perinatal transmission of toxoplasma has been observed among women with chronic toxoplasma infection (transmission rate: <4%), presumably because of reactivation of replication of the organism among women with severe immune suppression (46--49). AIDS-defining infection of the central nervous system (CNS) with Toxoplasma gondii is uncommon amongHIV-infected children. It was reported as an AIDS-indicator condition in <1% of pediatric AIDS cases, even before the advent of HAART (52). In most cases of Toxoplasma encephalitis among HIV-infected children, infection is considered to have occurred in utero (53,54). More rarely, it has also been reported among older HIV-infected pediatric patients, presumably with primary acquired toxoplasmosis (54--56). Clinical Manifestations In studies of nonimmunocompromised infants with congenital toxoplasmosis, the majority of infants (70%-90%) are asymptomatic at birth; however, the majority of asymptomatic children develop late sequelae (e.g., retinitis, visual impairment, and intellectual or neurologic impairment) with the interval until the onset of their symptoms ranging from several months to years. When symptoms do occur in newborns, either of two presentations can be observed: generalized disease or predominantly neurologic disease. Symptoms can include maculopapular rash, generalized lymphadenopathy, hepatosplenomegaly, jaundice, hematologic abnormalities including anemia, thrombocytopenia and neutropenia, and substantial CNS disease, including hydrocephalus, intracerebral calcification, microcephaly, chorioretinitis, and seizures (57). Similarly, toxoplasmosis acquired after birth is most often initially asymptomatic. When symptoms occur, they are frequently nonspecific and can include malaise, fever, sore throat, myalgia, lymphadenopathy (cervical), and a mononucleosis-like syndrome featuring a maculopapular rash and hepatosplenomegaly. Toxoplasma encephalitis should be considered among all HIV-infected children with new neurologic findings. Although focal findings are more typical, the initial presentation can be variable and reflect diffuse CNS disease. Other symptoms include fever, reduced alertness, and seizures. Isolated ocular toxoplasmosis is rare and usually occurs in association with CNS infection. As a result, a neurologic examination is indicated for children who have had Toxoplasma chorioretinitis diagnosed. Ocular toxoplasmosis appears as white retinal lesions with little associated hemorrhage; visual loss might be observed initially. Less frequently observed presentations among HIV-infected children with reactivated chronic toxoplasmosis include systemic toxoplasmosis, pneumonitis, hepatitis, and cardiomyopathy/myocarditis (49,58). Diagnosis HIV-infected women might be at increased risk for transmitting Toxoplasma gondii to their fetuses, and serologic testing for Toxoplasma should be performed on all HIV-infected pregnant women. All infants whose mothers are both HIV-infected and seropositive for Toxoplasma should be evaluated for congenital toxoplasmosis (59). Congenital toxoplasmosis can be diagnosed by using enzyme immunoassay or an immunosorbent assay to detect the presence of Toxoplasma-specific IgM, IgA, or IgE in neonatal serum within the first 6 months of life or persistence of specific IgG antibody beyond age 12 months. IgA might be more sensitive for detection of congenital infection than IgM or IgE (60--62). However, approximately 20%--30% of infants with congenital toxoplasmosis will not be identified in the neonatal period with IgA or IgM assays (63). Serologic testing is the major method of diagnosis, but interpretation of available assays is often confusing and difficult. Using the services of a specialized reference laboratory that is capable of performing serology, isolation of organisms, and PCR, and offers assistance in interpreting results, especially when attempting to diagnose congenital toxoplasmosis, can be helpful. Additional methods that can be used to diagnose infection in the newborn include isolation of the Toxoplasma parasite by mouse inoculation, or inoculation in tissue cultures of cerebrospinal fluid (CSF), urine, placental tissue, amniotic fluid, or infant blood. Toxoplasma gondii DNA can be detected by PCR performed in a reference laboratory on body fluids (e.g., white blood cells, CSF, amniotic fluid, or tissues) (60,62,63). If a possible diagnosis of congenital toxoplasmosis at the time of delivery is uncertain, an evaluation of the neonate should be undertaken and include ophthalmologic, auditory, and neurologic examinations; lumbar puncture; and imaging of the head (either computerized tomography or magnetic resonance imaging scans) to determine whether hydrocephalus or calcifications are present. In the United States, routine Toxoplasma serologic screening of HIV-infected children whose mothers do not have toxoplasmosis is not recommended because of its low prevalence. However, in regions with high incidence of Toxoplasma infection, serologic testing might be selectively considered for HIV-infected children aged >12 months. HIV-infected adolescents without a history of previous Toxoplasma infection should undergo serologic testing. A presumptive diagnosis of CNS toxoplasmosis is based on clinical symptoms, serologic evidence of infection, and the presence of a space-occupying lesion on imaging studies of the brain. Cases of Toxoplasma encephalitis have been reported in persons without Toxoplasma-specific IgG antibodies; therefore, negative serology does not exclude that diagnosis. Computerized tomography of the brain might indicate multiple, bilateral, ring-enhancing lesions in CNS toxoplasmosis, especially in the basal ganglia and cerebral corticomedullary junction. Magnetic resonance imaging is more sensitive and will confirm basal ganglia lesions in the majority of patients. F-fluoro-2-deoxyglucose-positive emission tomography can be helpful in distinguishing Toxoplasma abscesses from primary CNS lymphoma, but the accuracy is not high and this test is not widely available. Definitive diagnosis of Toxoplasma encephalitis requires histologic or cytologic confirmation by brain biopsy, which might demonstrate leptomeningeal inflammation, microglial nodules, gliosis, and Toxoplasma cysts. Biopsy should be considered when early neurologic deterioration is present despite empiric treatment or among children who fail to respond to anti-Toxoplasma therapy after 10--14 days. Treatment Pregnant women with suspected or confirmed primary toxoplasmosis and newborns with possible or documented congenital Toxoplasmosis should be managed in consultation with an appropriate specialist. If an HIV-infected woman has a symptomatic Toxoplasma infection during pregnancy, empiric therapy of the newborn should be strongly considered whether or not the mother was treated during pregnancy (BIII). The preferred treatment for congenital toxoplasmosis is pyrimethamine (loading dose of 2 mg/kg body weight/day for 2 days, then 1 mg/kg/day for 2--6 months, followed by 1 mg/kg adminstered three times a week) combined with sulfadiazine (50 mg/kg/dose twice daily), with supplementary leucovorin (folinic acid) to minimize pyrimethamine-associated hematologic toxicity (AII). Although the optimal duration of therapy is undefined, the recommended duration of treatment of congenital toxoplasmosis for infants without HIV infection is 12 months (AII). Absent definitive data, the same recommendation applies to HIV-infected children with congenital toxoplasmosis. For children without HIV infection who have mild congenital toxoplasmosis, certain experts alternate pyrimethamine/sulfadiazine/folinic acid monthly with spiramycin (50 mg/kg orally twice daily) from the seventh through the 12th month of treatment (CIII). However, among children with moderate to severe disease and those with HIV infection, the full 12-month regimen of pyrimethamine/sulfadiazine should be administered (AII). HIV-infected children with acquired CNS, ocular, or systemic toxoplasmosis should be treated with pyrimethamine (2 mg/kg/day for 3 days, followed by 1 mg/kg/day) and leucovorin (10--25 mg/day) plus sulfadiazine (25--50 mg/kg/dose given four times daily) (AI). Acute therapy should be continued for 6 weeks, assuming clinical and radiological improvement (BII). Longer courses of treatment might be required in cases of extensive disease or poor response after 6 weeks. Pyrimethamine can be associated with rash (including Stevens-Johnson syndrome) and nausea. The primary toxicity of pyrimethamine is reversible bone marrow suppression (i.e., neutropenia, anemia, and thrombocytopenia). A complete blood count should be performed at least weekly while the child is on daily pyrimethamine and at least monthly while on less than daily dosing (AIII). Leucovorin (folinic acid) should always be administered with pyrimethamine; increased doses of leucovorin might be required in the event of marrow suppression. Because of the long half-life of pyrimethamine, leucovorin should be continued 1 week after pyrimethamine has been discontinued. Adverse effects of sulfadiazine include rash, fever, leukopenia, hepatitis, gastrointestinal symptoms (nausea, vomiting, and diarrhea), and crystalluria. The primary alternative for sulfadiazine in patients who develop sulfonamide hypersensitivity is clindamycin (5.0--7.5 mg/kg orally 4 times daily; maximum 600 mg/dose), adminstered with pyrimethamine and leucovorin (AI). Clindamycin can be associated with fever, rash, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea, including pseudomembranous colitis) and hepatotoxicity. Azithromycin (900--1,200 mg/day) also has been used with pyrimethamine and leucovorin among sulfa-allergic adults instead of clindamycin (BII), but this regimen has not been studied among children (CIII). Another alternative in adults is atovaquone (1,500 mg orally twice daily, adminstered with meals) plus pyrimethamine and leucovorin, or atovaquone with sulfadiazine alone, or atovaquone as a single agent among patients intolerant to both pyrimethamine and sulfadiazine (BII); however, these regimens have not been studied among children (CIII). Trimethoprim-sulfamethoxazole (5 mg/kg trimethoprim plus 25 mg/kg sulfamethoxazole intravenously or orally adminstered twice daily) alone has been used as an alternative to pyrimethamine-sulfadiazine among adults (BI), but this has not been studied among children (CIII). Corticosteriods (e.g., dexamethasone or prednisone) have been used among children with CNS disease when CSF protein is very elevated (i.e., >1,000 mg/dL) or with focal lesions with substantial mass effects (BIII). Because of the potential immunosuppressive effects of steroids, they should be discontinued as soon as possible. Among HIV-infected children, lifelong suppression is indicated after treatment for toxoplasmosis to prevent recurrence; details on secondary prophylaxis (i.e., maintenance therapy) have been published (4). Safety of discontinuation of secondary prophylaxis after immune reconstitution with HAART among children has not been studied extensively. Cryptosporidiosis/MicrosporidiosisEpidemiology Cryptosporidium species are protozoal parasites that mainly cause enteric illness in humans and animals. The three most common species infecting humans are C. hominis (formerly C. parvum genotype 1 or human genotype), C. parvum (formerly C. parvum genotype 2 or bovine genotype), and C. meleagridis. In addition, infections with C. canis, C. felis, C. muris, and Cryptosporidium pig genotype have been reported in immunocompromised patients. Cryptosporidium parasites invade the gut mucosa, causing severe profuse, nonbloody, watery diarrhea leading to dehydration and malnutrition in immunocompromised hosts. The parasite is transmitted by ingestion of oocysts excreted in the feces of infected animals and humans. The parasite is highly infectious, with an ID50 ranging from nine to 1,042 oocysts, depending on the isolate (64). Infection occurs when the ingested oocyst releases sporozoites, which attach to and invade the intestinal epithelial cells. The parasite has a predilection for the jejunum and terminal ileum (64). Person-to-person transmission is common in child care centers. Outbreaks have been associated with ingestion of contaminated drinking water in large metropolitan areas and with public swimming pools. Foodborne and person-to-person spread also have been documented (64). Cryptosporidiosis has been reported in 3%--4% of HIV-infected children in the United States, but occurs more frequently among children outside of the United States, particularly in Africa (65). Microspora species are obligate, intracellular, spore-forming protozoa that primarily cause moderate to severe diarrhea among children. Enterocytozoon bieneusi and Encephalitozoon intestinalis are the most common microsporidia that cause infection among patients with HIV infection. In addition to the Enterocytozoon and Encephalitozoon microsporidia genera, Nosema, Pleistophora, Trachipleistophora, Brachiola, and Vittaforma species have been implicated in human infections. The Microspora parasites develop in enterocytes and are excreted with feces and, like C. parvum, are transmitted by the fecal-oral route, which can include ingestion of contaminated food or water (66). Microsporidiosis has been reported in up to 7% of HIV-infected Thai children with acute and chronic diarrhea (67). Clinical Manifestations Frequent, usually nonbloody, watery, persistent diarrhea is the most common manifestation of both cryptosporidial and microsporidial infection, with abdominal cramps, fatigue, vomiting, anorexia, weight loss, and poor weight gain. Fever and vomiting are relatively common in children, mimicking viral gastroenteritis (65). Among immunocompromised children, chronic severe diarrhea can result in malnutrition, failure to thrive, and substantial intestinal fluid losses, resulting in severe dehydration and even death. Clinical history or physical examination does not allow differentiation of cryptosporidial or microsporidial infection from those caused by other pathogens. Cryptosporidium can migrate into the bile duct and result in inflammation of the biliary epithelium, acalculous cholecystitis, and sclerosing cholangitis. Symptoms include fever and right upper abdominal pain and elevated alkaline phosphatase. Although infection is usually limited to the gastrointestinal tract, pulmonary or disseminated infection also can occur among immunocompromised children. In addition to acute and chronic diarrhea, microsporidia species have been described in cases of hepatitis, peritonitis, keratoconjunctivitis, myositis, cholangitis, sinusitis, and disseminated CNS disease. Diagnosis For diagnosis of cryptosporidiosis, stool samples are concentrated by using the sucrose flotation or formalin-ethyl acetate method to concentrate the oocysts. A sample is then stained by using a modified Kinyoun acid-fast stain and examined for small (4--6 um in diameter) acid-fast positive oocysts. Monoclonal antibody-based fluorescein-conjugated stain for oocysts in stool and an enzyme immunoassay to detect antigen in stool are preferred to staining methods because of enhanced sensitivity and specificity. Oocyst excretion can be intermittent; therefore, the parasite might not be detected in every stool. At least 3 stool samples should be submitted for oocyst evaluation. Organisms also can be identified on small intestinal biopsy or intestinal fluid samples. For diagnosis of microsporidia infection, thin smears of unconcentrated stool-formalin suspension or duodenal aspirates can be stained with modified trichrome stain. Chemofluorescent agents (e.g., Calcofluor white) are helpful for the quick identification of spores in stool samples. Microsporidia spores are stained pink to red and are 1--3 um in size and ovoid and contain a distinctive equatorial-belt-like stripe. Urine sediment examination by light microscopy can be used to identify microsporidia spores in disseminated disease with Encephalitozoonidae and Trachipleistophora. Enterocytozoon bieneusi is not associated with disseminated disease. Transmission electron microscopy is needed for speciation. Endoscopy should be considered for all patients with chronic diarrhea of >2 months duration and negative stool examinations. Touch preparations are useful for rapid diagnosis (i.e., within 24 hours). Sensitive assays using PCR amplification of parasite DNA sequences extracted from stool or biopsy specimens have been developed for Cryptosporidium and Enterocytozoon bieneusi (68,69), but are research tools and not commercially available. Treatment Immune reconstitution resulting from HAART frequently results in clearance of Cryptosporidium and Microsporidium infections. Effective HAART is the recommended treatment for these infections (70) (AII). Supportive care with hydration, correction of electrolyte abnormalities, and nutritional supplementation should be provided (AIII). Antimotility agents should be used with caution among young children (CIII). No consistently effective therapy is available for either cryptosporidiosis or microsporidiosis, and duration of treatment among HIV-infected persons is uncertain. Certain agents have demonstrated efficacy in decreasing the severity of symptoms among children. Nitazoxanide is approved for treatment of diarrhea caused by Cryptosporidium and Giardia lamblia among children and is available in a liquid formulation (BI for uninfected children and CIII HIV-infected children). An Egyptian clinical trial among 100 HIV-uninfected patients, half of them children, randomized patients to a 3-day course of nitazoxanide or placebo. Nitazoxanide therapy reduced the duration of both diarrhea and oocyst shedding; among children, clinical response was 88% with nitazoxanide and 38% with placebo (71). No substantial adverse events were reported, and adverse events that were reported were similar in the treatment and placebo groups in this study. A study in Zambia among 100 children aged 12--35 months, half HIV-infected, reported a clinical response of 56% with treatment compared with 23% with placebo among HIV-uninfected children, but among HIV-infected children, the drug was no more effective than placebo (72). However, in a study among HIV-infected adults in Mexico, 14 days of nitazoxanide resulted in 67% response using 1,000 mg twice daily and 63% using 500 mg twice daily, compared with 25% with placebo (73). One study among HIV-infected adults demonstrated clinical response in patients with CD4 cell count >50/µL but not those with CD4 cell count <50/µL (74). The recommended dose for children is 100 mg orally twice daily for children aged 1--3 years and 200 mg twice daily for children aged 4--11 years. A tablet preparation is not yet approved. Certain specialists recommend paromomycin (25--35 mg/kg body weight/day orally in 2--4 divided doses; maximum dose: 500 mg four times daily) for the treatment of cryptosporidiosis in HIV-infected children (CIII). However, in a placebo-controlled trial in HIV-infected adults, paromomycin was no more effective than placebo for the treatment of symptomatic cryptosporidiosis (75). Azithromycin has demonstrated some activity against C. parvum infection in a limited number of HIV-infected children (76) (CIII). An azithromycin regimen of 10 mg/kg per day on day 1 and 5 mg/kg per day on days 2--10 was successful in rapidly resolving enteric symptoms in three of four HIV-infected children with cryptosporidiosis (76). Oral hyperimmune bovine colostrum and oral immune globulin have variable benefits among immunocompromised patients with cryptosporidiosis (CIII). For treatment of microsporidia infection, albendazole (dosage for person weighing <60 kg is 7.5 mg/kg orally twice daily; maximum dose: 400 mg orally twice daily) decreases diarrhea, sometimes with eradication of the organism (AII). Albendazole appears to be more effective for cases caused by Encephalitozoon intestinalis (77) and other microsporidia species but is not active against Enterocytozoon bienesi. Nitazoxanide has been used for treatment of Enterocytozoon bienesi infection among HIV-infected adults (78). Fumagillin® (Sanofi-Synthelabo Laboratories, Gentilly, France) is an antibiotic derived from the fungus Aspergillus fumigatus, which has been used to treat microsporidiosis in animals and humans. In a placebo-controlled study of immunocompromised adults (including HIV-infected adults) with Enterocytozoon bieneusi microsporidiosis, fumagillin (20 mg/dose orally three times daily for 2 weeks) was associated with decreased diarrhea and clearance of microsporidial spores, which was not observed in placebo patients (79). Dose-related bone marrow toxicity was the principal adverse effect of fumagillin, with reversible thrombocytopenia and neutropenia being the most frequent adverse events. No data are available on use of fumagillin among HIV-infected children (CIII), and the drug is not available in the United States. Ocular infections caused by microsporidia among HIV-infected adults have been treated topically with fumagillin eye drops prepared from Fumidil-B®, a commercial product (Mid-Continent Agrimarketing, Inc., Olathe, KS) used to control a microsporidial disease of honeybees. Mycobacterium tuberculosisEpidemiology In 2002, approximately 15,000 new cases of TB disease were diagnosed in the United States. Of these, 6% were among children aged <15 years (80). Although the number of cases in this age group has been decreasing since 1992, the number coinfected with HIV is uncertain because only a limited number of U.S. children who have TB have been tested for HIV infection. In a study of TB cases in the United States during 1993--2000, more adults than children had an HIV test reported (41.4 versus 16.1%); the majority of children (83.9%) did not have a reported HIV test result (81). Overall, 12.9% of adults were reported to be coinfected with HIV, compared with 1.1% of all childhood TB cases (81). Incident case rates of TB disease among HIV-exposed or -infected children were reported in one study from the early 1990s to be up to 100-fold higher than those in comparably aged children in the general U.S. population (8). Data from international studies indicate an increased risk for TB disease among HIV-infected children; coinfection with HIV occurred in up to 48% of hospitalized South African children with culture-proven TB (82,83). In New York City, 3% of approximately 1,400 HIV-infected children and 0.5% of HIV-exposed uninfected children had active TB diagnosed during 1989--1995 (84). Extrapulmonary and miliary TB are more common among younger children (aged <4 years) (85,86) who do not have HIV infection.Younger children are also more likely to progress more rapidly from infection to active disease than older children and adults and might not be recognized as having TB disease because they might have negative skin tests and fewer symptoms of disease (87). Despite evidence that extrapulmonary TB occurs more frequently among HIV-infected rather than uninfected adults, this is less clear among children in areas where TB is endemic (88,89). Congenital TB is rare but has been reported among children born to HIV-infected women with active TB (90). The true incidence and whether the rate is higher among these children compared with children born to non-HIV-infected women with active TB are unknown. Congenital TB can result from hematogenous dissemination of M. tuberculosis after maternal mycobacteremia, rupture of a placental tubercule into the fetal circulation, or ingestion of infected amniotic fluid or maternal blood at delivery. The mother might not have symptoms of TB disease, and subclinical maternal genital TB also can result in an infected neonate (90). Children with TB disease are almost always infected by an adult in their daily environment, and their infection represents primary infection rather than the reactivation disease observed among adults (8,91). Identification and treatment of the source patient and evaluation of all exposed children is particularly important; all confirmed and suspected TB cases should be reported to state and local health departments, who will assist in contact evaluation. In addition, other exposed members of the household should be evaluated because other secondary TB cases and latent infections with M. tuberculosis often are found. Latent infections should be treated to prevent cases. HIV counseling and testing should be offered to TB contacts because coexisting HIV infection, which increases the risk for TB disease, can reduce the sensitivity of the tuberculin test. Multidrug-resistant TB is unusual among U.S.-born TB patients. Data from U.S. surveillance during 1993--2001 among pediatric patients with TB indicate that M. tuberculosis with resistance to any of the first-line anti-TB drugs was identified in 15.2% of children with culture-positive M. tuberculosis, with higher rates among foreign-born children (19.2%) than U.S.-born children (14.1%) (81). However, the prevalence of multidrug resistance (e.g., at least isoniazid and rifampin) was lower: 2.8% in foreign-born children and 1.4% in U.S.-born children with TB. Data are limited about the incidence of drug resistant strains of TB among HIV-infected U.S. children. In one retrospective survey study of 70 PACTG sites, multiple resistant strains of M. tuberculosis were observed in 15%--20% of HIV-infected children with TB.The majority of cases were from one state with a peak in incidence of disease (8), reflecting the increase in drug-resistant TB among adults infected with TB to which the children were exposed (92,93). Drug-resistant M. tuberculosis is as transmissible as drug-susceptible M. tuberculosis and remains drug resistant in a new host. Contacts to drug-resistant TB should be treated under the assumption that any newly diagnosed infections are similarly drug resistant. Clinical Manifestations Clinical signs of infection in the infant with congenital TB disease are nonspecific. Predominant early symptoms are inadequate feeding and failure to gain weight during the first weeks of life; upper respiratory symptoms and progressive hepatosplenomegaly might appear later with fever, progressive pneumonia, and meningitis. Certain infants might present more acutely with progressive respiratory distress, apnea, jaundice, and abdominal distention (94). Children with pulmonary TB might have little or no symptoms. Symptoms, when present, might be nonspecific (e.g., weight loss, fever, and failure to thrive). TB among young children rarely manifests with the typical apical lung infiltrates and late cavitation observed among adults with TB. More commonly, pulmonary TB appears as a localized pulmonary infiltrate with associated hilar lymphadenopathy. Multiple lobes are involved in up to 25% of children. Concomitant atelectasis might result from hilar adenopathy compressing bronchi or from endobronchial granulomas. Clinical presentation of TB disease among children with HIV infection is similar to that among children without HIV infection (86,95). Signs and symptoms might be consistent with acute pneumonia, with nonspecific radiological opacities without hilar adenopathy (83,88,96). Older HIV-infected children and adolescents might have clinical presentations more similar to that seen in HIV-infected adults (97). In countries with a high burden of TB, the incidence of treatment failure and mortality is higher among HIV-infected children (86,98). Commonly reported sites of extrapulmonary disease among children include lymph nodes, hematogenous (military), CNS, bone, pericardium, peritoneum, and pleura (86,96,99,100). Diagnosis Because of the difficulty of definitively diagnosing TB disease among children, a high index of suspicion is important. M. tuberculosis can be detected in culture of gastric aspirate samples from approximately 50% of children without HIV infection who have TB disease (101). Suspicion for and diagnosis of TB in HIV-infected children is further complicated by the frequent presence of pre-existing or coincidental fever, pulmonary symptoms, and radiographic abnormalities (e.g., chronic lymphoid interstitial pneumonitis or coincident pulmonary bacterial infection) in this population (102,103). Because of the difficulty in obtaining a definitive culture-proven diagnosis of TB disease among children, the diagnosis of TB disease usually involves linking the child to an adult with confirmed pulmonary TB together with a positive tuberculin skin test (TST) and an abnormal radiograph or physical examination in the child (101). However, a negative TST result cannot exclude TB disease among children because approximately 10% of children without HIV infection but with culture-positive TB disease do not react initially to a TST (59). HIV infection further decreases TST reactivity. Therefore, a TST result among children, particularly HIV-infected children, is less useful than in adults. Although a positive test is useful to confirm the diagnosis, a negative test would not rule out the possibility of TB disease. Because children with HIV infection are considered at high risk for TB, annual Mantoux tuberculin skin testing of this population is recommended, beginning at age 3--12 months, using intradermally injected 5 TU purified protein derivative (PPD) (59). Among children and adults with HIV infection, >5 mm of induration is considered a positive (diagnostic) reaction. Multiple puncture TB skin tests (e.g., Tine) are not recommended. The use of control skin antigens at the time of PPD testing to identify anergy is of uncertain value and no longer routinely recommended; however, if anergy testing is performed, mumps and candida are appropriate control antigens (104). Even without HIV infection, approximately 10% of children and adults with culture-negative TB have a negative TST, and up to 50% with miliary TB and meningitis have an initially negative TST (101). Children aged <2 years or those who have HIV infection might be more likely to have a negative skin test (99,100). Although acid-fast stained sputum smears are positive in 50%--70% of adults with pulmonary TB, children with TB disease rarely produce sputum voluntarily and typically have a low bacterial load (101). Acid-fast stains of early morning gastric aspirates are positive in 0-20% of children with TB, and in children with extrapulmonary TB, acid-fast stains of samples such as lymph node, CSF, and joint fluid are usually positive in <25% of children. Although the sensitivity of stained specimen smears is less among children than adults, a positive smear is indicative of mycobacteria, although it does not differentiate M. tuberculosis from other mycobacterial species. Smears of all specimens should be prepared, stained (using either the Ziehl-Neelsen method or auramine-rhodamine staining in conjunction with fluorescence microscopy), and evaluated for the presence of acid-fast organisms. Auramine-rhodamine staining followed by fluorescent microscopy is more efficient than traditional carbol fuchsin stains (101). A definitive diagnosis of TB disease requires isolation of M. tuberculosis from expectorated sputum, bronchoalveolar lavage (BAL) fluid, aspirated gastric fluid (obtained in the early morning after the child fasts overnight), biopsied lung, peripheral lymph node or other tissue (depending on location of disease), or mycobacterial blood culture (105,106). In addition, availability of an isolate allows drug susceptibility testing to be performed. Three consecutive morning gastric aspirates yield a positive culture of M. tuberculosis in up to 70% of infants and 30%--50% of children with clinical pulmonary TB (101). Gastric lavage samples, collected on three consecutive mornings, has a higher yield on culture (50%) than a single sample collected by bronchoalveolar lavage (10%) (107). Nasopharyngeal aspiration was evaluated in one study of 64 children aged 1 month--16 years and was found to have comparable sensitivity and specificity as gastric aspirate culture (108). Sputum induction was safe and almost twice as effective in identifying M. tuberculosis compared with gastric lavage specimens in one South African study of children aged <5 years with community-acquired pneumonia (109). The culture yield from other fluids and tissues from children with extrapulmonary TB is <50%, even with optimal samples. Strenuous efforts should be made to obtain diagnostic specimens (three each of sputum or gastric aspirate specimens or an induced sputum for culture) whenever a presumptive diagnosis of TB is made or when it is highly suspected despite negative skin testing results (i.e., because of a history of exposure to a person with active TB). Specimens should be cultured with radiometric culture methods and DNA probes for species identification. M. tuberculosis can be isolated and identified in 7--14 days. Antimycobacterial drug susceptibility testing should be performed on the initial M. tuberculosis isolate and on subsequent isolates if treatment failure or relapse is suspected; the radiometric culture system has been adapted to perform rapid sensitivity testing. Before obtaining results of susceptibility testing and if an organism has not been able to be isolated from specimens from the child, the antimycobacterial drug susceptibility of the M. tuberculosis isolate from the source case can be used to define the probable drug susceptibility of the child's organism and to design the empiric therapeutic regimen for the child. Diagnosis can be facilitated by use of PCR amplification techniques that allow rapid amplification of mycobacterial-species specific target sequences that are detected by a molecular probe. Two commercial kits are available for rapid, direct detection of M. tuberculosis, and both are labeled for use on sputum only. One is labeled for use on sputum only with AFB detected on the smear. When these tests are used for other specimens, sensitivity and specificity might be unsatisfactory. PCR assays provide adjunctive, but not primary, diagnosis for evaluation of children with TB, because a negative PCR does not rule out TB as a diagnostic possibility and a positive result does not represent absolute confirmation of M. tuberculosis infection. However, it might be useful in suggesting the diagnosis of TB among HIV-infected children with unexplained pulmonary disease, when both culture and tuberculin skin tests may be falsely negative. Although both DNA and RNA amplification systems are available, only DNA systems have been used in published pediatric studies. Results for PCR testing for M. tuberculosis on gastric aspirates from children have been disappointing with sensitivity varying between 45%--83% (110--112). In one study, PCR specificity was only 80%; false positives occurred in children with Mycobacterium avium-intracellulare disease, which is not an uncommon infection among HIV-infected children (112). Treatment Principles for treatment of TB in the HIV-infected child are the same as for the HIV-uninfected child. However, optimal therapy has not been defined, and modified treatment durations, schedules, and medications are recommended for specific instances. Because of overlapping drug toxicities, children being treated for both HIV and TB should be managed by a specialist with expertise in treating both these conditions (AIII). Because of the high risk for dissemination among children aged <4 years, TB treatment should be initiated as soon as the diagnosis of TB is suspected (AIII). Although the optimal timing of initiation of antiretroviral therapy during TB treatment is unknown, in the setting of antiretroviral naïve HIV-infected children, treatment of TB should be initiated 4--8 weeks before initiating antiretroviral medications to improve adherence and better differentiate potential side effects (BIII). For children already receiving antiretroviral therapy who have had TB diagnosed, the child's antiretroviral regimen should be reviewed and altered, if needed, to ensure optimal treatment for both TB and HIV and to minimize potential toxicities and drug-drug interactions (BIII). The major problem limiting successful treatment is inadequate adherence to prescribed treatment regimens. Use of directly observed therapy (DOT) decreases the rates of relapse, treatment failures, and drug resistance. Therefore, DOT is recommended for treatment of children and adolescents with TB in the United States (59) (AII). For the first 2 months of treatment, DOT should be adminstered daily (induction phase). After this, DOT is usually given two to three times weekly (continuation phase). For patients on rifampin- or rifabutin-based regimens and who have severe immunosuppression, three-times weekly regimens are preferred because of concerns about development of rifamycin resistance by M. tuberculosis (BII). However, data on the efficacy of three-times weekly regimens among children are limited. Initial empiric treatment of active disease (induction phase) should generally consist of a 4-drug regimen (isoniazid, rifampin, pyrazinamide, and either ethambutol or streptomycin) to allow for the possibility of a drug-resistant organism (AI). Ethionamide can be used as an alternative to ethambutol in cases of TB meningitis because ethionamide has better CNS penetration than ethambutol (AIII). Subsequent modifications of therapy should be based on susceptibility testing if possible. The drug susceptibility pattern from the isolate of the adult source case might help guide treatment in cases where an isolate is not available from the child (59). If the organism is susceptible to isoniazid, rifampin, and pyrazinamide during the 2-month period of induction therapy, ethambutol can be discontinued and induction therapy completed using 3 drugs (AI). Following the 2-month induction phase, for treatment of M. tuberculosis known to be sensitive to isoniazid and rifampin, therapy is continued with isoniazid and rifampin to complete therapy (AI); daily or intermittent (2--3 times weekly) therapy is acceptable (59,112). However, children with severe immunosuppression should receive either daily or thrice weekly treatment during the continuation phase, because TB treatment regimens with once- or twice-weekly dosing have been associated with an increased rate of acquisition of rifamycin resistance among HIV-infected adults with low CD4 cell counts (<100 cells/µL) (113) (AII). Many clinicians report high rates of treatment failure and relapse when only 6 months of treatment is administered (the recommended duration of therapy for children without HIV infection) (59,112,114). For HIV-infected children with active pulmonary disease, the minimum recommended duration of antituberculous drug treatment is 9 months; for children with extrapulmonary disease involving the bones or joints, CNS, or miliary disease, the minimum recommended duration of treatment is 12 months (59,112,115) (AIII). These recommendations assume that the organism is susceptible to the medications, that compliance with the medications has been assured, and that the child has had a clinical and microbiologic response to therapy. For treatment of drug-resistant TB, a minimum of three drugs should be administered, including at least 2 bactericidal drugs to which the isolate is susceptible (AII). Regimens can include three to six drugs with varying levels of activity. Children infected with multidrug-resistant TB (e.g., resistance to at least isoniazid and rifampin) should be managed in consultation with an expert in this condition (AIII). If the strain is resistant only to isoniazid, isoniazid should be discontinued and the patient treated with 9--12 months of a rifampin- or rifabutin-containing regimen (e.g., rifampin, pyrazinamide and ethambutol (BII); ethionamide or streptomycin can be substituted for ethambutol if the M. tuberculosis isolate is sensitive to these agents). If the strain is resistant only to rifampin, risk for relapse and treatment failure is increased. Rifampin should be discontinued and a 2-month induction phase of isoniazid, pyrazinamide, ethambutol and streptomycin administered, followed by an additional continuation phase of isoniazid, pyrazinamide, and ethambutol to complete a minimum of a 12-month course of therapy, with the exact length of therapy based on clinical and radiologic improvement (BIII). Among older adolescents with rifampin monoresistant strains, isoniazid, ethambutol, and a fluoroquinolone can be administered, with pyrazinamide added for the first 2 months (BIII); an injectable agent (e.g., aminoglycoside such as streptomycin or amikacin) also can be included in the first 2--3 months for patients with severe disease (BIII). When the strain is resistant to isoniazid and rifampin (multidrug-resistant TB), therapeutic regimens must be individualized based on the resistance pattern, relative activities of the drugs, extent of disease, and any co-morbid conditions. Therapy frequently requires 12--24 months. Isoniazid (10--15 mg/kg body weight adminstered orally once daily [maximum dose: 300 mg/day]) is available as syrup, but certain specialists advise against using it because the syrup is unstable and frequently causes diarrhea. When isoniazid is adminstered in a dosage exceeding 10 mg/kg in combination with rifampin, the incidence of hepatic toxicity might be increased. Pills can be pulverized at the time of administration and mixed with a small amount of appealing food immediately before giving it to the child. Dose for two--times weekly administration is 20--30 mg/kg/dose (maximum dose: 900 mg). Gastric upset during the initial weeks of isoniazid treatment occurs frequently. Hepatotoxicity is the most common adverse effect and includes subclinical hepatic enzyme elevation and clinical hepatitis that can be reversible when drug is discontinued or rarely progresses to hepatic failure. Hepatotoxicity is less frequent in children than in adults. Transient asymptomatic serum transaminase elevations have been noted in 3%--10% and clinical hepatitis in <1% of children receiving isoniazid, although <1% of children required treatment discontinuation (115,116). However, the rate of hepatotoxicity might be higher in children on multiple hepatotoxic medications. Other toxicities reported with isoniazid include peripheral neuritis, mild CNS effects, and rare hypersensitivity reactions. Pyridoxine is recommended for children and adolescents on meat- and milk-deficient diets and children with nutritional deficiencies, including all symptomatic HIV-infected children (AII). Rifampin (10-20 mg/kg adminstered orally once daily [maximum dose: 600 mg/day]) is available only as a capsule. It can be administered by opening the capsule and sprinkling the contents on food. Alternatively, a suspension can be formulated by the pharmacy, although the stability of the ad hoc suspension is unknown. Dose for twice weekly administration is 10--20 mg/kg per dose (maximum dose: 600 mg). Rifampin is excreted in urine, tears, sweat, and other body fluids and colors them orange; contact lenses may be stained. The most common adverse reaction to rifampin therapy is gastrointestinal upset. Other reactions include skin rash, hepatitis, thrombocytopenia, and cholestatic jaundice. An influenza-like syndrome, hemolytic anemia, and acute renal failure have been reported among adults receiving high doses of rifampin. Rifampin induces hepatic cytochrome P450 enzymes and can accelerate clearance of drugs metabolized by the liver (e.g., protease inhibitors and non-nucleoside reverse transcriptase inhibitors), resulting in subtherapeutic levels of the drug. As a result, concurrent administration of rifampin and single protease inhibitors, with the exception of ritonavir, is not recommended (117) (EII). Coadministration of ritonavir-boosted saquinavir, with 400 mg ritonavir boosting, with rifampin is possible, but low-dose ritonavir-boosted dual protease inhibitor regimens should not be used. Concurrent administration of rifampin with the non-nucleoside reverse transcriptase inhibitor delavirdine also is contraindicated because of similar drug interactions (EII). However, concomitant administration of rifampin with efavirenz (and perhaps nevirapine) is possible (117). Rifampin- and nevirapine-containing regimens should be used only when no other options are available and close clinical and virologic monitoring can be performed because of the decrease in nevirapine levels that can occur with concomitant administration (117). Rifabutin (10--20 mg/kg adminstered orally once daily) is a suitable alternative to rifampin in children on HAART that proscribes the use of rifampin; however, experience in children is limited (BIII). Major toxicities of rifabutin include leukopenia, gastrointestinal upset, polyarthralgias, rash, elevated transaminases, and skin and secretion discoloration (pseudojaundice). Anterior uveitis has been reported among adults and children receiving rifabutin as prophylaxis or a part of a combination regimen for treatment, usually when adminstered at higher doses (118). Rifabutin also increases hepatic metabolism of many drugs but is a less potent inducer of cytochrome P450 enzymes than rifampin and has fewer problematic drug interactions than rifampin. However, adjustments in doses of rifabutin and the coadministered antiretroviral drugs might be necessary for certain combinations (117). Coadministration of rifabutin with certain protease inhibitors can result in increased rifabutin concentration and thus potential toxicity; therefore, a decrease in rifabutin dose by 50% is required when coadministered with ritonavir, indinavir, nelfinavir, amprenavir, or ritonavir-boosted saquinavir. An increased dose (by 50%--100%) of rifabutin is needed when coadministered with efavirenz (117) because of decreased rifabutin levels with coadministration. Rifabutin should not be coadministered with delavirdine or hard gel capsule saquinavir without ritonavir boosting because of substantial decreases in the concomitant protease inhibitor drug levels (EII). Other drugs that inhibit hepatic metabolism (e.g., fluconazole) also can increase concentrations of rifabutin and consequent toxicity and might require dose adjustment or discontinuation of rifabutin. Pyrazinamide (20--40 mg/kg/day is adminstered orally once daily [maximum dose: 2 g/day]) is available only as a scored tablet. It is generally administered during the first 2 months of TB therapy. If pyrazinamide is to be continued on a two- to three-times-weekly schedule, it should be administered at a dose of 50--70 mg/kg/dose (maximum dose: 2 g). Adverse effects include hepatotoxicity and hyperuricemia, arthralgias, skin rash, and gastrointestinal intolerance. Ethambutol (15--25 mg/kg administered orally in single oral dose [maximum dose: 1.0 g]) is available only as a scored tablet. Although not approved for use among children because of concern for optic nerve toxicity that might not be easily recognizable with pediatric use, it has been used in children without toxicity (BIII). Dose for twice weekly administration is 50 mg/kg per dose (maximum dose: 1.0 g). The major toxicity is optic neuritis, with symptoms of blurry vision, central scotomata, and red-green color blindness, which is usually reversible and rare at doses of 15 mg/kg among children with normal renal function. Children receiving ethambutol should have monthly monitoring of visual acuity and color discrimination if possible (AIII). Other toxicities include headache, nausea, peripheral neuropathy, rash, and hyperuricemia. Secondary drugs used in treatment of resistant TB have not been well studied in children. These medications should be used in consultation with a TB specialist. Ethionamide (15--20 mg/kg adminstered orally divided into 2--3 doses per day [maximum dose: 1.0 g/day]) is available only in tablet formulation. Data are unavailable to support intermittent (e.g. twice or three times weekly) dosing of this drug. Ethionamide might be useful for children with drug-resistant TB or TB meningitis because the drug achieves increased concentration in CSF (59). Nausea, vomiting, loss of appetite, and abdominal pain are the most common adverse effects. Other adverse effects include hepatitis, arthralgias, gynecomastia, photosensitive dermatitis, and a metallic taste in the mouth. Hypothyroidism has been reported with ethionamide use, and periodic (e.g., monthly) monitoring of thyroid hormone serum concentrations is recommended (AIII). Streptomycin (20--40 mg/kg/day adminstered intramuscularly once daily [maximum dose: 1 g/day]) is an alternative drug that can be substituted for ethambutol (BIII). It also is used in combination quadruple therapy with rifampin, isoniazid, and pyrazinamide for CNS TB (meningitis and tuberculoma). Dosage for twice weekly administration is 20 mg/kg per dose intramuscularly (maximum dose: 1 g). If streptomycin is not available, kanamycin (15--30 mg/kg adminstered intramuscularly once daily [maximum dose: 1 g/day]) or amikacin (15--30 mg/kg adminstered intravenously or intramuscularly once daily [maximum dose: 1 g/day]) are active against most strains of streptomycin-resistant M. tuberculosis. Amikacin has the advantage of a lower rate of ototoxicity and has largely replaced kanamycin in the treatment of adults. Major adverse effects of aminoglycoside drugs are oto- and nephrotoxicity. Periodic audiometry, monitoring of vestibular function (if possible), and blood urea nitrogen and creatinine are recommended. Capreomycin (15--30 mg/kg adminstered intravenously or intramuscularly once daily [maximum dose: 1 g/day]) is a secondary drug used for drug-resistant TB. The major adverse effect is toxicity to the eighth cranial nerve. Renal toxicity also might be seen, with electrolyte disturbances secondary to tubular damage and elevated serum creatinine. Monitoring of hearing with audiograms monthly, periodic examinations of vestibular function, and regular monitoring of blood urea nitrogen and creatinine are recommended (AIII). Quinolones such as ciprofloxacin (10--15 mg/kg adminstered orally twice daily [maximum dose: 1.5 g/day]), ofloxacin (400--800 mg total given orally once daily in adults [maximum dose: 800 mg/day]) levofloxacin (500--1,000 mg adminstered orally once daily in adults) and moxifloxacin (400 mg adminstered orally once daily in adults) can be used. Adverse effects of quinolones include gastrointestinal upset, diarrhea, rash, and headache. Cartilage damage has been observed with use of the fluoroquinolone drugs in animals and, theoretically, these drugs could have an effect on growing cartilage in children; they are not approved for persons aged <18 years and use in younger persons requires an assessment of potential risks and benefits (119) (CIII). Ciprofloxacin has had the greatest use among children and appears to be well tolerated and not associated with arthropathy (120). Cycloserine (10--20 mg/kg adminstered orally once daily [maximum dose: 1 g]) is another second-line antimycobacterial that might be needed for treatment of drug-resistant infections. The major adverse reactions are emotional and behavioral disturbances, and periodic assessment of mental status is recommended. Convulsions and peripheral neuropathy can occur, especially if adminstered with isoniazid, and coadministration of pyridoxine (150 mg/day) is recommended (AII). Para-amino salicylic acid (200-300 mg/kg adminstered orally divided into 3 or 4 daily doses [maximum dose: 10 g/day]) also can be used for treatment of drug-resistant TB. The adverse effects of the drug are predominantly gastrointestinal (nausea, vomiting, and diarrhea). Hypersensitivity reactions occur in 5%--10% of persons, and hepatitis can occur. Hepatic enzyme monitoring is recommended (AIII). Thiacetazone can cause severe and often fatal reactions among HIV-infected children, including severe rash and aplastic anemia, and should not be used (EIII). Unlike the majority of children without HIV infection, HIV-infected children on anti-TB medications should have liver enzymes obtained at baseline and monthly for the first few months of therapy (AIII). If symptoms of drug toxicity develop, a physical examination and repeat liver enzyme measurement should be performed (AIII). Mild elevations in serum transaminases (e.g., 2--3 times upper limit of normal) do not require discontinuation of drugs if other findings are normal (AII). Adjunctive treatment with corticosteriods is indicated for children with tuberculous meningitis; dexamethasone lowers mortality and long-term neurologic impairment (AII). These drugs might be considered for children with pleural or pericardial effusions, severe miliary disease, and substantial endobronchial disease (BIII). Appropriate antituberculous therapy must be administered concomitantly. Most experts use 1 to 2 mg/kg/day of prednisone or its equivalent for 6--8 weeks. Monthly monitoring of clinical and bacteriologic response to therapy is important (AII). For children with pulmonary TB, chest radiographs should be obtained after 2--3 months of therapy to evaluate response (AIII). Hilar adenopathy might persist for as long as 2--3 years despite successful antituberculous therapy, and a normal radiograph is not a criterion to discontinue therapy. Follow-up radiographs after completion of therapy are not necessary unless clinical symptoms recur. An immune reconstitution syndrome in patients receiving anti-TB therapy in the setting of HAART has been reported in HIV-infected adults (121--123). New onset of systemic symptoms, especially high fever, expanding CNS lesions, and worsening adenopathy, pulmonary infiltrates, or pleural effusions have been reported in the setting of HAART up to several months after starting TB therapy. Persons with mild-to-moderate symptoms of immune reconstitution syndrome have been treated symptomatically with nonsteroidal anti-inflammatory drugs while continuing anti-TB and HIV therapies. In certain cases, use of systemic corticosteriods steroids for 1--2 weeks results in improvement while continuing to receive TB/HIV therapies (121--123) (CIII). Mycobacterium avium Complex DiseaseEpidemiology Mycobacterium avium complex (MAC) refers to multiple related species of nontuberculous mycobacteria (e.g., M. avium, M. intracellulare, M. paratuberculosis) that are widely distributed in the environment. MAC is the cause of the second most common opportunistic infection among children with HIV infection after PCP, and is presumably acquired by common environmental exposures through inhallation, ingestion, or inoculation (124). Respiratory and gastrointestinal colonization can act as portals of entry that can lead to disseminated infection (125). The proportion of children with AIDS and disseminated MAC has been higher among children with hemophilia or transfusion-acquired HIV infection (approximately 13%--14%) than those with perinatal HIV infection (5%) (124). The median age at diagnosis of disseminated MAC in children with hemophilia or transfusion-associated AIDS is 9 years, compared with 3 years in those with perinatal infection. Data on the incidence of and risk factors for MAC among children receiving HAART are limited. MAC can appear as isolated lymphadenitis among HIV-infected children. Presentation with isolated MAC pulmonary disease is a marker of high risk for dissemination; 72% of children develop disseminated MAC within a mean time of 8 months (126). Disseminated infection with MAC in pediatric HIV infection rarely occurs during the first year of life; its frequency increases with age and declining CD4+ count, and it is a frequent complication of advanced immunologic deterioration among HIV-infected children (124,127,128). Disseminated MAC can occur at higher CD4+ cell counts among younger HIV-infected children than older children or adults, especially among children aged <2 years. Age-related CD4+ cell counts levels considered as high risk for MAC warranting consideration of prophylaxis are <750/µL among HIV-infected children <1 year old; <500/µL for children aged 1--2 years; <75/µL for children aged 2--6 years; and <50/µL for children aged >6 years (4,129--131). Clinical Manifestations Respiratory symptoms are uncommon among HIV-infected children with disseminated MAC, and isolated pulmonary disease is rare (125). Symptoms commonly associated with disseminated MAC infection among children include recurrent fever, weight loss or failure to thrive, neutropenia, night sweats, fatigue, chronic diarrhea, malabsorption, and persistent or recurrent abdominal pain. Lymphadenopathy, hepatomegaly, and splenomegaly can be found. Laboratory abnormalities include anemia, leukopenia, and thrombocytopenia. Serum chemistries are usually normal, although certain children might have elevations in alkaline phosphatase or lactate dehydrogenase. Diagnosis Procedures used to diagnose MAC in children are the same as used in HIV-infected adults. Definitive diagnosis is accomplished by isolation of the organism from the blood or from biopsy specimens from normally sterile sites (e.g., bone marrow, lymph node, or other tissues). Multiple mycobacterial blood cultures over time might be required to yield a positive result. Recovery of organisms from blood is enhanced by use of a radiometric broth medium or lysis-centrifugation culture technique. Histology demonstrating macrophage-containing acid-fast bacilli strongly indicates MAC in a patient with typical signs and symptoms, but culture is essential to differentiate nontuberculous mycobacteria from M. tuberculosis and to determine which nontuberculous mycobacteria are the cause of infection and the antimycobacterial drug susceptibilities of the organism. The Bactec method for radiometric susceptibility testing can be used. Susceptibility thresholds for clarithromycin are minimal inhibitory concentrations (MIC) of >32 ug/ml and an MIC of >256 ug/ml for azithromycin (132). Identification of MAC in stool or respiratory tract secretions indicates colonization but not necessarily invasive disease. Although not available widely, use of PCR might be of future value for diagnostic purposes (133,134). Treatment Treatment of disseminated MAC infection should be done in consultation with a pediatric infectious disease specialist with expertise in pediatric HIV infection (AIII). Combination therapy with a minimum of 2 drugs is recommended (AI). Monotherapy with a macrolide results in emergence of high-level drug resistance within weeks. The most effective way to prevent disseminated MAC among HIV-infected children is to preserve immune function through use of effective antiretroviral therapy. In addition, improved immunologic status is important for control of MAC disease among children with disseminated disease; potent antiretroviral therapy should be initiated among children with MAC disease who are antiretroviral-naïve. However, the optimal time to start HAART in this situation is unknown; certain clinicians treat MAC 1--2 weeks before starting HAART to try to minimize the occurrence of immune reconstitution syndrome, although whether this makes a difference is unknown (CIII). HAART should be continued and optimized for those already being treated. Prolonged survival among HIV-infected adults with MAC has been associated with receiving therapy that included clarithromycin and receiving combination antiretroviral therapy that included a protease inhibitor (135). Initial empiric therapy should include at least two drugs: clarithromycin or azithromycin plus ethambutol (AI). Certain specialists use clarithromycin as the preferred first agent, reserving azithromycin for patients with substantial intolerance to clarithromycin or when drug interactions with clarithromycin are a concern (AII). Rifabutin can be added as a third drug to the clarithromycin/ethambutol regimen, particularly in patients with more severe symptoms or disseminated disease (AI). A study in adult patients demonstrated a survival benefit with the addition of rifabutin to clarithromycin plus ethambutol. Drugs that can increase cytochrome P3A activity (e.g., rifabutin) can lead to increased clearance of other drugs (e.g., protease inhibitors and non-nucleoside reverse transcriptase inhibitors), and increased toxicity might be observed with concomitant administration of drugs competing for the same metabolic pathways. Therefore, drug interactions should be checked carefully, and more intensive toxicity monitoring might be warranted if such drugs are given concomitantly (AIII). A decrease in rifabutin dosage by 50% is required when coadministered with ritonavir, indinavir, nelfinavir, amprenavir, or ritonavir-boosted saquinavir; an increased dose (by 50%--100%) of rifabutin is needed when coadministered with efavirenz (117). Rifabutin should not be coadministered with delavirdine or hard gel capsule saquinavir without ritonavir boosting because of substantial decreases in the concomitant protease inhibitor drug levels (EII). Additional drugs can be considered depending on severity of illness. In a patient with severe illness, if rifabutin cannot be administered, ciprofloxacin, levofloxacin and amikacin or streptomycin have been used (CIII). In one study in HIV-infected adults, amikacin did not provide additional clinical or microbiologic benefit in a clinical trial of disseminated MAC therapy (136,137). In other studies, clofazamine was not associated with clinical or microbiologic benefit and was associated with increased mortality and is therefore not recommended (EII). Clarithromycin is adminstered at a dose of 7.5--15.0 mg/kg body weight orally twice daily (maximum dose: 500 mg twice daily) (AI). Major toxicities include nausea, diarrhea, and abdominal pain. Uncommon toxicities include headache, leukopenia, altered taste, and elevated transaminases. Clarithromycin can inhibit hepatic metabolism of other drugs cleared by the liver, thus potential drug interactions with concomitantly administered drugs need to be considered. Azithromycin is adminstered at a dose of 10--12 mg/kg orally once daily (maximum dose: 500 mg daily) and can be given as an alternative to clarithromycin (AII). Major toxicities include nausea, diarrhea, abdominal pain, and possible ototoxicity; uncommon adverse effects include headache, leukopenia, and elevated transaminases. Azithromycin has a minor effect on hepatic metabolism of other drugs and has less drug interactions than clarithromycin (138). Ethambutol is adminstered at a dose of 15--25 mg/kg and is adminstered in single oral dose (maximum dose: 1.0 g) (AI). It is available only as a scored tablet. Although not approved for use among children because of concern for optic nerve toxicity that might not be easily recognizable with pediatric use, it has been used among children without a high incidence of toxicity. The major toxicity is optic neuritis, with symptoms of blurry vision, central scotomata, and red- green color blindness, which is usually reversible and is rare at doses of 15 mg/kg. Children receiving ethambutol should have monthly monitoring of visual acuity and color discrimination if possible (AII). Other toxicities include headache, nausea, peripheral neuropathy, rash, and hyperuricemia. Rifabutin is adminstered at a dose of 10--20 mg /kg orally once daily (maximum dose: 300 mg/day) (AI). The drug is not available in a liquid formulation, but a suspension (10 mg/mL in cherry or simple syrup) can be formulated from the contents of capsules. Major toxicities of rifabutin include leukopenia, gastrointestinal upset, polyarthralgias, rash, elevated transaminases, and skin and secretion discoloration (pseudojaundice). Anterior uveitis has been reported in adults and children receiving rifabutin as prophylaxis or a part of a combination regimen for treatment, usually when adminstered at higher doses (139). Ciprofloxacin is adminstered at a dose of 20--30 mg/kg intravenously or orally once daily (maximum dose: 1.5 grams). Adverse effects of quinolones include gastrointestinal upset, diarrhea, rash, and headache. Cartilage damage has been observed with use of the fluoroquinolone drugs in animals, and theoretically, these drugs can have an effect on growing cartilage in children. They are not approved for persons aged <18 years and use in younger persons requires an assessment of potential risks and benefits (119) (CIII). Of the quinolone drugs, ciprofloxacin has had the greatest use among children and appears to be well-tolerated and not associated with arthropathy (120). Amikacin can be adminstered at a total daily dose of 15--30 mg/kg/day divided every 12--24 hours (maximum dose: 1.5 grams) (CIII). Amikacin is available only for intravenous administration and might be useful as a second-line agent. Ototoxicity and renal toxicity are adverse effects. Most patients demonstrate substantial clinical improvement during the first 4--6 weeks of therapy. Microbiologic response can be monitored by blood cultures every 4 weeks during initial therapy (BIII). However, elimination of the organism from the blood might require up to 12 weeks of effective therapy. An immune reconstitution syndrome in patients receiving MAC therapy in the setting of HAART has been reported among HIV-infected adults (140). New onset of systemic symptoms, especially fever or abdominal pain, leukocytosis, and focal lymphadenitis (cervical, thoracic, or abdominal) associated with pre-existing but relatively asymptomatic MAC infection, has been seen after starting HAART. Before initiation of HAART among HIV-infected children with low CD4+ cell counts, consideration should be given for an assessment for MAC and treatment if MAC is identified. However, recent data indicate that MAC prophylaxis with azithromycin did not prevent the development of immune reconstitution disease (141). Children with moderate symptoms of immune reconstitution syndrome can be treated symptomatically with nonsteroidal anti-inflammatory drugs or, if unresponsive to nonsteroidals, a short course (e.g., 4 weeks) of systemic corticosteroid therapy while continuing to receive HAART (CIII). Among HIV-infected children with MAC disease, after initial therapy, lifetime chronic suppressive maintenance therapy for MAC (secondary prophylaxis) is required. Although discontinuation of secondary prophylaxis in adults receiving HAART has been evaluated, the safety of discontinuation of secondary prophylaxis after immunologic recovery with HAART among children has not been studied extensively. Serious and Recurrent Bacterial InfectionsEpidemiology In an evaluation of opportunistic infections diagnosed in approximately 3,000 HIV-infected children participating in Pediatric AIDS Clinical Trials Group protocols in the pre-HAART era, serious bacterial infections were the most commonly diagnosed infection, with an event rate of 15/100 child-years (9). Pneumonia was the most common bacterial infection (11 per 100 child-years), followed by bacteremia (three/100 child-years), and urinary tract infection (two/100 child-years). Other serious bacterial infections, including osteomyelitis, meningitis, abscess, and septic arthritis, occurred at rates <0.2/100-child years. Acute pneumonia was associated with increased risk for long-term mortality among HIV-infected children in one study, although multiple episodes of acute pneumonia probably represent a marker for progressive disease and immunologic dysfunction rather than being causally associated with increased long-term mortality (142). Because of difficulties obtaining appropriate specimens (e.g., sputum) from young children, bacterial pneumonia is most often a presumptive diagnosis in a child with fever, pulmonic symptoms, and an abnormal chest radiogram unless an accompanying bacteremia exists. In a study of intravenous immune globulin prophylaxis of bacterial infections, only 12% of acute presumed bacterial pneumonia episodes had a bacterial pathogen identified (142). Chronic lung disease might predispose persons to development of acute pneumonia; in one study, the incidence of acute lower respiratory tract infection in HIV-infected children with chronic lymphoid interstitial pneumonitis was approximately 10-fold higher than in a community-based study of non-HIV--infected children (143). In a study of 1,215 hospitalized South African children with lower respiratory tract infections, HIV infection was identified in 45.1%; bacteremia occurred in 14.9% of HIV-infected and 6.5% of uninfected children with pneumonia (144). The estimated relative incidence of bacteremic pneumonia caused by Streptococcus pneumoniae, Haemophilus influenzae type b (Hib), Staphylococcus aureus, or Escherichia coli was higher in HIV-infected than uninfected children. These organisms were more likely to be resistant to common antibiotics (e.g., methicillin, penicillin, and trimethoprim/sulfamethoxazole) in HIV-infected children. Mortality was higher among HIV-infected than uninfected children with pneumonia (13.1% versus 2.1%, respectively). Streptococcus pneumoniae is the most prominent invasive bacterial pathogen in children with HIV infection both in the United States and worldwide, accounting for >50% of bacterial blood-stream infections in HIV-infected children (9,145--148). HIV-infected children have a markedly increased risk for pneumococcal infection compared with those who are not HIV-infected (149,150). The incidence of invasive pneumococcal disease is 6.1 cases/100 patient-years among HIV-infected children through age 7 years (151). In studies from Malawi and South Africa of approximately 600 children (36% HIV-infected) with acute bacterial meningitis, HIV-infected children were substantially more likely than those without HIV infection to have S. pneumoniae as the cause of their meningitis (58% and 74% of HIV-infected children in Malawi and South African studies, respectively, compared with 32% and 29% in children without HIV infection) (152,153). The high incidence of invasive pneumococcal infections among HIV-infected children does not appear to be caused by increased rates of asymptomatic colonization with S. pneumoniae (154,155). Among children with invasive pneumococcal infections, studies vary on whether penicillin-resistant pneumococci strains are more commonly isolated from HIV-infected than uninfected persons (147,151,156--158). Although reports among children without HIV infection have not demonstrated a difference in the case-fatality rate in those with penicillin-susceptible and nonsusceptible pneumococcal infections (case-fatality rate was associated with severity of disease and underlying illness) (159), in a multivariate analysis of mortality in HIV-infected, predominantly adult patients with pneumococcal bacteremia, high-level penicillin-resistance, the severity of illness, and Hispanic ethnicity were independently associated with mortality (160). Hib also has been reported to be more common in HIV-infected children before availability of Hib vaccine. In a study in South African children who had not received Hib conjugate vaccine, the estimated relative annual rate of overall invasive Hib disease in children aged <1 year was 5.9 times greater among HIV-infected than uninfected children, and HIV-infected children were also at greater risk for having bacteremic pneumonia (161). Although the frequency of gram-negative bacteremia is lower than gram-positive bacteremia among HIV-infected children, gram-negative bacteremia is more common among children with advanced HIV disease or immunosuppression or those with central venous catheters. However, in children aged <5 years, gram-negative bacteremia also was observed among children with milder levels of immune suppression. In a study of 680 HIV-infected children in Miami, Florida, through 1997, a total of 72 (10.6%) had 95 episodes of gram-negative bacteremia; the predominant organisms identified in those with gram-negative bacteremia were Pseudomonas aeruginosa (26%), nontyphoidal Salmonella (15%), Escherichia coli (15%), and Haemophilus influenzae (13%) (162). The relative frequency of the organisms varied over time, with the relative frequency of P. aeruginosa bacteremia increasing from 13% before 1984 to 56% during 1995--1997 and Salmonella from 7% before 1984 to 22% during 1995--1997. However, H. influenzae was not observed after 1990 (presumably decreasing after incorporation of Hib vaccine into routine childhood vaccinations). The overall case-fatality rate for gram-negative bacteremia was 43%. The presence of a central venous catheter increases the risk for bacterial infections in HIV-infected children, but the incidence is similar to that observed among children with cancer (163). S. aureus is the most commonly isolated pathogen in catheter-associated bacteremia in HIV-infected children (163). P. aeroginosa also is common. Other organisms associated with catheter-associated bacteremia include S. epidermidis, Enterococcus, and Bacillus cereus. Clinical Manifestations Clinical presentation will be dependent on the particular type of recurrent bacterial infection (e.g., bacteremia/sepsis, osteomyelitis/septic arthritis, pneumonia, meningitis, and sinusitis/otitis media) (164). HIV-infected children with invasive bacterial infections typically have a clinical presentation similar to children without HIV infection, with an acute presentation and fever (150,151,165). Studies have indicated that HIV-infected children might be less likely than children without HIV infection to have leukocytosis (151). The classical signs, symptoms, and laboratory test abnormalities that usually indicate invasive bacterial infection (e.g., fever and elevated white blood cell count) are usually present but might be lacking among HIV-infected children having reduced immune competence (150,164). One third of HIV-infected children who experience acute pneumonia have recurrent episodes (142). In studies in Malawian and South African children with acute bacterial meningitis, the clinical presentation of children with and without HIV infection was similar (152,153). However, in the Malawi study, HIV-infected children were 6.4-fold more likely to have recurrent meningitis than children without HIV infection, although the study did not differentiate recrudescence from new infections (152). In both studies, HIV-infected children were more likely to die of meningitis than children without HIV infection. Diagnosis Attempted isolation of a pathogenic organism from normally sterile sites (e.g., blood, CSF, and pleural fluid) is strongly recommended. This is particularly important because of an increasing incidence of antimicrobial resistance, including penicillin-resistant S. pneumoniae and community-acquired methicillin-resistant S. aureus. The diagnosis of pneumonia is most typically made on the basis of clinical (e.g., fever, dyspnea, tachypnea, cough, and rales) and radiographic findings, although differenciating viral from bacterial pneumonia clinically is difficult (166). Sputum induction obtained by nebulization with hypertonic (5%) saline has been evaluated for diagnosis of pneumonia in 210 South African infants and children (median age: 6 months), 66% of whom had HIV infection (167). The procedure was well-tolerated, and identified an etiology in 63% of children with pneumonia (identification of bacteria in 101, M. tuberculosis in 19, and PCP in 12 children). Culture of blood and pleural fluid, if present, should be done. Among children with bacterimia, a source for the bacteremia should be sought. In addition to routine chest radiographs, other diagnostic radiologic evaluations might be necessary (e.g., chest, abdomen, and ultrasound studies) among HIV-infected children with compromised immune systems to identify less apparent foci of infection (e.g., bronchiectasis and internal organ abscesses) (168--170). Among children with central venous catheters, both a peripheral and catheter blood culture should be obtained; if the catheter is removed, the catheter tip should be sent for culture. Assays for detection of bacterial antigens or evidence by molecular biology techniques are important for the diagnostic evaluation of HIV-infected children in whom unusual pathogens might be involved or difficult to identify or culture by standard techniques. For example, Bordetella pertussis and Chlamydia pneumoniae can be identified by a PCR assay of nasopharyngeal secretions (166). Treatment The local prevalence of resistance to common infectious agents (i.e., penicillin-resistant S. pneumoniae and methicillin-resistant S. aureus) and the recent use of prophylactic or therapeutic antibiotics should be considered when initiating empiric therapy. When the organism is identified, antibiotic susceptibility testing should be performed and therapy based on the results of susceptibility testing (AII). HIV-infected children whose immune systems are not seriously compromised (CDC Immune Class I) and who are not neutropenic can be expected to respond similarly to HIV-uninfected children and should be treated with the usual antimicrobial agents recommended for the most likely bacterial organisms (AIII). For example, for HIV-infected children outside of the neonatal period with suspected community-acquired bacteremia, bacterial pneumonia, or meningitis, empiric therapy with an extended-spectrum cephalosporin such as ceftriaxone (80--100 mg/kg body weight in 1 or 2 divided doses [maximum daily adult dose: 4 g]), cefotaxime (150--200 mg/kg divided into 3 or 4 doses [maximum daily adult dose: 8--10 g]), or cefuroxime (100--150 mg/kg divided into 3 doses [maximum daily adult dose: 4--6 g]) is reasonable until culture results are available (166,171) (AIII). Initial empiric therapy of HIV-infected children with suspected catheter sepsis should include coverage for both gram-positive and enteric gram-negative organisms, such as ceftazidime (125--150 mg/kg divided into 3 doses [maximum daily adult dose: 6 g]), which has anti-Pseudomonas activity, and vancomycin (40--60 mg/kg divided into 4 doses [maximum daily adult dose: 2--4 g]) to cover methicillin-resistant S. aureus (AIII). Severely immunocompromised HIV-infected children with invasive or recurrent bacterial infections might require expanded empiric antimicrobial treatment covering a broad range of resistant organisms similar to that chosen for suspected catheter sepsis pending results of diagnostic evaluations and cultures (AIII). HIV-infected children aged <5 years should receive Hib and heptavalent pneumococcal conjugate vaccines (AII). In a placebo-controlled trial of a 9-valent pneumococcal conjugate vaccine among South African children, although vaccine efficacy was somewhat lower among children with HIV infection than those without (65% versus 85%, respectively), the incidence of invasive pneumococcal disease was substantially decreased among HIV-infected vaccine recipients (157). HIV-infected children aged >2 years also should receive the 23-valent pneumococcal polysaccharide vaccine (>2 months after their last conjugate vaccine dose), with a single revaccination with the pneumococcal polysaccharide vaccine 3--5 years later if the child is aged <10 years or after 5 years if the child is aged >10 years (4,172) (AIII). SyphilisEpidemiology Treponema pallidum can be transmitted from mother to child at any stage of pregnancy or during delivery. Untreated or inadequately treated primary and secondary syphilis during pregnancy leads to congenital infection in 60%--100% of infants. Treatment of the mother for syphilis >30 days before delivery is required for effective in utero treatment. Congenital syphilis has been reported despite adequate maternal treatment. Factors that contribute to treatment failure include maternal stage of syphilis (early stage), advancing gestational age at treatment, higher VDRL (Venereal Disease Research Laboratory) titers at treatment and delivery, and short interval from treatment to delivery (<30 days) (173,174). In 2000, the rate of congenital syphilis among HIV uninfected infants was 13.4 cases/100,000 live-born infants compared with 27.8 cases in 1997 (175). During that same time, the prevalence of primary and secondary syphilis in women of reproductive age also decreased substantially. Drug use during pregnancy, particularly cocaine, is substantially associated with an increased risk for maternal and congenital syphilis (176). Infants born to HIV-infected women have a substantially higher rate for congenital syphilis than in the general population. One large U.S. study conducted during 1988--1994 reported the rate of congenital syphilis was approximately 50 times greater among infants born to HIV-infected women (177). Although mother-to-child HIV transmission does not appear to be increased when syphilis is effectively treated before pregnancy (178), concurrent coinfection during pregnancy might increase the rate for perinatal HIV transmission (177,178). Half of all new HIV infections in the United States occur among persons aged 15--24 years, with most infections transmitted sexually. In addition, approximately two thirds of the sexually transmitted diseases diagnosed annually in the United States occur among persons aged <24 years. As a result, the prevalence and incidence of syphilis among HIV-infected youth and of HIV infection among youth with syphilis is expected to be higher than the general population. In a study of 320 HIV-infected and uninfected adolescents aged 12--19 years in the United States, the prevalence of syphilis was 9% among HIV-infected girls and 6% among HIV-infected boys (179). In a meta-analysis of 30 studies, the median HIV seroprevalence among persons infected with syphilis in the United States was 15.7% (27.5% among men and 12.4% among women with syphilis) (180). Clinical Manifestations Untreated early syphilis during pregnancy can lead to spontaneous abortion, stillbirth, hydrops fetalis, preterm delivery, and perinatal death in up to 40% of pregnancies (181). In a study of 148 infants born to mothers with untreated or inadequately treated syphilis, 47% had clinical, radiographic, or conventional laboratory findings consistent with a diagnosis of congenital syphilis, and 44% had a positive rabbit infectivity test, PCR assay, or IgM immunoblot of serum, blood, or CSF (182). At birth, approximately 60% of infants with congenital syphilis are asymptomatic (183). If untreated, symptoms can occur within 3 weeks--6 months after birth and might include hepatosplenomegaly, jaundice, mucocutaneous lesions, skin rash, nasal discharge, pseudoparalysis of an extremity, anemia, thrombocytopenia, and osteochondritis. Late manifestations of congenital syphilis (after age 2 years) involve CNS, bones, teeth, eyes, and skin. Manifestations include mental retardation, interstitial keratitis, cranial nerve deafness, anterior bowing of the skin, frontal bossing, mulberry molars, Hutchinson teeth, saddle nose, rhaades, and Clutton joints. HIV-infected persons with acquired early syphilis might be at increased risk for neurological complications and uveitis and have higher rates of treatment failure. Diagnosis The standard serologic tests for syphilis in adults are based on the measurement of IgG antibody. Because IgG antibody in the infant reflects transplacental passively transferred antibody from the mother, interpretation of reactive serologic tests for syphilis among infants is difficult. Therefore, the diagnosis of neonatal congenital syphilis depends on a combination of results from physical, radiologic, serologic, and direct microscopic examinations. All infants born to women with reactive nontreponemal and treponemal test results should be evaluated with a quantitative nontreponemal test (e.g., VDRL slide test, rapid plasma regain (RPR), and the automated regain test). Testing should be performed on neonatal serum because of the potential for maternal blood contamination of the umbilical cord blood specimens. Performing specific treponemal tests, such as the fluorescent treponemal antibody absorption (FTA-ABS) test and T. pallidum particle agglutination (TP-PA) test, is not necessary for evaluation of congenital syphilis in the neonate. No commercially available IgM test is recommended for diagnostic use. Darkfield microscopic examination or direct fluorescent antibody staining of lesions or body fluids should be performed, although false-negative results are common. Definitive diagnosis of congenital syphilis can be made if T. pallidum is detected in umbilical cord, placenta, nasal discharge, or skin lesion material. Pathologic examination of placenta and umbilical cord with specific fluoroscent antitreponemal antibody staining is recommended. Evaluation of suspected cases of congenital syphilis should include a physical examination, complete blood count, differential and platelet count, and CSF analysis for VDRL, cell count, and protein. HIV-infected infants might have increased cell counts and protein concentrations even in the absence of neurosyphilis. Other tests should be performed as clinically indicated (e.g., long-bone radiographs, chest radiograph, liver-function tests, cranial ultrasound, ophthalmologic examination, and auditory brainstem response). A presumptive case of syphilis is defined as an infant born to a mother with untreated or inadequately treated syphilis at delivery, regardless of findings in the infant, or any infant who has a reactive treponemal test result and clinical signs or symptoms of congenital syphilis on physical examination, or an abnormal CSF finding without other cause or positive CSF VDRL. For diagnosis of acquired syphilis, a reactive nontreponemal test must be confirmed by a specific treponemal test such as FTA-ABS or TP-PA. These tests will remain positive for life, even with successful treatment. The prozone phenomenon (a weakly reactive or falsely negative) reaction might occur more frequently in HIV-infected persons (184). Treponemal antibody titers do not correlate with disease activity and should not be used to monitor treatment response. CSF evaluation should be performed among HIV-infected adolescents with acquired syphilis who have neurologic or ocular symptoms or signs, although some clinicians recommend a CSF examination for all HIV-infected patients. Treatment Data are insufficient about whether infants who have congenital syphilis and whose mothers are coinfected with HIV require different evaluation, therapy, or follow-up for syphilis than is recommended for all infants (185). Some studies in adults have shown a lag in serological improvement in appropriately treated patients with HIV infection (186). Infants should be treated if mothers have untreated or inadequately treated syphilis (including treatment with erythromycin or any other nonpenicillin regimen) or no documentation of having received treatment; received treatment <4 weeks before delivery; been treated with penicillin but titers did not decrease by four-fold; or have four-fold or greater increase in nontreponemal antibody titer suggesting relapse or reinfection (185) (AII). Infants should be treated regardless of maternal history if an abnormal examination consistent with congenital syphilis, positive darkfield or fluorescent antibody test of body fluid(s), or serum quantitative nontreponemal serologic titer that is the same or four-fold greater than maternal titer are observed (185) (AII). Treatment for proven or highly probable congenital syphilis is aqueous crystalline penicillin G at a dose of 100,000--150,000 units/kg/day, administered as 50,000 units/kg body weight/dose intravenously every 12 hours during the first 7 days of life and every 8 hours thereafter for a total of 10 days (AII). If congenital syphilis is diagnosed after 1 month of life, the dose of aqueous penicillin G should be increased to 200,000--300,000 units/kg intravenously every 6 hours for 10 days (AII). An alternative to aqueous penicillin G is procaine penicillin G at a dose of 50,000 units/kg/dose intramuscularly/day in a single dose for 10 days (BII). However, aqueous penicillin G is preferred because of its higher penetration into the CSF. Asymptomatic infants born to mothers who have had adequate treatment and response to therapy and normal physical examination and CSF findings but who have a serum quantitative nontreponemal serologic titer that is the same or four-fold higher than maternal titer might be treated with a single dose of benzathine penicillin G 50,000 units/kg/dose intramuscularly with careful clinical and serologic follow-up (BII). However, certain health-care providers would treat such infants with the standard 10 days of aqueous penicillin because physical examination and laboratory test results cannot definitively exclude congenital syphilis in all cases (BII). Infants with treated congenital syphilis should be examined at age 1, 2, 3, 6, and 12 months, with serologic nontreponemal tests performed at age 3, 6 and 12 months after conclusion of treatment or until results become nonreactive (AIII). If initial CSF examination was abnormal, repeat lumbar puncture should be conducted every 6 months until results are normal. Nontreponemal antibody titers should decline by age 3 months and be nonreactive by age 6 months if the infant was adequately treated or not infected (e.g., passive antibody transfer from mother). Children with increasing titers or persistently positive titers (even if low levels) at age 6--12 months should be evaluated and considered for retreatment (AIII). Children with congenital syphilis who also are HIV-infected might take longer to become nonreactive and might require retreatment. Acquired syphilis is treated with a single dose of benzathine penicillin G 50,000 units/kg intramuscularly for early stage disease (e.g., primary, secondary, and early latent disease) (AII). For late latent disease, 3 doses of benzathine penicillin G 50,000 units/kg should be administered intramuscularly once weekly for 3 doses (AIII). Alternative therapies (e.g., doxycycline, ceftriaxone, or azithromycin) have not been evaluated among HIV-infected patients and should not be used as first-line therapy (185) (EIII). Neurosyphilis should be treated with aqueous penicillin G 200,000--300,000 units/kg intravenously every 6 hours (maximum dose: 18--24 million units/day) for 10--14 days (AII). Children and adolescents with acquired syphilis should have clinical and serologic response monitored at age 3, 6, 9, 12, and 24 months after therapy (BIII). Nontreponemal test titers should decline by at least four-fold by 6--12 months after successful therapy. If initial CSF examination was abnormal, repeat lumbar puncture should be conducted at 3 and 6 months after therapy and then every 6 months until results are normal and VDRL is negative. Candida InfectionsEpidemiology The most common fungal infections among HIV-infected children are caused by Candida species. Oral thrush and diaper dermatitis occur among 50%--85% of HIV-infected children. Candida albicans is the most common cause of mucosal and esophageal candidiasis. Candida esophagitis is reported as the AIDS-defining condition in approximately 12%--16% of children aged <13 years in the United States, and continues to be seen in the post-HAART era among children who are not responding to therapy (187,188). The condition is often associated with low CD4+ cell count (<100/µl), high viral load, and neutropenia (<500/µL) within 4 weeks of the episode (9,187,189). In the pre-HAART era, concomitant oropharyngeal candidiasis (OPC) occurred in 94% of children with candida esophagitis (187). In one study of HIV-infected children, the strongest risk factors for esophageal candidiasis were OPC and low CD4+ count and percentage. Children who develop esophageal candidiasis despite being treated with HAART are less likely to have typical symptoms (e.g., odynophagia and retrosternal pain) or have concomitant OPC (189). Laryngeal and epiglottal candidiasis also have been reported (190). Disseminated candidiasis is infrequent among HIV-infected children, but Candida can disseminate from the esophagus particularly when coinfection with HSV or CMV is present (187,191). Fungemia occurs in up to 12% of HIV-infected children with chronically indwelling central venous catheters for total parental nutrition or intravenous antibiotics (188,192). Approximately 50% of reported cases of fungemia among HIV-infected children are caused by non-albicans Candida species including C. tropicalis, C. pseudotropicalis, C. parapsilosis, C. glabrata, C. krusei,and C. dubliniensis. A substantial number of children who develop fungemia have received systemically absorbed oral antifungal azole compounds (ketoconazole or fluconazole) for control of oral and esophageal candidiasis (188). Common complications of disseminated candidiasis include endophthalmitis, hepatosplenic, and renal candidiasis, and osteomyelitis. Early detection and treatment of candidemia can decrease mortality. Overall mortality was 90% in one study in children who had >14 days of fever and symptoms before diagnosis of disseminated infection with Candida species (191). Clinical Manifestations Clinical manifestations of OPC are variable and include pseudomembranous (thrush), erythematous (atrophic), hyperplastic (hypertrophic), and angular cheilitis. Thrush is the most classic form of oral candidiasis, appearing as creamy white curdlike patches with inflamed underlying mucosa that is exposed after removal of the exudate. It can be found on the oropharyngeal mucosa, palate, and tonsils. Erythematous OPC are flat erythematous lesions on the mucosal surface. Hyperplastic candidiasis is composed of raised white plaques appearing on the lower surface of the tongue, palate, and buccal mucosa and cannot be removed. Angular cheilitis occurs as red, fissured lesions in the corners of the mouth. Esophageal candidiasis can present with odynophagia, dysphagia, or retrosternal pain, which can be severe enough to cause dehydration and weight loss in children. Although common, evidence of oropharyngeal candidiasis might be absent among children with esophageal candidiasis, particularly those receiving HAART. Unlike adults, a substantial number of children might experience nausea and vomiting. New onset fever in an HIV-infected child with advanced disease and a central venous catheter is the most common clinical manifestation of candidemia. Renal candidiasis might appear with candiduria and ultrasonographically demonstrated renal parenchymal lesions without symptoms related to renal disease (188). Systemic fungemia can lead to endogenous endophthalmitis, and ocular examination by an ophthalmologist might be warranted among children with candidemia. Diagnosis Diagnosis of oral candidiasis can be made by a KOH preparation and culture with microscopic demonstration of budding yeast cells in wet mounts or biopsy specimens. For recurrent or refractory OPC, cultures with in vitro susceptibility testing can be used to guide antifungal treatment (193). Esophageal candidiasis has a classic cobblestoning appearance on barium swallow. In refractory symptomatic cases, endoscopy should be performed to rule out other causes of refractory esophagitis (e.g., herpes simplex virus [HSV], CMV, MAC, and azole-resistant Candida species). Endoscopy might show few small white raised plaques to elevated confluent plaques with hyperemia and extensive ulceration. Diagnosis of candidemia is best made with blood cultures using lysis-centrifugation techniques (188) or automated broth based systems (194). When fungemia is present, retinal examination for endophthalmitis, abdominal CT or ultrasound for hepatic or renal involvement, and bone scans if osteomyelitis is clinically suspected might be appropriate. TreatmentOropharyngeal Candidiasis
Esophageal Disease
Invasive Disease
CryptococcosisEpidemiology Cryptococcal infections occur much less frequently among HIV-infected children (1%) than adults (191,222,223). Infection primarily occurs among HIV-infected children aged 6--12 years and most frequently in those with CD4+ cell counts indicating severe immunosuppression. Clinical Manifestations Meningoencephalitis is the most common initial manifestation of cryptococcosis. It typically evolves over days to weeks with fever, headache, and altered mental status. In the United States, meningismus, photophobia, and focal neurological signs are uncommon presenting symptoms in children (191,223). However, in Zimbabwe, cryptococcal meningoencephalitis presents more acutely, with up to 70% of children in one study presenting with nuchal rigidity, approximately 40% with seizure activity, and approximately 20% having focal neurologic signs (224). CNS mass lesions (cryptococcomas) have not been reported among children. The skin might be secondarily involved in disseminated cryptococcosis. Lesions might be small, translucent umbilicated papules indistinguishable from molluscum contagiosum, nodules, or ulcers or infiltrated plaques resembling cellulitis. Cutaneous lesions have been reported as the presenting manifestation of disseminated cryptococcosis in an HIV-infected African child (225). Pulmonary cryptococcus without dissemination is unusual among children but can present as unexplained recurrent fever, cough with scant sputum, intrathoracic lymphadenopathy, and focal or diffuse pulmonary infiltrates. It might be asymptomatic with pulmonary nodules found on routine chest radiograph. Diagnosis Among HIV-infected children with CNS disease, CSF cell count, glucose, and protein might be virtually normal, but the opening pressure is usually elevated (226). Similarly, results of computerized tomography scan usually are nonspecific but might show signs of increased intracranial pressure, hydrocephalus, or focal lesions, especially in the basal ganglia. For diagnosis of suspected CNS disease, microscopic examination of CSF on India ink-stained wet mounts should be performed. Cryptococcal antigen can be detected in CSF, serum, or bronchoalveolar lavage fluid by latex agglutination test. However, CSF antigen detection might be negative in culture-positive cryptococcal meningitis; high titers of antigen (prozone effect), low levels of antigen, or nonencapsulated strains can contribute to this effect (227,228). Cryptococcal antigen titers in CSF can be helpful in evaluating response to therapy or ongoing relapse; however, changes in serum antigen titers do not correlate with clinical response. A rise in CSF antigen titer during suppressive therapy is associated with relapse. Specifically, a CSF titer of >1:8 after completion of therapy appears to be indicative of treatment failure or pending relapse (229). Fungal cultures from CSF, sputum and blood might identify the organism, with the lysis-centrifugation method being the most sensitive for blood specimens. Acapsular variants of Cryptococees neoformans can be identified using culture-based methods (230). In selected cases (e.g., in patients with refractory disease or relapse) susceptibility testing of the C. neoformans isolate can be beneficial. Diffuse pulmonary disease can be diagnosed through bronchoalveolar lavage and direct examination of India ink-stained specimens, culture, and antigen detection. Focal pulmonary and skin lesions might require biopsy with culture and staining. Molecular PCR-based assays using primers derived from 5.8s rRNA and 18S rRNA have been used on an investigational basis. Treatment Without treatment, cryptococcosis is fatal. Treatment for cryptococcal disease has not been studied in a controlled manner in pediatric patients. Data from the adult literature have resulted in a recommendation for the use of combination therapy for severe cryptococcosis and cryptococcal meningitis (231) (AI). For children with severe disease that is isolated to the lungs, amphotericin B induction therapy, usually combined with an initial 2 weeks of flucytosine, is recommended until symptoms are controlled (AI). After treatment of acute pulmonary disease, maintenance therapy with fluconazole or itraconazole is recommended. Adverse effects of amphotericin B are primarily nephrotoxicity; permanent nephrotoxicity is related to cumulative dose. Nephrotoxicity can be ameliorated by hydration with 0.9% saline intravenously over 30 minute before the amphotericin B infusion. Infusion-related fevers, chills, nausea, and vomiting can occur, although they are less frequent in children than adults. Onset is usually within 1--3 hours after the infusion is started, typical duration is <1 hour, and the febrile reactions tend to decrease in frequency over time. Pretreatment with acetaminophen or diphenhydramine might alleviate febrile reactions. Idiosyncratic reactions including hypotension, arrhythmias, and allergic reactions, including anaphylaxis, occur less frequently. Hepatic toxicity, thrombophlebitis, anemia, and neurotoxicity (manifested as confusion or delirium, hearing loss, blurred vision, or seizures) also might occur. Flucytosine has the potential for considerable toxicity, especially affecting the bone marrow (anemia, leukopenia, and thrombocytopenia), liver, gastrointestinal tract, kidney, and skin. Levels should be monitored and doses adjusted to keep the level between 40--60 µg/mL, particularly in patients with renal impairment where toxic levels can result in bone marrow suppression; the drug should be avoided among children with severe renal impairment (EIII). For children with mild-to-moderate cryptococcosis that is isolated to the lungs, fluconazole alone can be used for treatment of HIV-infected children, followed by life-long suppressive therapy with fluconazole (BII). Alternatively, itraconazole can be used for treatment and suppressive therapy (BII). Fluconazole and the other azoles have relatively low rates of toxicity, but have substantial drug interactions that can limit their use. Because of their ability to inhibit the cytochrome P-450-dependent hepatic enzymes, the potential for drug interactions, particularly with antiretroviral drugs, should be carefully evaluated before initiation of therapy (AIII). Skin rash and pruritis might be seen with all azole drugs, and rare cases of Stevens-Johnson syndrome have been reported with fluconazole. Asymptomatic increases in transaminases and, less frequently, hepatitis can occur; rare cases of fatal hepatitis have been reported. Thrombocytopenia and leukopenia have been reported with itraconazole. For meningeal and extrameningeal cryptococcosis, initial therapy with the combination of amphotericin B (at a dose of 0.7--1.5 mg/kg body weight/day) plus flucytosine (25 mg/kg/dose adminstered four times daily) for a minimum of 2 weeks (induction) is recommended (AI). This regimen was superior to single-drug therapy with either amphotericin B or fluconazole in two clinical trials among HIV-infected adults (232). In cases of cryptococcal meningitis where flucytosine cannot be administered, amphotericin B alone can be administered (BI). Doses of 0.5--1.5 mg/kg/day of amphotericin B among children being treated for cryptococcal meningitis have been well tolerated (222). Lipid formulations of amphotericin B have been used for treatment of cryptococcal meningitis among adults and might be useful among patients with impaired renal function, although the optimal dose has not been determined; liposomal amphotericin B (AmBisome) at a dose of 4 mg/kg daily has been effective (AI). Children treated with liposomal amphotericin B (AmBisome) at a dose of 2 mg/kg/day have had a good effect, and doses to 7.5 mg/kg/day have been used for refractory cases (233,234). Acute, infusion-related reactions might be seen in about 20% of patients receiving lipid formulations, including chest pain, dyspnea, hypoxia, severe abdomen, flank or leg pain, and flushing and urticarial. The majority of reactions occur within the first 5 minutes of infusion and rapidly resolve with temporary interruption of the amphotericin infusion and administration of intravenous diphenhydramine (221). Premedication with diphenhydramine can reduce the incidence of these reactions. Fluconazole plus flucytosine (235) is superior to fluconazole alone and provides an alternative option to amphotericin B for acute therapy of invasive disease; however, little data is available on this combination among children (232) (CIII). The combination regimen has more toxicity than treatment with fluconazole alone (232). After successful acute induction therapy in stable patients, amphotericin B and flucytosine can be discontinued and consolidation therapy with fluconazole (5-6 mg/kg/dose intravenously or orally adminstered twice daily) administered for a minimum of 8 weeks or until CSF cultures are stable (193,232) (AI). Fluconazole has more rapid clearance and shorter half-life among children than adults (236), and higher doses of fluconazole are recommended for treatment of disseminated disease. Following induction and consolidation therapy, maintenance suppressive therapy with lower dose fluconazole should be instituted (AI). If fluconazole cannot be administered, itraconazole is an alternative for consolidation (2--5 mg/kg/dose administered twice daily), but might be less active than fluconazole (237) (BI). In cases of refractory cryptococcal meningitis where systemic antifungal administration has failed, intrathecal or intraventricular amphotericin B has been used (CII). Oral acetazolamide should not be used for reduction of elevated intracranial pressure in cryptococcal meningitis (DIII); it was associated with an excess of severe acidosis, hypokalemia, and other adverse effects compared with placebo in a clinical trial among adults (238). Recommendations for the management of elevated intracranial pressure are the same as for adults (4,232). Prevention of relapse after successful treatment requires lifelong suppressive treatment; details on secondary prophylaxis (maintenance therapy) have been published (4). Safety of discontinuation of secondary prophylaxis following immune reconstitution with HAART among children has not been studied extensively. HistoplasmosisEpidemiology The incidence of disseminated histoplasmosis among HIV-infected pediatric patients living in the United States is 0.4% (239). Infection among children might be greater in Latin American countries with reports ranging from 2.7% to 3.8% in Argentina, Brazil and Mexico (240,241). No evidence exists of histoplasmosis dissemination in the fetus or that infection is more severe during pregnancy. Clinical Manifestations The most common presenting symptom among children and adults with AIDS with disseminated histoplasmosis is prolonged fever. In comparison with adults, children predominantly have malaise, weight loss, and nonproductive cough (242,243). In addition, interstitial pneumonitis as seen in adults is rarely observed among children, but a primary pulmonary focus frequently leads to widespread dissemination among HIV-infected children. The most frequent physical finding is hepatosplenomegaly (89% in infants) (242). Cutaneous lesions that are erythematous and nodular might occur. CNS involvement with meningitis and focal brain lesions is common among HIV-infected adults and might result from reactivated infection in the setting of low CD4+ cell number. Anemia and thrombocytopenia are the most common hematologic abnormalities found, although pancytopenia has been reported. Elevated liver transaminases also occur. Diagnosis Culture of the organism is the definitive method of diagnosis. Blood cultures using lysis-centrifugation blood cultured system are highly sensitive in HIV-associated disseminated histoplasmosis, but the organism might require up to 6 weeks to grow. Identification of Histoplasma capsulatum can be shortened in cultures through the use of a DNA probe. Although PCR and DNA probes have been studied for detection of H. capsulatum DNA in tissues and body fluids, they have not been validated and are used only for research (244). Detection of H. capsulatum polysaccharide antigen, using an enzyme-linked immunoassay (EIA), in urine, bronchoalveolar lavage or CSF, and/or serum is a rapid, sensitive, and specific method for diagnosis; it can be detected before culture positivity and, in acute histoplasmosis, is positive before antibody detection (244). EIA sensitivity is greatest among patients with disseminated disease or acute pulmonary infection and less with chronic or reactivation disease; however, primary infection with acute disease is most common in children. EIA sensitivity is greater in urine than serum because patients rarely exhibit antigenemia without antigenuria; patients with pulmonary disease might have high antigen levels in bronchoalveolar lavage fluid (244). Antigen concentrations decline with treatment, and failure to decline in both urine and serum indicates treatment failure; similarly, after a decline, an increase in antigen (>2--4 units) in urine or serum indicates relapse (244). Cross-reacting antigens can occur in blastomycosis, paracoccidioidmycosis, and Penicillium marneffei infections. Serologic tests to detect antibody to Histoplasma are positive in the majority of patients without HIV infection who have histoplasmosis; however, serologic tests are not useful for diagnosis of acute histoplasmosis and might be undetectable in immunosuppressed patients (193). Histoplasmin skin tests are no longer available and were not useful for diagnosis of disseminated disease because >70% of tests are negative (242,243). Diagnosis of CNS disease is difficult, particularly if the patient has isolated meningitis without disseminated disease. The highest sensitivity is achieved by testing CSF for Histoplasma antigen and antibody and culture: CSF culture is only positive in 20%--60% of patients, CSF antigen is positive in 40%--70%, and CSF antibody is positive in 70%--90% (244). Treatment Disseminated histoplasmosis is fatal without antifungal treatment. Treatment for disseminated histoplasmosis has not been studied in a controlled manner among pediatric patients. Pediatric treatment recommendations for HIV-infected children are based on data from the adult literature. Among nonimmunocompromised HIV-infected adults who do not require hospitalization and have mild symptoms of histoplasmosis, itraconazole alone has been used, administered for 3--4 months (245) (AII). Although experience with itraconazole among children is limited, itraconazole capsules at doses of 6--8 mg/kg body weight/day given for 3--12 months has been used effectively for treatment of mild disseminated histoplasmosis in a limited number of nonimmunocompromised children without HIV infection (246). High-dose fluconazole is an alternative for patients with mild histoplasmosis who cannot take itraconazole, but is less effective, and the organism can develop drug resistance (247) (CII). Because of the ability of the azole drugs to inhibit the cytochrome P-450-dependent hepatic enzymes, the potential for drug interactions should be carefully evaluated before initiation of therapy (AIII). For example, cardiac toxicity among patients receiving terfenadine or astemizole has been reported with concomitant itraconazole. The most frequent adverse effects of the azoles drugs are gastrointestinal, including nausea and vomiting. Skin rash and pruritis might be seen with azole drugs, and rare cases of Stevens-Johnson syndrome have been reported with fluconazole. Asymptomatic increases in transaminases might be observed in 1%--13% of patients receiving azole drugs, and less frequently, hepatitis; rare cases of fatal hepatitis have been reported. Thrombocytopenia and leukopenia have been reported with itraconazole. Among HIV-infected adults or children with more severe disseminated histoplasmosis who require hospitalization or who are immunocompromised, amphotericin B is recommended for the initial phase of induction therapy (AI). Amphotericin B at a dose of 1 mg/kg for an average of 30 days has been effectively used for treatment of disseminated histoplasmosis in immunocompromised non-HIV--infected children (243). The duration of amphotericin B therapy among HIV-infected children is usually 4--6 weeks, followed by itraconazole chronic suppressive therapy. Adverse effects of amphotericin B are primarily nephrotoxicity; permanent nephrotoxicity is related to cumulative dose. Nephrotoxicity can be ameliorated by hydration with 0.9% saline intravenously over 30 minute before the amphotericin B infusion. Infusion-related fevers, chills, nausea, and vomiting might occur, although they are less frequent in children than adults. Onset is usually within 1--3 hours after the infusion is started, typical duration is <1 hour, and the febrile reactions tend to decrease in frequency over time. Pretreatment with acetaminophen or diphenhydramine can alleviate febrile reactions. Idiosyncratic reactions including hypotension, arrhythmias, and allergic reactions, including anaphylaxis, occur less frequently. Hepatic toxicity, thrombophlebitis, and anemia, and rarely neurotoxicity (manifested as confusion or delirium, hearing loss, blurred vision, or seizures), also might occur. Certain health-care providers limit amphotericin B therapy to 2--3 weeks, followed by 3--6 months of consolidation therapy with itraconazole after the patient is clinically stabilized and afebrile (59) (AII). After successful treatment of acute disease, itraconazole chronic suppressive therapy (secondary prophylaxis) should be instituted. However, children with confirmed H. capsulatum meningitis, amphotericin B therapy should be continued for 12--16 weeks, followed by chronic suppressive therapy (secondary prophylaxis) with itraconazole (AII). Liposomal amphotericin B is an alternative for patients who cannot tolerate conventional amphotericin and in one randomized trial was associated with improved treatment response and survival and less toxicity compared with conventional amphotericin B induction therapy (247--249) (AI). Acute, infusion-related reactions might be observed in approximately 20% of patients receiving lipid formulations, including chest pain, dyspnea, and hypoxia; severe abdomen, flank or leg pain; or flushing and urticarial. The majority of reactions occurs within the first 5 minutes of infusion and rapidly resolve with temporary interruption of the amphotericin infusion and administration of intravenous diphenhydramine (221). Premedication with diphenhydramine can reduce the incidence of these reactions. Prevention of relapse after successful treatment requires lifelong suppressive treatment; details on secondary prophylaxis (maintenance therapy) have been published (4). Safety of discontinuation of secondary prophylaxis following immune reconstitution with HAART among children has not been studied extensively. CoccidioidomycosisEpidemiology Children with HIV infection are at increased risk for infection with Coccidioides immitis in areas where coccidioidomycosis is endemic (e.g., southwestern United States, northern Mexico, and Central and South America). Primary infection of the newborn occurs rarely. However, infection of the genital tract of the mother can result in placental involvement, coccidioidal endometritis, and aspiration of infected amniotic fluid by the fetus. Both in utero and perinatal transmission of Coccidioides immitis have been reported. Clinical Manifestations Fever and dyspnea are common presenting symptoms among children, along with chills, weight loss, lymphadenopathy, chest pain, and headache (250,251). With pulmonary disease, chest radiographs exhibit bilateral diffuse reticulonodular pulmonary infiltrates. Patients might experience persistent pulmonary nodules or thin-walled cavities. Diffuse pneumonia caused by C. immitis is usually accompanied by fungemia, and patients should be evaluated for systemic disease and extrapulmonary lesions (e.g., meningitis). Disseminated disease with diffuse erythematous maculopapular rash, erythema multiforme, erythema nodosum, or arthralgias and infection in bones, joints, and CNS can occur. Diagnosis Diagnosis can be made by direct examination and culture of respiratory secretions, CSF, or by biopsy of suspicious pulmonary or cutaneous lesions to reveal characteristic double-contoured spherules with endospores and without budding. Blood cultures are positive <15% of the time in HIV-associated coccidioidomycosis. A DNA probe can identify C. immitis in cultures. IgM antibody detected by latex agglutination, enzyme immunoassay, immunodiffusion, or tube precipitin appears early and is an indication of acute infection. IgG antibody gradually appears over the first few months after primary infection and does not disappear in the presence of disseminated disease. Titers of >1:16--1:32 are associated with disseminated disease, except in cases of isolated meningitis (194). Serological tests (e.g., complement fixation, tube precipitation and immunodiffusion assays) might have reduced diagnostic use in severely immunosuppressed HIV-infected patients. Treatment Experience is limited in treating coccidioidomycosis among HIV-infected children, and recommendations are generally based on experience with adults. On the basis of data from HIV-infected adults for treatment of diffuse pulmonary or disseminated disease, induction therapy with amphotericin B at a dose of 0.5--1.0 mg/kg body weight/day is recommended until clinical improvement is observed (AII); several weeks of therapy often are required to produce clear evidence of improvement (252). Following acute therapy, chronic suppressive therapy with fluconazole or itraconazole is recommended (AII). Alternative treatment for disseminated nonmeningitic infection that is stable includes fluconazole (5--6 mg/kg administered twice daily) or itraconazole (4--10 mg/kg twice daily for 3 days followed by 2--5 mg/kg administered twice daily) (BIII). CNS infections, including meningitis, should be treated with high-dose fluconazole (5--6 mg/kg/dose admistered twice daily) because, unlike amphotericin B, it crosses the blood brain barrier well (AII). For CNS infections unresponsive to fluconazole, intravenous amphotericin B is used and augmented by intrathecal amphotericin B (CI). Consultation with a specialist is recommended when treating children with meningeal disease. Fluconazole can inhibit the cytochrome P-450-dependent hepatic enzymes, and the potential for drug interactions should be evaluated carefully before initiation of therapy (AIII). The most frequent adverse effects of fluconazole are gastrointestinal, including nausea and vomiting. Skin rash and pruritis might be observed and rare cases of Stevens-Johnson syndrome have been reported with fluconazole. Asymptomatic increases in transaminases can be observed in 1%--13% of patients receiving azole drugs, and less frequently, patients with hepatitis; rare cases of fatal hepatitis have been reported. Surgical debridement or excision of localized, persistent, progressive, or resistant lesions in bone and lung might be helpful. Lung cavities with recurrent bleeding and those larger than 6 cm in diameter are at greater risk for rupture and require surgery. As with other disseminated fungal infections, continued chronic suppressive therapy with fluconazole or itraconazole is recommended following completion of initial therapy; details on secondary prophylaxis (maintenance therapy) have been published (4). Safety of discontinuation of secondary prophylaxis after immune reconstitution with HAART among children has not been studied extensively. CytomegalovirusEpidemiology Infection with human CMV is common and usually inapparent; acquisition of CMV can occur during infancy, early childhood, or adolescence. Transmission can occur from an infected woman to her offspring; horizontally by contact with virus-containing saliva, urine, or sexual fluid; or through transfusion of infected blood or transplantation of infected organs. CMV is the most common perinatally transmitted infection, occurring in 0.2%--2.2% of live-born infants in the United States (253). In early childhood, infection usually occurs secondary to exposure to infected saliva or urine. Infection occurs at younger ages in locations where sanitation is less optimal. Among adolescents, sexual transmission is the major mode of CMV acquisition. Congenital (in utero) CMV infection occurs most commonly among infants born to women with primary CMV infection during pregnancy. Following primary infection during pregnancy, the rate of transmission to the fetus is approximately 30%--40% (254,255). In comparison, the rate of congenital infection after recurrent CMV infection is lower (range: 0.15%--1.0%) (253,256,257). In utero transmission with recurrent infection can occur because of reactivation of infection among women infected before pregnancy or reinfection with a different CMV strain among women who are CMV seropositive (258,259). CMV also can be transmitted during the intrapartum or postpartum periods from mother to infant. Up to 57% of infants whose mothers shed CMV at or around the time of delivery become infected with CMV, and up to 53% of children who are breastfed with milk that contains infectious virus can become CMV infected. However, symptomatic CMV disease in the infant is much less common when CMV is acquired intrapartum or through breastfeeding. In the United States, the overall prevalence of CMV infection among women of childbearing age is 50%--80%, with the highest prevalence in women in lower socioeconomic strata (254,260). The prevalence of CMV infection among HIV-infected pregnant women is higher than in the general population, with approximately 90% of HIV-infected pregnant women coinfected with CMV (261,262). HIV-infected women with CMV infection have a higher rate of CMV shedding from the cervix than women without HIV infection (52%--59% compared with 14%--35% in HIV-uninfected cohorts) (263). The risk for mother to child transmission of CMV might be increased among infants born to women dually infected with CMV and HIV. In one study of 440 infants born to HIV-infected women in the United States, the overall rate of in utero infection was 4.5%, higher than the <2% rate of in utero infection in the general U.S. population. HIV-infected children appear to be at higher risk for acquisition of CMV infection during early childhood than children without HIV infection (7). The rate of CMV acquisition in HIV-infected children appears to be particularly high during the first 12 months of life but continues to remain higher among HIV-infected than uninfected children through age 4 years as they become exposed to CMV infection in other children in child care or school settings. CMV disease is less frequent among HIV-infected children than HIV-infected adults but contributes substantially to morbidity and mortality. CMV causes 8%-10% of pediatric AIDS-defining illness. In a group of 189 HIV-infected children followed at the National Cancer Institute, the overall prevalence of CMV infection was 30%, with a prevalence of symptomatic CMV-related disease of 9%, one half of which was CMV retinitis (264). Among patients with positive CMV urine cultures, 25% had evidence of CMV disease. Children with positive CMV cultures had lower survival rates than those without positive CMV cultures. Symptomatic HIV-infected children with CMV coinfection have a higher rate of CMV viruria than asymptomatic HIV-infected or HIV-exposed children. Overall, one third of HIV-infected children shed CMV (up to 60% with AIDS) compared with 15%--20% of CMV-infected, HIV-exposed but uninfected children and <15% of CMV-infected infants not exposed to HIV (265). Clinical Manifestations Approximately 10% of infants with in utero CMV infection are symptomatic at birth with congenital CMV syndrome (CMV inclusion disease); mortality of children with symptomatic disease is as high as 30%. Newborns with symptomatic congenital CMV infection are usually small for gestational age and might have purpura/petechiae, jaundice, hepatosplenomegaly, chorioretinitis, microcephaly, intracranial calcifications, and hearing impairment. Approximately 90% of infants with symptomatic disease at birth who survive have late complications, including substantial hearing loss, mental retardation, chorioretinitis, optic atrophy, seizures, or learning disabilities (254). Although the majority of children with in utero CMV infection do not have symptoms at birth, 10%--15% are at risk for developing later developmental abnormalities, sensorineural hearing loss, chorioretinitis, or neurologic defects. HIV-infected children with CMV coinfection appear to have accelerated progression of HIV disease compared with those without CMV infection (7,264,266). In one study, 53% of infants with HIV/CMV coinfection had progression to AIDS or death by age 18 months, compared with 22% of HIV-infected children without CMV infection; those with CMV coinfection also were more likely to have central nervous disease (36% versus 9%). The relative risk for HIV disease progression in those with CMV coinfection compared with those without coinfection was 2.6 (95% CI = 1.1--6.0) (7). CMV retinitis is the most frequent severe manifestation of CMV disease among HIV-infected children, accounting for approximately 25% of CMV AIDS-defining illnesses. CMV retinitis among young HIV-infected children is frequently asymptomatic and discovered on routine examination. Older children with CMV retinitis present similarly to adults with floaters, loss of peripheral vision, or reduction in central vision. Diagnosis of CMV retinitis is based on clinical appearance with white and yellow retinal infiltrates and associated retinal hemorrhages. A more indolent, granular retinitis also might occur. HIV-infected children with CD4+ cell counts <100/µL are more likely to develop CMV retinitis than those with higher CD4+ cell counts, but CD4+ cell count is less predictive of risk for CMV disease in young infants, and systemic and localized CMV disease also can occur among HIV-infected infants with higher age-adjusted CD4+ cell counts (265,267). End-organ CMV disease has been reported in the lung, liver, gastrointestinal tract, pancreas, kidney, sinuses, and CNS (267--270). In children with extraocular CMV disease, predominantly nonspecific symptoms (e.g., fever, poor weight gain, and loss of developmental milestones with laboratory abnormalities of anemia, thrombocytopenia, and elevated lactic dehydrogenase) are initially observed, although the extent to which CMV or HIV infection itself are contributing to these findings is unclear (25). Gastrointestinal manifestations among HIV-infected children include CMV colitis (most common GI manifestation), oral, and esophageal ulcers, hepatic involvement, ascending cholangiopathy, or gastritis. Sigmoidoscopy in CMV colitis is nonspecific, demonstrating diffuse erythema, submucosal hemorrhage, and diffuse mucosal ulcerations. The role of CMV in pulmonary disease among HIV-infected children is difficult to assess because it is often isolated with other organisms (e.g., P. jiroveci). Histologic evidence of CMV disease is needed to determine if active disease is present. CMV pneumonia is an interstitial process with gradual onset of shortness of breath and dry, nonproductive cough; auscultatory findings might be minimal. CNS manifestations of CMV include subacute encephalopathy, myelitis, and polyradiculopathy (primarily observed in adults but rarely reported among children). CSF findings are nonspecific and might indicate a polymorphonuclear predominance (>50% of patients), elevated protein (75%), and low glucose (30%). However, up to 20% of children have completely normal CSF. Diagnosis CMV infection versus disease might be difficult to differentiate in HIV-infected children. Because of transplacental transfer of antibody from mother to child, a positive CMV antibody assay in an infant aged <12 months is indicative of maternal infection but not necessarily infection of the infant. In an infant aged >12 months, a positive CMV antibody assay indicates previous infection with CMV but not necessarily active disease. At any age, a positive CMV culture is indicative of infection, but not necessarily of disease. CMV can be isolated in cell culture from peripheral blood leukocytes or body fluids (e.g., urine) or tissues. Using centrifugation-assisted shell vial culture amplification techniques, CMV can be detected within 16--40 hours of culture inoculation. A positive blood buffy-coat culture establishes a diagnosis of CMV infection and increases the likelihood that disease or symptoms were caused by CMV, because children with positive blood cultures are at higher risk for developing end-organ disease. Different methods have been used to detect viral antigen or DNA directly and identify patients at risk for development of CMV disease, including detection of pp65 antigenemia, qualitative and quantitative PCR, and DNA hybridization. The DNA assays are more sensitive than buffy-coat or urine cultures for detecting CMV and can be used to identify patients at higher risk for development of clinically recognizable disease. CMV DNA detection in CSF by DNA PCR is highly sensitive for CMV disease. Quantitative DNA PCR can be used as a marker of risk for disease and to monitor response to therapy (271). Recovery of virus from tissues (e.g., endoscopically guided biopsies of gastrointestinal or pulmonary tissue) provides evidence of infection in symptomatic patients. The limitation of this method is that it takes 1--6 weeks to detect visible cytopathic effects in cell culture. Staining of shell vial culture with CMV monoclonal antibodies or immunostaining for CMV antigens can allow earlier diagnosis of infection. Histopathology demonstrates characteristic "owl's eye" intranuclear and smaller intracytoplasmic inclusion bodies in biopsy specimens; staining with CMV monoclonal antibodies or immunostaining for CMV antigens also can be done. The same procedure can be used on cells obtained from bronchoalveolar lavage. Some pediatric HIV experts recommend testing all infants with HIV infection for CMV infection with a urine culture during the first months of life to identify infants with congenital, perinatal, or early postnatal infection. In addition, annual CMV antibody testing of previously seronegative and culture negative infants and children, beginning at age 1 year, will identify those who develop occult CMV infections, permitting appropriate screening for retinitis. Children with dual HIV/CMV infection should have a dilated retinal examination performed by an ophthalmologist experienced in this diagnosis every 4--6 months after they are severely immunocompromised. Older children and adolescents should be counseled to report "floaters" and visual changes. Treatment Treatment of newborns with symptomatic congenital CMV disease with ganciclovir (4--6 mg/kg administered intravenously every 12 hours for 6 weeks) has been evaluated in a phase II study (272). The higher dose of 12 mg/kg body weight/day led to a substantial decrease in the overall quantity of virus in the urine and other sites (BI). In a phase III trial of ganciclovir treatment of infants with symptomatic congenital CMV infection, ganciclovir begun in the neonatal period resulted in more rapid resolution of liver enzyme abnormalities and less hearing loss at age 6--12 months compared with no treatment, although approximately two thirds of the infants had substantial neutropenia during therapy (273). Neutropenia was severe enough to require dose modification in 48% and treatment with granulocyte colony stimulating factor in 7% and was complicated by gram-negative sepsis in one neonate. The drug of choice for initial treatment of disseminated CMV disease, including CMV retinitis, in HIV-infected children is intravenous ganciclovir (AI). The dose is 5 mg/kg/dose twice daily administered intravenously over 1--2 hours for 14--21 days followed by lifelong maintenance therapy. With long-term therapy, the emergence of ganciclovir-resistant CMV strains has occurred. The major side effect of ganciclovir is myelosuppression (i.e., anemia, neutropenia, and thrombocytopenia). Dose reduction or interruption might be necessary in up to 40% of patients because of hematologic toxicity; granulocyte colony-stimulating factor can be used to ameliorate marrow suppression. Renal toxicity, as seen by increased serum creatinine, also can occur and might require ganciclovir dose modification. Other toxic reactions include CNS effects, gastrointestinal dysfunction, thrombophlebitis, and elevated liver enzymes. An alternative drug to treat CMV disease or for use in ganciclovir-resistant CMV infections in HIV-infected children is foscarnet (AI). Foscarnet when used for suppression has been associated with increased length of survival relative to ganciclovir in HIV-infected adult patients. The pediatric dose is 60 mg/kg/dose every 8 hours administered intravenously over 1--2 hours for 14--21 days followed by lifelong maintenance therapy. The dose of foscarnet should be administered slowly over the course of 2 hours (i.e., no faster than 1 mg/kg/minute). Infusing foscarnet with saline fluid loading can minimize renal toxicity. Doses should be modified among patients with renal insufficiency. The main toxicity of foscarnet is decreased renal function; up to 30% of patients experience an increase in serum creatinine levels. Renal toxicity and foscarnet binding to divalent metal ions such as calcium leads to metabolic abnormalities in approximately one third of patients, and serious electrolyte imbalances (including abnormalities in calcium, phosphorus, magnesium, and potassium levels) and secondary seizures, cardiac dysrhythmias, abnormal liver transaminases, and and CNS symptoms can occur. Combination therapy with ganciclovir and foscarnet delays progression of retinitis in certain patients failing monotherapy (267,274--276) and can be used as initial therapy among children with sight-threatening disease (BIII). Combination therapy also has been used for adult patients with retinitis that has relapsed on single-agent therapy. Valganciclovir, a prodrug of ganciclovir, is one of the first-line treatments for HIV-infected adults with CMV retinitis at an induction dose of 900 mg orally twice daily for 21 days, followed by 900 mg orally once daily as maintenance (276) (AI). Valganciclovir is well absorbed from the gastrointestinal tract and rapidly metabolized to ganciclovir in the intestine and liver. Its major adverse effect is myelosuppression. However, data on appropriate dosage of this drug for children are not available (CIII). Before the availability of valganciclovir, oral ganciclovir in combination with an intraocular ganciclovir implant had been used for maintenance treatment of CMV retinitis in adults. Oral ganciclovir has been studied in a limited number of children. Children require a higher oral dose of ganciclovir than adults to achieve target serum levels because of low bioavailability and greater clearance. A dose of 30 mg/kg administered orally every 8 hours produced serum levels similar to the dose effective for maintenance treatment of CMV retinitis in adults (1 g orally every 8 hours) (277). The combination of oral ganciclovir with a ganciclovir sustained release intraocular implant, replaced every 6--9 months, could be considered for treatment and chronic suppression of CMV retinitis in older children (4) (BIII). Among children old enough to receive the adult dosage, valganciclovir would be the preferred drug instead of oral ganciclovir (AI). Cidofovir (5 mg/kg intravenously once a week for 2 weeks, then 5 mg/kg once every 2 weeks for maintenance therapy) is effective in treating CMV retinitis among adult patients who are intolerant of other therapies (AI). However, cidofovir has not been studied in pediatric patients with CMV disease (CIII). The major side effect of cidofovir is nephrotoxicity; the drug produces proximal tubular dysfunction including Fanconi syndrome and acute renal failure. To minimize nephrotoxicity, probenicid should be administered before each infusion, and intravenous hydration with normal saline should be administered before and after each cidofovir infusion; renal function should be carefully monitored. Neutropenia also has been reported. Other reported adverse effects include anterior uveitis and ocular hypotony; ophthalmologic monitoring for anterior segment inflammation and intraocular pressure is needed while receiving the drug. Intravitreous injections of ganciclovir, foscarnet, or cidofovir have been used for control of retinitis but require biweekly intraocular injections. Data are limited in children, and biweekly injection is impractical for use in most children (DIII). Implantation of an intravitreous ganciclovir medication release device in the posterior camber of the eye also has been used in HIV-infected adults and adolescents. Intraocular implants should not be used in children aged <3 years because of the small size of the eyes in young children (EIII). Fomivirsen (Vitravene) is an antisense nucleotide that binds to CMV mRNA, has potent anti-CMV activity, and is available as an aqueous solution for intravitreous injection. The drug has been studied in a controlled trial in HIV-infected adults (278) and is approved for intraocular use in adults with AIDS who have persistent active CMV retinitis despite other anti-CMV therapies or who cannot tolerate other treatments (AI); no studies have been conducted among pediatric patients (CIII). Complications of intraocular therapy include vitreous hemorrhage, retinal detachment, and endophthalmitis. Intraocular maintenance therapy does not prevent extension to the opposite eye or development of systemic disease and therefore, when used, should be combined with oral ganciclovir or oral valganciclovir to provide systemic therapy. Among HIV-infected children with CMV disease, after initial induction therapy, lifetime chronic suppressive maintenance therapy for CMV (secondary prophylaxis) is required; detailed recommendations have been published (4). Safety of discontinuation of secondary prophylaxis following immune reconstitution with HAART in children has not been studied extensively. Herpes simplex VirusEpidemiology HSV can be transmitted from an HSV-infected mother to her infant resulting in neonatal infection, in addition to the classic person-to-person HSV transmission observed amongolder children and adults through direct contact with infected oral secretions or lesions. Neonatal HSV infection occurs at a rate of one case/2,000--5,000 deliveries (279). Neonatal transmission occurs primarily through exposure of the infant to HSV-infected maternal genital fluids during passage through the birth canal, by ascending infection, or through use of invasive procedures, such as fetal scalp monitoring, that disrupt fetal skin integrity during labor (280). Congenital (in utero) HSV acquisition is rare but can result in devastating cutaneous, ocular, and CNS damage. Maternal HSV antibody status before delivery influences both the severity and the likelihood of transmission to the infant (280). The risk for neonatal HSV infection is greatest when an infant is born to a woman with primary HSV infection (range: 30%--50%) (279). The risk is much lower (0--5%) for infants born to women shedding HSV caused by reactivated infection (281). Genital shedding of HSV at the time of delivery is associated with increased risk for transmission, and prolonged rupture of membranes (>6 hours) also increases the risk for HSV transmission to the infant, probably as a result of ascending HSV infection from the cervix (279). Cesarean delivery substantially lowers the risk for transmission (279,280). In the United States, 75% of neonatal infections are caused by genital herpes, HSV type 2, and HSV type 1. HSV-2 seroprevalence among the overall population of childbearing-aged women is approximately 26% (281). HSV-2 infection rates might be higher in HIV-infected than HIV-uninfected women. Women infected with HIV, particularly those with low CD4+ cell count, shed HSV from the vulva and cervix more commonly than women not infected with HIV; the majority of this shedding is asymptomatic (282,283). Among women who are not infected with HIV, the rate of HSV reactivation is about 25% during the last month of pregnancy, but only about 2%--3% will be shedding on the day of delivery (284). In comparison, in women who are coinfected with HIV and HSV, an estimated 10% have cervical shedding of HSV on the day of delivery (282). The risk for genital HSV reactivation and shedding increases as HIV-related immunosuppression progresses. No evidence exists to indicate that in utero HSV infection of the infant occurs more frequently in the HIV-infected pregnant woman; whether infants born to women with HIV/HSV-2 coinfection have an increased risk for perinatal (intrapartum) HSV infection is unknown. Recurrent or persistent HSV infection is the AIDS-indicator condition in approximately 6% of pediatric AIDS cases. As in HIV-infected adults, HIV-infected children might have more frequent and severe episodes of HSV reactivation. From 5%--10% of children with AIDS and primary gingivostomatitis develop frequent recurrences, which can be associated with severe ulcerative disease and symptoms similar to primary infection (285). Children with HIV infection also can have more prolonged shedding of virus with both primary and reactivation HSV infection than children without HIV infection. Clinical Manifestations Neonatal HSV can appear as disseminated multiorgan disease (occurring in approximately 25% of neonates with HSV infection); localized disease of the CNS (approximately 35% of neonates); or disease localized to the skin, eyes, and mouth (approximately 40% of neonates) (279). Infants with disseminated disease usually appear at age 9--11 days; encephalitis occurs in 60%--75% of these infants. Vesicular rash is present in approximately 80% of children with localized skin, eye, or mouth disease, but only in approximately 60% of children with CNS or disseminated disease (279,286). Localized disease usually appears at age 10--11 days, and even with treatment, neonates with skin lesions might have cutaneous recurrences during the first 6 months after treatment (279). Outside of the neonatal period, the most common appearance of herpes virus infection in children is orolabial disease. Fever, irritability, tender submandibular lymphadenopathy, and superficial, painful ulcers in the gingival and oral mucosa and perioral area characterize primary HSV gingivostomatitis. HIV-infected children who experience primary infection when they are immunocompromised can have severe local lesions or, more rarely, disseminated HSV with visceral involvement and generalized skin lesions with primary infection. Other sites of involvement among HIV-infected children with severe immunocompromise include the esophagus, CNS, and genitals and disseminated disease involving the liver, adrenals, lung, kidney, spleen, and brain. Diagnosis Clinical diagnosis is based on the typical appearance of vesicles and ulcers. The virus can be isolated in culture and can usually be detected in tissue culture cells within 1--3 days. For the diagnosis of neonatal HSV infection, culture specimens should be obtained from blood and skin vesicles, mouth or nasopharynx, eyes, urine, and stool or rectum; positive cultures from any of the latter sites >48 hours after birth indicates viral replication rather than contamination after intrapartum exposure. CSF should be tested for HSV nucleic acid by amplifying an HSV DNA sequence common to both HSV-1 and HSV-2 using PCR assays. Direct immunofluorescence for HSV antigen can be conducted on cells collected from skin, conjunctiva, or mucosal lesion scrapings. Giemsa staining (Tzanck preparation) of lesion cell scrapings might show multinucleated giant cells and eosinophilic intranuclear inclusions, but this does not differentiate HSV type or HSV from varicella-zoster virus infection and is not routinely recommended. Among children with suspected HSV encephalitis, detection of HSV DNA by PCR in the CSF has replaced brain biopsy as the diagnostic test of choice in such patients (287). Cultures of the CSF for HSV usually are negative. Definitive diagnosis of HSV esophagitis requires endoscopy with biopsy (histologic evidence of multinucleated giant cells with intranuclear viral inclusion) and culture. Treatment Acyclovir is the drug of choice for treatment of HSV among infants and children, regardless of HIV-infection status. Both oral and intravenous preparations are available. Neonatal HSV disease should be treated with high-dose intravenous acyclovir (20 mg/kg body weight/dose three times daily) administered for 21 days for CNS and disseminated disease and for 14 days for skin, eye, and mouth disease (286) (AI). Acyclovir therapy should not be discontinued in neonates with CNS disease unless a repeat CSF HSV DNA PCR assay is negative at day 19--21 of treatment (BIII). Although treatment has reduced morbidity and mortality, infants with neonatal HSV infection remain at risk for neurologic sequelae, with the most severe neurologic sequelae seen in those with CNS disease. A limited percentage (2%--6%) of infants with localized skin, eye, or mucus membrane disease might have later neurologic sequelae after apparently successful treatment (279,288). Disseminated HSV disease or encephalitis outside of the neonatal period should be treated with intravenous acyclovir with a dose of 10 mg/kg/dose or 500 mg/m2/dose three times daily for 21 days (AII). HIV-infected children with symptomatic HSV gingivostomatitis should be treated with intravenous acyclovir (5--10 mg/kg/dose three times daily) or oral acyclovir (20 mg/kg/dose three times daily) for 7--14 days (AII). HIV-infected children who have severe oral HSV recurrences (more than 3--6 severe episodes a year) can be considered for secondary suppressive therapy with oral acyclovir (4) (AI). Acyclovir is primarily excreted by the kidney; as a result, dose adjustment based on creatinine clearance is needed in patients with renal insufficiency or renal failure. Primary toxicities of acyclovir are phlebitis, renal toxicity, nausea, vomiting, and rash. In infants receiving high-dose acyclovir for neonatal disease, the major toxicity was neutropenia (absolute neutrophil count <1,000/mm3) (289). Grade 3 or higher nephrotoxicity was observed in 6%. Pediatric experience with the oral preparation of acyclovir is limited for children aged <2 years. Among infants who received long-term oral acyclovir suppressive therapy (300 mg/m2 body surface area/dose given 2--3 times daily) following treatment for neonatal HSV infection, a relatively high rate of neutropenia (46%) was observed, although in most cases this was self-limited and did not require dose modification or drug discontinuation (290). Among HIV-infected children with acyclovir-resistant HSV infection, intravenous foscarnet is recommended at a dose of 120 mg/kg/day in 2--3 divided doses administered intravenously over 1--2 hours until the infection resolves (AI). The dose of foscarnet should be administered slowly over the course of 2 hours (or no faster than 1 mg/kg/minute). Infusing foscarnet with saline fluid loading can minimize renal toxicity. Doses should be modified in patients with renal insufficiency. The main toxicity of foscarnet is decreased renal function; up to 30% of patients experience an increase in serum creatinine levels. Renal toxicity and foscarnet binding to divalent metal ions such as calcium leads to metabolic abnormalities in approximately one third of patients, and serious electrolyte imbalances (including abnormalities in calcium, phosphorus, magnesium, and potassium levels) and secondary seizures, or cardiac dysrhythmias can occur. Abnormal liver transaminases and CNS symptoms also can occur. Valacyclovir is a prodrug of acyclovir with improved bioavailability; bioavailabililty is 50%--55% in adults (3--5 times higher than that of oral acyclovir). Valacyclovir is rapidly converted to acyclovir after absorption and is not active against acyclovir-resistant HSV strains. It is approved for use in adults and adolescents for treatment of genital herpes at a dose of 1 g twice daily for 7--10 days (AII). Dose adjustment (based on creatinine clearance) is needed among patients receiving valacyclovir who have renal insufficiency or renal failure. Data are limited on valacyclovir in children (291) (CIII). In a study of valacyclovir among children with leukemia, bioavailability of acyclovir was 45% after a median oral dose of 34.1 mg/kg of valacylcovir (292). In a second study in 28 immunocompromised children aged 5--12 years, children were randomized to receive 250 mg (9.4--13.3 mg/kg) or 500 mg (13.9--27.0 mg/kg) valacyclovir twice daily; the overall estimated acyclovir bioavailability was 48% (293). An oral dose of valacylcovir of 30 mg/kg/dose administered three times a day to a child with normal renal function was estimated to provide acyclovir concentrations similar to those achieved with standard acyclovir dosing of 10 mg/kg/dose intravenously three times daily. Valacylcovir is available only in caplet formulation, but a suspension formulation can be prepared in Ora-Sweet® or Syrpalta® syrups (to yield a final concentration of 50 mg/mL of the hydrochloride salt) that is stable for 21 days if stored in amber glass bottles (294). Adverse effects of valacyclovir are similar to acyclovir; nausea and vomiting are most common. Thrombotic microangiopathy (thrombotic thrombocytopenic purpura/hemolytic uremic syndrome) has been reported in HIV-infected adults with advanced disease who received high-dose valacyclovir (8 grams per day) (291). However, it was not reported in other studies in which valacyclovir was adminstered in lower doses (250 mg--1,000 mg/day). Penciclovir is an acyclic guanine analog derivative with similar activity and mechanism of action as acyclovir, although penciclovir has a longer intracellular half-life. It is not active against acyclovir-resistant HSV strains. It has poor bioavailability and is only available as a 1% cream for topical application. It is approved for treatment of recurrent herpes labialis in immunocompetent adults (applied every 2 hours while awake). No data are available about use of penciclovir treatment in children. Famciclovir is the oral prodrug of penciclovir; its bioavailability is approximately 75% compared with 5% for penciclovir. It is approved for treatment of recurrent mucocutaneous HSV infection in HIV-infected adults and adolescents at a dose of 500 mg orally twice daily for 7 days (AII). Famciclovir is available only in tablet form, and no specific data on children are available (CIII). Dose adjustment (based on creatinine clearance) is needed among patients with renal insufficiency or renal failure. Adverse effects are rare; these include gastrointestinal disturbances, rash, and CNS complaints (e.g., confusion, hallucinations and disorientation), neutropenia, and elevated liver transaminases. Varicella-Zoster VirusEpidemiology In the United States, 9% of children aged <10 years experienced varicella annually before widespread use of varicella vaccine; by adulthood, >95% of persons have antibodies to varicella-zoster virus (VZV), implying a past history of primary infection (295,296). Varicella has the potential to cause greater morbidity and mortality in HIV-infected immunocompromised children than among the general population of children. Mother-to-child transmission of VZV can occur; however, because most adults are immune, varicella is a rare complication of pregnancy. It is unknown whether mother-to-child VZV transmission occurs more frequently among HIV-infected women with primary varicella. In one study, 13% of HIV-infected pregnant women lacked immunity to VZV (296). Congenital varicella syndrome occurs in approximately 2% (95% CI = 0--5%) of infants born to women who have primary varicella during the first trimester (297). It is not seen among women who develop herpes zoster during pregnancy. Fewer than 100 cases of congenital VZV have been reported. VZV can be transmitted to the fetus in later gestation, resulting in neonatal varicella. When the mother develops varicella from 4 days before to 2 days after delivery without passive antibody prophylaxis, the attack rate for infants is approximately 20% and mortality is approximately 30% (298). In comparison, if maternal varicella precedes delivery long enough to allow transfer of VZV IgG antibodies across the placenta, infants might be born with cutaneous varicella lesions or have them in the first 5 days of life, but they are usually not at risk for serious complications. Zoster occurs only among children previously infected with VZV. Zoster is unusual in HIV-infected children who had primary varicella infection when their CD4+ cell counts were normal or mildly suppressed. However, among HIV-infected children with low CD4+ cell counts (i.e., CD4+ <15%) at the time of primary varicella, the rate of subsequent zoster may be as high as 70% (299,300). The incidence of zoster among HIV-infected children who had primary varicella when they were immunocompromised is 467/1,000 child-years, substantially higher than the 98/1,000 person-years observed in immunocompromised HIV-infected adults and the 25/1,000 child-years seen among children with leukemia. As in adults, current CD4+ cell count in children correlates with the frequency of zoster recurrences (301). Clinical Manifestations Congenital infection is characterized by cicatricial skin scarring, limb hypoplasia, and neurologic (microcephaly, cortical atrophy, seizures, and mental retardation), eye (chorioretinitis, microophthalmia, and cataracts), and renal (neurogenic bladder, hydroureter, hydronephrosis) abnormalities and swallowing dysfunction and aspiration pneumonia (298,302,303). Initial reports of varicella among HIV-infected children suggested more severe disease manifestations (304), but more recent studies support less complicated courses, particularly in children receiving antiretroviral therapy or with higher CD4+ cell counts at the time of infection (299,300,305,306). However, the duration of disease might be longer, and the rate of complications is still higher than in normal children hospitalized with varicella (306). HIV-infected children also might experience chronic infection with continued appearance of new lesions for >1 month after primary or recurrent VZV infection (307). The lesions are characteristically varicelliform at onset but evolve into nonhealing ulcers that become necrotic, crusted, and hyperkeratotic. Chronic VZV is reported in 14% of HIV-infected children with VZV, usually in children with low CD4+ cell counts (301). Viral isolates might become resistant to acyclovir during prolonged therapy (308). The classical clinical presentation of varicella (i.e., a generalized pruritic vesicular rash and fever) is diagnostic. However, persistent lesions might be atypical and lack a vesicular component. The classical clinical presentation of zoster (i.e., a frequently painful vesicular eruption with a dermatomal distribution) is diagnostic. However, less typical rashes, including those that extend beyond dermatomal boundaries or that are bilaterally distributed or are generalized, also might represent zoster in HIV-infected children. HIV-infected children can have recurrent episodes of reactivated VZV infection that present with a disseminated rash more similar to chickenpox than zoster but without visceral dissemination; they might have multiple episodes of recurrent disease (301). VZV retinitis is a rare complication of VZV infection among HIV-infected children and might be confused with cytomegalovirus retinitis. Progressive encephalitis caused by VZV in the absence of a zosteriform rash caan occur (309). VZV should be suspected among children with unilateral vesicular rashes, retinitis when CMV cannot be implicated, or with progressive and otherwise unexplained encephalitis and a history previous varicella. Diagnosis Clinical diagnosis of varicella and zoster infections is based on the typical appearance of generalized pruritic vesicular rash and fever in the former and a frequently painful vesicular rash in a dermatomal pattern in the latter. Direct immunofluorescence for VZV antigen can be conducted on cells collected from skin, conjunctiva, or mucosal lesion scrapings. Giemsa-staining (Tzanck preparation) of cell scrapings from lesions is nonspecific, as detection of multinucleated giant cells is suggestive of VZV but is also observed with HSV infection. The optimal sensitivity of these methods requires obtaining cells from the base of a lesion after unroofing a fresh vesicle. Direct and indirect immunofluorescence or immunoperoxidase methods also can be used for detection of VZV-infected cells in tissue sections of lung, liver, brain, or other organs. VZV can be isolated by cell culture from vesicular fluid or ulcer swabs, but is a very liable virus. The specimen must undergo rapid processing or be kept on dry ice or frozen at --70ºC (--94ºF) if storage for more than a few hours is required (298). Five to seven days after inoculation to detect typical cytopathic effects; confirmation by staining by virus-specific antiserum is needed. Shell viral cultures combine centrifugation and staining with fluorescein-conjugated monoclonal antibodies to detect synthesis of VZV proteins in infected cells. This allows results in 1--3 days after inoculation before a cytopathic effect is visible. Culture is necessary if testing of the virus for antiviral susceptibility is needed. PCR can be used to detect VZV in samples, is extremely sensitive and specific, and can differentiate wild-type and vaccine VZV, but is available only in research laboratories. Serologic tests can be used to diagnose VZV infection, noting a substantial increase in antibody titer during convalescence (2--3 weeks after onset of illness) or the presence of VZV IgM antibody. VZV reactivation can induce VZV-IgM antibodies in many patients so their presence does not differentiate primary from recurrent VZV infections (298). Treatment On the basis of controlled trials among children with malignancies, acyclovir is the drug of choice for treatment of VZV infection among HIV-infected children (AI). With primary varicella, acyclovir should be initiated as soon as possible after initial lesions appear. New lesions can continue to appear for 72 hours after initiation of acyclovir and crusting of all lesions might take 5--7 days. Intravenous acyclovir is recommended for treatment of primary varicella among HIV-infected children with moderate or severe immunosuppression or who have high fever or numerous or deep, necrotic, or hemorrhagic skin lesions (AIII). For children aged <1 year, the dose of acyclovir is 10 mg/kg body weight/dose adminstered intravenously every 8 hours as a 1-hour infusion. Some health-care providers adminster the same dose for children aged >1 year, and others use acyclovir based on body surface area among children aged >1 year old (500 mg/m2/dose intravenously every 8 hours as a 1-hour infusion) (59). Administration is for 7 days or until no new lesions have appeared for 48 hours. Oral administration should be used only for treatment of primary varicella among HIV-infected children with normal or only slightly decreased CD4+ cell counts or in children with mild disease (BIII). The dose is 20 mg/kg per dose adminstered orally 4 times daily (maximum dose: 800 mg). Acyclovir is the treatment of choice for zoster among HIV-infected children (AII). With zoster, oral acyclovir can be adminstered because the chance for disseminated, life-threatening disease is less with zoster than varicella. Initial intravenous administration should be considered for HIV-infected children with severe immunosuppression, trigeminal nerve involvement, or extensive multidermatomal zoster (AII). Acyclovir is primarily excreted by the kidney, and dose adjustment (based on creatinine clearance) is needed among patients with renal insufficiency or renal failure. Primary toxicities of acyclovir are phlebitis, renal toxicity, nausea, vomiting, and rash. Among infants receiving high-dose acyclovir for neonatal HSV disease, the major toxicity was neutropenia (absolute neutrophil count <1,000/mm3), which was observed in 21% of children (288). Grade 3 or higher nephrotoxicity was observed in 6% of children. Pediatric experience with the oral preparation of acyclovir is limited in children aged <2 years. In infants who received long-term oral acyclovir suppressive therapy (300 mg/m2 body surface area/dose administered 2--3 times daily) following treatment for neonatal HSV infection, a relatively high rate of neutropenia (46%) was observed, although in the majority of cases this was self-limited and did not require dose modification or drug discontinuation (290). Children who continue to develop lesions or whose lesions fail to heal might be infected with acyclovir-resistant VZV. HIV-infected children with acyclovir-resistant VZV can be treated with intravenous foscarnet (BII). The dose is 40--60 mg/kg per dose 3 times daily administered intravenously over 1--2 hours for 7 days or until no new lesions have appeared for 48 hours. The dose of foscarnet should be administered slowly over the course of 2 hours (i.e., no faster than 1 mg/kg/minute). Infusing foscarnet with saline fluid loading can minimize renal toxicity. Doses should be modified among patients with renal insufficiency. The main toxicity of foscarnet is decreased renal function; up to 30% of patients experience an increase in serum creatinine levels. Renal toxicity and foscarnet binding to divalent metal ions (e.g., calcium) leads to metabolic abnormalities in approximately one third of patients, and serious electrolyte imbalances (including abnormalities in calcium, phosphorus, magnesium, and potassium levels) and secondary seizures, cardiac dysrhythmias, abnormal liver transaminases, and CNS symptoms can occur. Valacyclovir is a prodrug of acyclovir with improved bioavailability. It is not active against acyclovir-resistant VZV strains. It is approved for treatment of zoster among adults at a dose of 1,000 mg given orally 3 times a day for 7 days (AII). However, data are limited for its use in children (291) (CIII). Valacyclovir is available only in caplet formulation, but a liquid formulation that is stable for 21 days can be prepared in Ora-Sweet and Syrpalta syrups and stored in amber glass bottles (291). Adverse effects of valacyclovir are similar to those with acyclovir; nausea and vomiting are most common. Thrombotic microangiopathy (thrombotic thrombocytopenic purpura/hemolytic uremic syndrome) has been reported among HIV-infected adults with advanced disease who received high-dose valacyclovir (8 g/day) (291). However, this condition has not been reported in other studies in which valacyclovir was adminstered in lower doses (250--1,000 mg/day). Famciclovir is the oral prodrug of penciclovir. It is not active against acyclovir-resistant VZV strains. Famciclovir is approved for the treatment of zoster in immunocompetent adults in a dose of 500 mg orally every 8 hours for 7 days (AII). It is comparable in efficacy to oral acyclovir in treatment of immunocompromised adults with localized zoster, although it has not been approved for this indication. It is available only in tablet form. No specific data about dosing information in children are available, although immunocompromised persons aged 12--18 years were included in a study of famciclovir for localized zoster (291) (CIII). Human PapillomavirusEpidemiology Human papillomavirus (HPV) infects cutaneous and mucosal squamous epithelium. Approximately 100 distinct types of HPV exisits, nearly half of which were first identified in the genital epithelium (310,311). They can be categorized on the basis of the site they occur (genital versus cutaneous) and also as high or low risk on the basis of their potential to induce malignant proliferation (e.g., HPV 16, 18, 31, 33, and 35 are most often associated with intraepithelial neoplasia) (311). HPV types that cause nongenital warts are usually distinct from those causing genital infections; however, genital HPV types can cause conjunctival, nasal, and oral and laryngeal warts. Children with compromised cellular immunity might have intense and widespread appearance of warts. Transmission of HPV-associated cutaneous warts occurs by close person-to-person contact and might be facilitated by minor trauma to the skin. HPV-associated anogenital warts are transmitted by sexual contact but also might be acquired at the time of delivery or transmission from nongenital sites. Genital warts (condylomata accuminata) in young children might be a sign of sexual abuse (312,313). Mother-to-child transmission of HPV can occur. Latent HPV infection has been identified in 5%--42% of pregnant women without HIV infection, with the higher rates among pregnant women with a history of sexually transmitted diseases (314--316). Some studies have demonstrated an increased prevalence of detectable HPV DNA during pregnancy, with a decline in the postpartum period (310,316). Among nonpregnant women, HPV DNA is detected more frequently among HIV-infected than uninfected women, with reported prevalence rates as high as 95%, but data related to HPV prevalence in HPV/HIV co-infected pregnant women are not available (317). HPV DNA has been detected in cord blood peripheral blood cells and amniotic fluid, indicating the potential for in utero infection (318--320). Duration of membrane rupture has been associated with mother-to-child HPV transmission, and some studies have indicated higher infant HPV infection rates among infants delivered vaginally than by cesarean section (321,322). Reported rates of HPV detection in nasopharyngeal aspirates, buccal brush swabs, or genital swabs from infants born to HPV-infected mothers have varied (range: 2%--80%) (321--325). In general, no neonatal clinical abnormalities have been associated with HPV detection. The presence of HPV DNA in newborn nasopharyngeal samples could represent contamination with infected maternal cells; however, persistence of HPV DNA in oral secretions or buccal swabs has been reported in 0--80% of infants with initially positive specimens who were followed through age 6 months (322,323,325--327). Infant laryngeal papillomas and juvenile laryngeal papillomatosis are thought to be secondary to HPV transmitted from mother to child through aspiration of infectious maternal genital secretions during delivery (322--324). HPV can be detected in the genital tract of 13%--60% of sexually active adolescent girls (328--332). In one U.S. study, cervical HPV infection was detected in 77% of HIV-infected girls compared with 55% of girls without HIV infection (333). The predominant risk factors for HPV infection in youth include number of lifetime and recent partners (330,331,334,335). Although the incidence of anogenital HPV infection in sexually active youth is high, longitudinal studies have demonstrated that 40%--80% of infections among youth without HIV infection might be transient and spontaneously regress, although recurrent infections might be observed (331,332,336,337). Infection with multiple types or high-risk types of HPV (e.g., 16 and 18), older age, and duration of HPV detection for >12 months were risk factors for persistent infection. Published data are limited about the rate of persistence among HIV/HPV co-infected youth. Persistent infection with HPV, particularly HPV-16, 18, 31, and 33, is associated with a high risk for developing cervical and anal intraepithelial neoplasia and risk for cervical, vulvovaginal, and anal carcinoma in both women and men. Adolescent girls might have biologic differences from adult women (e.g., cervical squamous metaplasia) that might increase their susceptibility to either development of persistent infection or disease (333). The risk for HPV-associated cervical abnormalities might be increased among HIV-infected youth. In one study, 70% of adolescent HIV-infected girls with HPV infection had abnormal cytologic findings; neither CD4+ cell count nor HIV viral load correlated with HPV infection or squamous intraepithelial lesions (333). Clinical Manifestations HPV causes hyperplastic, papillomatous, and verrucous squamous epithelial lesions on skin and mucus membranes, including anal, genital, oral, nasal, conjunctiva, gastrointestinal, and respiratory tract mucosa. Wart lesions appear as verrucous papules; lesions also can be smooth and flat or pedunculated. They can be soft, pink or white "cauliflower-like" sessile growths on moist mucosal surfaces (condylomata accuminata), or keratotic lesions on squamous epithelium of the skin, with a thick, horny layer. They might resemble common papular warts that are flesh-colored 1--4 mm, dome-shaped papules or flat-topped papules that appear macular to slightly raised on either moist mucus membrane surfaces or skin. Most frequently the hands, feet, face, and genitalia are involved. Diagnosis Most cutaneous and anogenital warts can be diagnosed by physical examination. Diagnosis of laryngeal papillomas requires laryngoscopy, and children with suspected respiratory tract papillomas need to be evaluated by a pediatric otolaryngologist. HPV DNA can be detected using a consensus PCR assay, with typing determined by hybridization assays or sequencing; alternatives include type-specific PCR assays, or HybridCaptureTM to determine high- versus low-risk group HPV types. However, detection and typing of HPV is not required for diagnosis or management of anogenital or cutaneous warts or papillomas (313). Treatment Multiple treatments for HPV-associated skin and external genital lesions exist; however, no single treatment is ideal for all patients or all lesions (313) (CIII). Standard topical therapy for HPV-associated lesions among HIV-infected children is often ineffective. Treatment can induce wart-free periods, but the underlying viral infection can persist and result in recurrence. In addition, topical treatments are seldom effective in patients with large or extensive lesions. However, individual lesions can be destroyed using cryotherapy or electrodesiccation. Topical treatments include podofilox solution and gel (0.5%) (antimitotic agent), imiquimod cream (5%) (topical immune enhancer that stimulates production of interferon and other cytokines), trichloroacetic or bichloroacetic acid 80%--95% aqueous solution (caustic agents that destroy warts by chemical coagulation of proteins), and podophyllin resin (contains antimitotic compounds and mutagens). Podofilox is applied to all lesions twice a day for 3 consecutive days and can be repeated weekly up to 4 weeks (BIII). Imiquimod is applied at bedtime and removed with water the following morning (BII). It should be applied 3 nonconsecutive nights a week for up to 16 weeks. HIV-infected patients with immunosuppression might have a lower response rate to imiquimod. Acid cauterization and podophyllin resin require application by a health-care provider. Acid cauterization should be discontinued if substantial improvement is not observed after three treatment sessions or complete clearance has not occurred after six treatments (BIII). Podophyllin resin is applied and removed by washing a few hours later; applications can be repeated weekly for up to 6 weeks (CIII). Systemic absorption can occur if applied to a large area, causing nausea, vomiting, or CNS effects. Efficacy of the various topical agents in patients without HIV infection ranges from 20%--80%. Major toxicity of topical agents is local pain or irritation of adjacent normal skin. Cidofovir topical gel (1%) is an experimental topical preparation that has been evaluated in a limited number of adults for treatment of anogenital HPV infection in a placebo-controlled trial; a complete response was observed in 47% of cidofovir recipients compared with none in the placebo group (311) (CIII). Successful use of cidofovir gel for treatment of severe molluscum contagiosum in children with HIV infection and congenital primary immunodeficiency has been reported (338,339). Topical cidofovir might have certain systemic absorption and be associated with renal toxicity (340). Individual lesions can be removed by using cryotherapy or electrodessication (BIII). Cryotherapy (application of liquid nitrogen or dry ice) must be applied until each lesion is thoroughly frozen. Treatment can be repeated every 1--2 weeks up to four times. The major toxicity is local pain. Curettage, electrosurgery, scissor excision, or laser vaporization also can be effective. Injectable therapy (e.g., interferon or 5-fluorouracil/epinephringel implant) has been used. Laryngeal papillomatosis is difficult to manage (341). Treatment is directed toward removing lesions obstructing the airway rather than at the elimination of disease. Lesions are removed by debridement or laser. Systemic interferon-alfa therapy or intralesional cidofovir has been used as an investigational treatment in children with frequent recurrences or extension into the trachea, bronchi, or lung parenchyma (CIII). Management of anogenital HPV infection accompanied by cytologic changes indicating dysplasia/carcinoma among children/adolescents is analogous to that for the adult population. HAART has not been consistently associated with a reduced risk for HPV-related cervical abnormalities in HIV-infected women. However, an "immune reconstitution"-like syndrome related to the occurrence of HPV-associated oral warts among HIV-infected adults has been observed in which the occurrence of oral warts was associated with a decrease in HIV RNA levels with highly active antiretroviral therapy (342). Immune reconstitution in response to viral load reduction might result in a return of marked inflammatory responses against latent oral HPV infection. Hepatitis CEpidemiology The prevalence of hepatitis C virus (HCV) infection among children aged 1--11 years in the United States is low (0.1%--0.2%) (343,344). The prevalence of HCV infection among HIV-infected children might be higher. In a serostudy of 535 HIV-infected children followed at pediatric HIV clinical trials sites, the prevalence of HCV infection was 1.5% (345). With the implementation of blood donor screening for HCV infection, the primary mode of acquisition of HCV infection among children is perinatal. The overall risk for mother-to-infant transmission of HCV is approximately 6% (346). The majority of HCV transmissions occur predominantly during or near delivery (347,348). The risk for vertical HCV transmission is increased by the presence of maternal HCV viremia at delivery. The role of quantitative HCV viral load has not been well defined, although data indicate that higher quantitative HCV levels might be associated with higher risk for transmission (349--356). Only one case of mother-to-infant transmission has been reported from a woman who was negative for HCV RNA; however, this was an older study and the PCR assay used might have been less sensitive than current assays. No association of HCV perinatal transmission with mode of delivery has been demonstrated in most studies (346,347,352,357--359). On the basis of current evidence, routine Cesarean delivery is not recommended for women with chronic HCV infection. Although vaginal delivery itself might not be a risk factor for HCV transmission, higher risk for transmission among vaginally delivered infants has been described with higher maternal HCV quantitative viral load, intrapartum exposure to virus-contaminated maternal blood by the occurrence of perineal or vaginal laceration, or neonatal hypoxia (354). Although HCV can be detected in breast milk, studies of infants born to HCV-infected women have not demonstrated an increased risk for HCV transmission in infants who are breastfed compared with those who were formula-fed (346,347,349,357,359,360). Therefore, breastfeeding is not contraindicated on the basis of HCV status alone (361). Other reported risk factors for mother-to-infant HCV transmission include intravenous drug use; overall transmission was 11% in drug users compared with 4% in nonusers (346). However, this has been an inconsistent finding. Available data do not indicate that HCV genotype is related to the risk for perinatal HCV transmission (358,362). The rate of mother-to-infant HCV transmission is increased among women co-infected with HIV (346--348,350,360,363--365). In an analysis comparing transmission rates from HCV-infected women with and without HIV infection, the crude rate of HCV vertical transmission was 22% for HIV-infected and 4% for HIV-uninfected women (346). In a meta-analysis of 10 studies involving 2,382 infants, the overall risk for HCV perinatal transmission was 2.8-fold higher (95% CI = 1.8--4.5) for HCV/HIV-coinfected women compared with HCV-infected women without HIV infection (365). In a subanalysis that included only infants born to HCV-viremic women, the risk for perinatal HCV infection remained two-fold higher for HCV viremic mothers who were co-infected with HIV compared with those without HIV infection (365). HCV RNA levels are higher among women co-infected with HIV than women infected with only HCV. This might, in part, account for the increased risk for mother-to-child HCV transmission from HCV/HIV co-infected women (366). Mother-to-infant transmission of HIV also might be more frequent in HIV-HCV co-infected mothers; in one study, the relative risk for HIV transmission was 1.7 times higher in HIV-HCV co-infected women than women with HIV alone (95% CI = 1.0--2.7) (367). Because HCV infection is highly associated with injection drug use, it is not known if the increased risk for HIV transmission is a direct effect of HCV or an effect of drug use, which also has been associated with the risk for perinatal HIV transmission. Co-transmission of both HIV and HCV can occur. Among children born to HIV-HCV co-infected women, HIV-infected children are more likely to be HCV-infected than children who were HIV-uninfected (350,362,363). Patterns of HCV viremia observed among HCV-infected children without HIV infection vary widely and include persistent viremia (52%), intermittent viremia (42%), and lack of detectable viremia despite persistent HCV antibody positivity (6%) (368,369). Compared with with HCV-infected adults without HIV infection, in which 2% demonstrated a loss of HCV RNA over follow-up of 3 years, spontaneous HCV clearance or transient viremia in infants has been reported in 17% of cases of mother-to-child HCV transmission (346,368). However, whether this represents true clearance of infection is unknown (370); >40% of those who become persistently HCV RNA-negative continue to demonstrate biochemical signs of hepatitis (368). Clinical Manifestations Children with HCV infection appear to have a more benign clinical course than persons who acquire the infection initially as adults, with less frequent and slower progression (371--373). In 67 children with transfusion-acquired HCV infection observed at 20 years post infection, 55% of HCV-antibody--positive children had detectable HCV RNA in their blood; all but one of these viremic children had normal alanine aminotransferase (ALT) levels, and in 17 children who had liver biopsies at a mean interval of 21 years after infection, only three had histologic evidence of progressive liver damage (374). However, longer-term follow-up of infected children is needed to determine eventual late outcomes (369). Older children and adolescents have a higher rate of fibrosis than younger children, indicating insidious progression of liver disease might occur over the course of years (373). In addition, certain children might have a more rapidly progressive course of disease (375). Histopathologic changes of chronic hepatitis can be present despite the lack of symptoms. Histologic findings vary; steatosis and portal lymphoid aggregation are most frequent findings and fibrosis, although rare, can be observed. Children typically have mild-to-moderate liver disease as determined by signs of structural alterations, inflammatory activity, and necrosis (368,373,376). No direct correlation has been determined between underlying liver disease and persistence of viremia or elevated ALT levels, so these are not good prognostic markers for liver disease. No evidence exists to indicate that children with HIV-HCV co-infection appear differently from HCV-infected children. Approximately 90% of children with vertically-acquired HCV infection are asymptomatic and appear to grow normally (368). Serum ALT levels are high during the first 2 years of life and decline substantially thereafter (368). Among children, ALT concentrations can fluctuate substantially. Among 16% of children, no abnormalities in ALT concentration are ever observed. After age 2 years, mean ALT concentrations are slightly higher among children with persistent viremia compared with those with intermittent or no viremia. However, ALT activity is not a reliable index of ongoing viral replication in HCV-infected children. Diagnosis Because of transplacental passage of maternal antibody, anti-HCV antibody positivity is only considered definitive evidence for HCV infection among infants aged >18 months (377). Among children aged >18 months and adults who are anti-HCV positive by a screening immunoassay, a recombinant immunoblot assay (RIBA) is used to confirm HCV infection. Case reports exist of HCV carriers with undetectable levels of HCV antibody in HIV co-infected children (347,364). For children aged <18 months, direct detection of HCV using a nucleic acid test for HCV RNA is required to diagnose HCV infection. HCV RNA can be detected by PCR, branched chain DNA (b-DNA) transcription mediated amplification, or other target or signal amplification techniques. Direct viral detection is required for diagnosis of infection in infants aged <18 months; however, because of passively transferred maternal HCV RNA, it should not be performed before age 1--2 months (377). Regardless of the result, the test should be repeated because positive results should be confirmed and a single negative HCV RNA result is not conclusive because HCV RNA might only be detected intermittently. HCV RNA can be measured qualitatively or quantitatively. Qualitative assays should be used for diagnosis. Quantitative assays are most useful for monitoring response to antiviral therapy. However, viral load does not necessarily correlate with the degree of histologic abnormalities on liver biopsy and therefore is not an accurate surrogate for disease severity. Liver transaminase levels are not elevated in direct proportion to the extent of liver disease and correlate only loosely with biopsy findings. Persistent elevation indicates liver inflammation and warrants further evaluation. Hepatotoxicity resulting from antiretroviral therapy or from a possible immune reconstitution syndrome after initiation of HAART also can contribute to an elevation in liver enzymes (378). Liver biopsy is the most specific test for diagnosis and assessment of hepatic pathology and is used to quantitate the amount of hepatic fibrosis present and stage the extent of disease. A liver biopsy should be strongly considered before initiating therapy for chronic hepatitis C. Treatment Hepatitis A vaccine should be administered to susceptible children aged >2 years with chronic viral hepatitis (AIII). Children with symptomatic chronic hepatitis C or histologically advanced pathologic features (bridging necrosis or active cirrhosis) should be considered for treatment (BI). Patients infected with HCV genotype 1 have a less favorable response to therapy than those infected with HCV genotypes 2 or 3 (372,379,380). In an analysis of published trials, sustained clearance of HCV RNA with interferon monotherapy was 26% in patients with HCV genotype 1 infection compared with 70% in patients with HCV genotype 2 or 3 infections (380). Quantitative HCV RNA levels are used to assess treatment response; a sustained virologic response is defined as absence of detectable HCV RNA at the end of treatment. In adults with chronic hepatitis C, HCV RNA levels usually are assessed at baseline (i.e., before therapy) and after 12 and 24 weeks of therapy. Persons with undetectable HCV RNA at completion of therapy should be retested 24 weeks after completion of therapy. In HIV-coinfected patients, certain health-care providers continue to do serial HCV RNA testing at 6-month intervals for an additional 1--2 years to exclude later virologic relapse. Among adults with HCV disease, regardless of HIV infection status, combination therapy (interferon plus ribavirin) is the preferred initial therapy because of substantially higher rates of virologic response than with interferon monotherapy (AI). On the basis of data from adults, treatment recommendations for children with HCV disease are similar (AI). Pegylated interferon-alfa requires once weekly administration and results in more sustained interferon blood levels than with standard interferon. Adult studies have demonstrated increased efficacy with once weekly subcutaneous administration of pegylated interferon-alfa-2B plus ribavirin compared with standard interferon-alfa-2b plus ribavirin (381). The combination of pegylated interferon-alfa-2b (1.5 mcg/kg body weight) or --2a (180 mcg) administered subcutaneously once weekly plus ribavirin (400 mg orally administered twice daily) is the preferred initial therapy for adults with HCV infection (382) (AI). Adults with contraindications to the use of ribavirin have been treated with pegylated interferon monotherapy, although the response rate is decreased (AII). However, data on the safety and dosing of pegylated interferon-alfa for children are not available (CIII). Interferon-alfa-2a or -2b monotherapy is the therapy that has received the most study among HCV-infected children; none of the studies specifically evaluated therapeutic response to interferon among children coinfected with HIV and HCV. In a review of 20 published studies of interferon-alfa monotherapy in HCV-infected pediatric patients without HIV infection, the average end-of-treatment response (negative HCV RNA PCR) rate was 54% (range: 0--91%) and the sustained response rate was 36% (range: 0--73%) (380). The youngest child treated in these studies was aged 2 years. Doses of interferon-alfa used in the pediatric studies have ranged from 1.75 to 5 million units (MU)/m2 (maximum dose: 3--5 MU) administered subcutaneously or intramuscularly three times weekly for 4--12 months (368,372,383--387). In one study, an induction dose of 0.1 MU/kg (maximum dose: 6 MU) was administered subcutaneously once daily for 2 weeks, followed by the same dose administered three times weekly for 22 additional weeks (384). In another study, treatment was initiated at 25%--50% of the final dose and advanced to the final dose over the first 1--1½ weeks (379). A commonly used regimen in children is 3--5 MU/m2 given subcutaneously three times a week (BII). Treatment with interferon-alfa-based therapies is contraindicated in children with decompensated liver disease, significant cytopenias, severe renal or cardiac disorders, and autoimmune disease (EII). Ribavirin oral solution has been approved for treatment of chronic hepatitis C among children aged >3 years with compensated liver disease and for use in combination with interferon alfa-2b, primarily based on data in adults (388--390) (AII). The dose of interferon-alfa-2b is 3--5 MU/m2 administered subcutaneously three times a week (388,389). Administration of ribavirin in a fixed dose by weight is recommended (59). Interferon-alfa monotherapy can be used among children with contraindications to ribavirin who cannot receive combination therapy (e.g., unstable cardiopulmonary disease, severe pre-existing anemia, or hemoglobinopathy) (BII). The ideal length of treatment for HIV-HCV coinfected children is unknown. On the basis of recommendations in HIV-uninfected adults, the duration of treatment using interferon-ribavirin combination therapy is 48 weeks for patients with HCV genotype 1 disease who demonstrate an early virologic response (a decrease of at least 2 log10 in HCV viral load as measured by quantitative HCV RNA levels) during the first 12 weeks of treatment (AI). Patients with genotype 1 disease who fail to achieve an early virologic response by week 12 can have treatment discontinued after 12 weeks because they have a limited chance of achieving a sustained virologic response, regardless of duration of therapy, and toxicity outweighs any potential benefit (BI). For patients who are not coinfected with HIV who have HCV genotype 2 or 3 disease, the recommended treatment duration is 24 weeks (BII). However, some health-care providers would treat HIV-HCV-- coinfected person with HCV genotype 2 or 3 disease with 48 weeks of combination therapy (CIII). Adverse effects of interferon-alfa in children, although frequent, are usually not severe; only 5% of children require treatment discontinuation. Toxicity is dose-related, with a higher rate of side effects with doses of 10 MU//m2 (391). The incidence of the majority of adverse effects decreases substantially during the first 4 months of therapy. Premedication with acetaminophen might reduce the incidence of side effects (BIII). The most common adverse effect of interferon-alfa is an influenza-like syndrome that can consist of fever, chills, headache, myalgia, and arthralgia, abdominal pain, nausea, and vomiting. Fever appears within 2--6 hours after interferon injection, and febrile seizures have occurred; influenza-like symptoms are most severe during the first month of treatment (391). Relapsing cases of epistaxis not associated with thrombocytopenia or prolonged prothrombin time have been reported among certain children and occurred more frequently in the first months of treatment. Certain children experience loss of appetite and a transient weight loss and impairment in height growth, which resolves after completion of therapy (392). Transient mild alopecia, usually first occurring after 2--3 months of therapy, also has been reported. Subtle personality changes have been reported in 42% of children; they resolve when therapy is discontinued (393). Neutropenia, which resolves upon discontinuation of therapy, is the most common laboratory abnormality; anemia and thrombocytopenia are less common. Certain children have experienced antinuclear autoantibodies. Periodic monitoring of a complete blood count is recommended among children receiving interferon-alfa therapy. Abnormalities in thyroid function (hypo- or hyper-thyroidism) have been reported with interferon-alfa therapy; periodic monitoring of TSH is recommended. Interferon should be permanently discontinued if a life-threatening toxicity occurs (AII). For severe but nonlife-threatening reactions, the drug can be temporarily discontinued and reinstated when the reaction has resolved in a stepwise fashion beginning with a maximum of 50% of the last administered dose; for moderate reactions, the dose can be reduced by 50% and then increased stepwise by 0.5 or 1 MU/m2 up to the full dose after the adverse effect has resolved (BIII). Side effects of ribavirin include those observed with interferon alone (e.g., influenza-like syndrome and neutropenia); adverse effects more specific to ribavirin include hemolytic anemia and lymphopenia. Depression and suicidal ideation have also been observed with this combination. The hemolytic anemia that occurs with the use of ribavirin is dose-dependent, might cause a substantial decrease in hemoglobin, and usually occurs within 1--2 weeks of therapy initiation. Therefore, hemoglobin or hemocrit should be obtained pretreatment and at week 2 and week 4 of therapy, or more frequently if clinically indicated. Ribavirin inhibits intracellular phosphorylation of pyrimidine nucleoside analogues (zidovudine, stavudine, zalcitabine) in vitro, but the clinical significance of this interaction in vivo is unclear. Ribavirin enhances intracellular phosphorylation of didanosine; case reports have indicated a potential increased risk for pancreatitis and mitochondrial toxicity with concomitant use, and this combination should be used with caution. Hepatitis BEpidemiology An important mode of hepatitis B virus (HBV) acquisition by children is perinatal or mother-to-infant transmission. All pregnant women, including HIV-infected women, should be tested for hepatitis B surface antigen (HBsAg) during an early prenatal visit in each pregnancy. Testing should be repeated in late pregnancy for HBsAg-negative women at high risk for HBV infection (e.g., injection-drug users, those with intercurrent sexually transmitted diseases, and those with multiple sexual partners). Whether HIV-HBV coinfected women are more likely to transmit HBV to their infants than women with HBV who are not infected with HIV is unknown. All infants born to HIV-HBV coinfected women should receive hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth, the second dose of vaccine at age 1--2 months, and the third dose at age 6 months. Postvaccination testing for antibody to HBsAg (anti-HBs) and HBsAg should be performed at age 9--15 months, and infants determined to be anti-HBs and HBsAg negative should be revaccinated (59). HIV-infected children also might be at risk for HBV infection through exposure to HBV-infected household contacts. Horizontal transmission might occur secondary to frequent interpersonal contact of nonintact skin or mucus membranes to blood or body fluids that contain blood (e.g., saliva) from sharing inanimate objects such as toothbrushes. All infants and previously unvaccinated children should receive the 3-dose hepatitis B vaccine as part of the recommended childhood vaccination schedule. HIV-infected adolescents are at increased risk for HBV infection through sexual activity or injection-drug use. In a study of HIV-infected youth at 43 Pediatric AIDS Clinical Trials Group centers, 19% were determined to be co-infected with HBV; the rate of HBV infection in HIV-infected females was twice the U.S. population-based rates, and for males, approximately seven times higher (394). Similarly, in a study of youth in Toronto, Canada, 16% were determined to be HIV-HBV co-infected (395). Risk for HIV-HBV coinfection was related to substance abuse and sexual activity, particularly among men who have sex with men. All HBV-susceptible adolescents should be vaccinated against hepatitis B. Risk for experiencing chronic HBV infection after acute infection in children without HIV infection is related inversely with age at the time of infection. In children without HIV infection, chronic HBV infection develops in up to 90% of infants, 30% of children aged 1--5 years, and 6% of older children and adolescents who become infected with HBV. In HIV-HBV co-infected adults, clearance rates of HBsAg and HBeAg are decreased compared with HBV-infected adults without HIV infection, and the risk for chronic HBV infection and cirrhosis is increased when HIV infection preceded the HBV infection (396--398); whether this is true for HIV-infected children is unknown. Chronic HBV infection can lead to chronic hepatitis, cirrhosis, and primary hepatocellular carcinoma. Among persons without HIV infection who are infected with HBV at birth, the lifetime risk for hepatocellular carcinoma is 50% for men and 20% for women (399); whether this risk is higher in HIV-HBV coinfected persons is unknown. Humoral response to hepatitis B vaccination is reduced in children with HIV infection. In multiple studies, approximately 25%--35% of HIV-infected children vaccinated with hepatitis B vaccine have protective antibody titers (400,401). Younger children and those with higher CD4+ cell counts are more likely to respond to vaccination than older, symptomatic, and immunodeficient HIV-infected children. Booster doses might increase response rates (402). HIV-infected infants, children, and adolescents should be tested for anti-HBs 1--2 months after completing the vaccination series, and if anti-HBs negative, revaccinated (59). Clinical Manifestations The majority of HBV infections in children are asymptomatic. Children with HIV-HBV co-infection might have a mild acute illness followed by a smoldering, persistent chronic infection. Symptoms of lethargy, malaise, fatigue, and anorexia can occur. Jaundice might be present and, less commonly, hepatomegaly and splenomegaly. Young children might experience a serum sickness-like prodrome marked by symmetrical arthropathy and skin lesions. Gianotti-Crosti (papular acrodermatitis), urticarial, or purpuric lesions can occur. Extrahepatic conditions associated with circulating immune complexes that have been reported with children with HBV infection include aplastic anemia, polyarteritis nodosa, and glomerulonephritis. Diagnosis HBsAg is the first marker detectable in serum; it precedes the elevation of serum aminotransferases and the onset of symptoms. Antibody to hepatitis B core antigen (anti-HBc) appears 2 weeks after HBsAg and persists for life. Passively transferred maternal anti-HBc can be detectable in the infant up to age 12 months. IgM anti-HBc is highly specific for acute infection but might not be observed in perinatally acquired infection. In self-limited infections, HBsAg is eliminated in 1--2 months, and anti-HBs develop during convalescence. Anti-HBs indicate immunity from HBV infection. After recovery from natural infection, both anti-HBs and anti-HBc are usually present; only anti-HBs develop in response to the hepatitis B vaccine. In persons who become chronically infected (i.e., persistently positive for HBsAg [and anti-HBc] beyond 24 weeks), anti-HBs is not detectable. Hepatitis B e antigen (HBeAg) correlates with viral replication, DNA polymerase activity, increased infectivity, and increased severity of liver disease. With clearance of HBeAg, antibody to HBeAg might be detectable. HBV DNA can be detected in serum and peripheral blood mononuclear cells by PCR or branched chain DNA amplification. Quantitative DNA assays can be helpful in evaluating response to therapy. Treatment Hepatitis A vaccine should be administered to susceptible children aged >2 years with chronic viral hepatitis (AIII). Early treatment, if started before integration of viral DNA in the majority of host hepatocytes nuclear DNA, might provide improved long-term outcome; however, whether treatment of acute HBV infection offers additional benefit over treatment after infection is known to be chronic is not known and requires further study (403,404). Indications for treatment of chronic HBV infection in HIV-coinfected children are the same as in HBV-infected children without HIV infection and include 1) evidence of ongoing viral replication, as indicated by the presence of detectable serum HBV DNA, with or without HBeAg positivity, for at least 6 months; 2) persistent elevation of serum transaminases (at least twice the upper limit of normal); and 3) evidence of chronic hepatitis on liver biopsy (BII). Patients without necroinflammation usually do not warrant antiviral therapy. The correlates of successful therapy are not well defined, but markers of improvement would include improved liver histology on biopsy, normalization of hepatic transaminases, substantial decrease in HBV viral load (HBV DNA levels), and loss of e antigen with development of e antibody in patients who are HBeAg positive (398). Although a decline in viral load correlates with response, no target HBV DNA level has been established as representing a successful virologic response. Monitoring for virologic response of therapy should include regular determination of serum levels of HBV DNA, HBsAg, HBeAg, anti-HBe antibody, and serum transaminases (AIII). Three therapies have been approved for chronic hepatitis B in adults: interferon-alfa, lamivudine (3TC), and adefovir. Interferon-alfa and 3TC are also approved for treatment of chronic hepatitis B in children. For treatment of chronic hepatitis B in HIV-HBV coinfected adults, some specialists recommend that interferon-alfa is the therapy of choice in persons who do not yet require antiretroviral therapy for HIV infection to preserve use of 3TC and tenofovir for later treatment of HIV infection (CIII). For HIV-HBV coinfected adults who are antiretroviral-naïve and require both HBV and HIV treatment, 3TC is considered by some specialists to be the therapy of choice for HBV, administered in HIV- suppressive doses and in combination with other antiretroviral drugs for treatment of HIV infection (BIII). Considerations would be similar for HIV-HBV co-infected children. Interferon-alfa-2a or -2b is the therapy that has received the most study in HBV-infected children and is recommended for the treatment of chronic hepatitis B with compensated liver disease in patients aged >2 years who warrant treatment (BII). Interferon-alfa therapy is contraindicated for children with decompensated liver disease, substantial cytopenias, severe renal or cardiac disorders, and autoimmune disease (405) (EII). None of the clinical studies of interferon-alfa therapy of chronic hepatitis B have specifically studied children with HIV-HBV co-infection. Certain studies of interferon-alfa therapy in HBV-HIV co-infected adults indicate that response to therapy might be less than in adults not infected by HIV (398). In a review of six randomized clinical trials in 240 HBV-infected children aged >1.5 years, interferon-alfa therapy resulted in HBV DNA clearance in 35% of treated children, HBeAg clearance in 10%, and normalization of serum transaminases in 39% at the end of therapy (406). Six to 18 months after therapy discontinuation, 29% of children had persistent HBV DNA clearance, and 23% HBeAg clearance. Interferon alfa-2a or --2b doses ranged from 3 MU/m2 to 10 MU/m2 administered subcutaneously three times weekly for 3--12 months. A commonly used regimen in children is 5 MU/m2 three times weekly for 6 months (393,406) (BII). In adults with chronic hepatitis who are HBeAg-negative, longer therapy (minimum: 12 months) is recommended because response to interferon therapy is lower than in HBeAg-positive patients (BIII). More prolonged interferon-alfa therapy is associated with better virologic response in children (405,407,408). Long-term response is also better in children with higher (approximately twice the upper limit of normal) serum transaminase levels and lower HBV DNA at baseline. Studies have indicated improved virologic response with higher dose (i.e., 10 MU/m2 three times weekly) interferon therapy as initial therapy or for retreatment of children who have failed before lower dose interferon therapy (409,410) (CII). Data from pediatric trials that initiated interferon therapy with prednisone priming had results comparable to those observed with interferon alone; therefore, prednisone administration is not recommended (411--413) (DII). Adverse effects of interferon-alfa in children, while frequent, are usually not severe; approximately 5% of children require treatment discontinuation. Toxicity is dose-related with a higher rate of side effects with doses of 10 MU/m2 (391). Incidence of the majority of adverse effects decreases substantially dduring the first 4 months of therapy. Premedication with acetaminophen might reduce the incidence of side effects. (BIII) The most common adverse effect of interferon-alfa is an influenza-like syndrome that can consist of fever, chills, headache, myalgia, arthralgia, abdominal pain, nausea, and vomiting. Fever usually appears within 2--6 hours after interferon injection, and rarely febrile seizures have occurred; the influenza-like symptoms are most severe during the first month of treatment (391). Relapsing cases of epistaxis (not associated with thrombocytopenia or prolonged prothrombin time) have been reported in certain children and occurred more frequently in the first months of treatment. Certain children experience loss of appetite and a transient weight loss and impairment in height growth, which resolves after completion of therapy (392). Transient mild alopecia, usually first occurring after 2--3 months of therapy, also has been reported. Subtle personality changes have been reported in 42% of children that resolve when therapy is discontinued (393). Neutropenia, which resolves after discontinuation of therapy, is the most common laboratory abnormality; anemia and thrombocytopenia are less common. Certain children have experienced antinuclear autoantibodies. Periodic monitoring of a complete blood count is recommended in children receiving interferon-alfa therapy. Abnormalities in thyroid function (hypo- or hyper-thyroidism) have been reported with interferon-alfa therapy; periodic monitoring of TSH is recommended. Interferon should be permanently discontinued if a life-threatening toxicity occurs (AII). For severe but nonlife-threatening reactions, the drug can be temporarily discontinued, and after the reaction has resolved, treatment can be reinstated in a stepwise fashion, beginning with a maximum of 50% of the last administered dose. For moderate reactions, the dose can be reduced by 50% and then increased stepwise by 0.5 or 1 MU/m2 up to the full dose after the adverse effect has resolved (BIII). Pegylated interferon alfa, which can be administered once weekly, is being studied in adults with HIV-HBV coinfection. The drug has been studied in a limited number of HIV-infected children for treatment of HIV, but data are not yet available (CIII). For children who have not responded to interferon-alfa, treatment with interferon-beta (5 MU/m2 intramuscularly three times a week for 6 months), which shares common biologic functions with interferon-alfa but is antigenically different, can be considered (CIII). In a limited number of children, therapy was well tolerated with a low-grade fever the most common side effect. At 18 months after completion of therapy, 45% of children were HBV DNA negative, 32% became anti-HBe antibody positive, and 50% normalized serum transaminase levels. Liver histopathology had substantial improvement in those children who responded to therapy (413,414). 3TC is approved for children and adults for the treatment of compensated chronic hepatitis B associated with evidence of HBV replication and active liver inflammation and would be the preferred therapy (as part of a fully suppressive HAART regimen) for chronic hepatitis B in HIV-infected children who require HIV therapy (BIII). 3TC treatment results in a rapid decline in HBV DNA levels and is well tolerated in HBV-infected children who are not HIV-infected, although persistent virologic response rates with 3TC monotherapy are low (415,416). In a study of children with HBV infection who were not HIV coinfected, 23% of 191 children who received 52 weeks of treatment with 3TC had a virologic response (i.e., the absence of HBe antigen and serum HBV DNA) compared with 13% of 91 who received placebo (417). 3TC has been used both as primary therapy and as secondary therapy for children without HIV infection who have not responded to interferon-alfa treatment (415--418). Reports of clinical and laboratory exacerbations of hepatitis after discontinuation of 3TC treatment have occurred among children with HBV infection who are not infected with HIV. The optimal duration of therapy is not known. Extended treatment with 3TC can lead to the development of 3TC-resistant HBV, with base pair substitutions at the YMDD locus of DNA polymerase. In one pediatric study, 19% of HBV-infected patients treated with 3TC for 1 year had emergence of the YMDD HBV-variant; a more recent study reported mutant variants in 65% of 3TC treated children unresponsive to interferon (419), compared with 16%--32% in HBV-infected adults treated with 3TC for 1 year, and 49% after 3 years (418,420). However, the emergence of variants containing the YMDD motif mutation did not prevent HBeAg seroconversion or result in substantial worsening of liver histology (420). 3TC resistance should be suspected if HBV replication (as measured by HBV DNA levels) increases or recurs while receiving treatment. Among children with HIV-HBV coinfection, 3TC should not be administered as monotherapy because resistance of HIV to 3TC develops (EI). The dose of 3TC approved to treat HBV infection (3 mg/kg body weight once daily) is lower than that required to treat HIV (4 mg/kg twice daily, maximum dose 150 mg twice daily). If 3TC is administered to HIV-HBV co-infected children at the lower dose, the resulting subtherapeutic blood levels of 3TC will result in the development of 3TC-resistant HIV; emergence of the M184V 3TC resistance mutation is observed after only 1--2 weeks of single drug therapy. In contrast, the dose of 3TC used to threat HIV as part of combination antiretroviral therapy is adequate to treat HBV. Thus, among HIV-HBV coinfected children, if 3TC is used to treat chronic hepatitis B, 3TC should be administered at the dose of 4 mg/kg twice daily in the context of a potent combination antiretroviral regimen (BIII). To reduce the development of resistance, some specialists in adult HIV infection recommend use of adefovir or tenofovir in addition to 3TC as part of a fully suppressive HAART regimen among HIV-infected adults who require treatment for both HIV and chronic hepatitis B, although data to support this approach are limited (CIII). No data are available on appropriate dosing and safety of adefovir or tenofovir in children. Because clinical and laboratory exacerbations of hepatitis might occur if 3TC is discontinued among children who are responding to therapy, after anti-HBV-HIV therapy has begun, it should be continued unless contraindicated or until the child has been treated for >6 months after HBeAg seroconversion and can be closely monitored on discontinuation (BIII). If 3TC is discontinued, careful monitoring of serum transaminases and HBV DNA is important. Combination with 3TC and interferon-alfa has not been demonstrated to be more effective than treatment with interferon alone in adult patients. Combined treatment with 3TC and interferon-alfa (10 MU/m² administered subcutaneously three times weekly) administered for 6 to 12 months has been studied in 57 HBV-infected children aged >2 years (421). Sustained complete responses (clearance of HBeAg, HBeAb seroconversion, and normalization of serum transaminases) was observed 6 months after completion of therapy in 20% of those receiving 6 months and 37% of those receiving 12 months of therapy. However, use of this combination in pediatric patients is not recommended until more data are available (DII). Adefovir dipivoxil (10 mg once daily) is a nucleotide analogue drug active against HBV. Adefovir at a dose of 10 mg daily, although active against HBV, has minimal anti-HIV activity, and HIV resistance has not been observed to develop in patients receiving adefovir at this dose for 48 weeks (422). Adefovir is now FDA approved for adults who require treatment for chronic hepatitis B but do not yet require treatment for their HIV infection (CIII). Adefovir can cause renal tubular disease when administered in high dosage, but this is less common at the 10 mg/day dose for treatment of chronic hepatitis B (398). Safety and effectiveness of adefovir in pediatric patients has not yet been established. Tenofovir, a nucleotide analogue similar to adefovir, has in vitro activity against HBV, but data is limited in subjects with HIV-HBV coinfection. It has been administered in certain adult patients at a dose used for treatment of HIV (tenofovir adminstered as 300 mg once daily). Similar to 3TC, tenofovir, when used for treatment of chronic hepatitis B among HIV-infected patients, should be administered as part of a fully suppressive HAART regimen and not as monotherapy (CIII). No data are available on safety and appropriate dosing of tenofovir among children. References
Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children Members who assisted in developing this report: Elaine Abrams, M.D., Harlem Hospital Center, New York, New York; Michael Brady, M.D., Columbus Children's Hospital, Columbus, Ohio; Carolyn Burr, Ed.D., Francois-Xavier Bagnoud Center, University of Medicine and Dentistry of New Jersey, Newark, New Jersey; Joseph Cervia, M.D., Long Island Jewish Medical Center, New Hyde Park, New York; Diana Clarke, Pharm.D., Boston Medical Center, Boston, Massachusetts; Marilyn Crain, M.D., University of Alabama at Birmingham, Birmingham, Alabama; Barry Dashefsky, M.D., Francois-Xavier Bagnoud Center, University of Medicine and Dentistry of New Jersey, Newark, New Jersey; Brian Feit, M.D., Health Resources and Services Administration, Rockville, Maryland; Mary Glenn Fowler, M.D., CDC, Atlanta, Georgia; Samuel Grubman, M.D., St. Vincent's Hospital and Medical Center of New York, New York; Peter Havens, M.D., Children's Hospital of Wisconsin, Milwaukee, Wisconsin; Nancy Hutton, M.D., Johns Hopkins School of Medicine, Baltimore, Maryland; George Johnson, M.D., Medical University of South Carolina, Charleston, South Carolina; Andrea Kovacs, M.D., LAC USC Medical Center, Los Angeles, California; Linda Lewis, M.D., Food and Drug Administration, Rockville, Maryland; Kathleen McGann, M.D. , Washington University School of Medicine, St. Louis, Missouri; James McNamara, M.D., National Institutes of Health, Bethesda, Maryland;. George McSherry, M.D., University of Medicine and Dentistry of New Jersey, Newark, New Jersey; Mark Mintz, M.D., Bancroft NeuroHealth, Cherry Hill, New Jersey; Charles Mitchell, M.D., University of Miami School of Medicine, Miami, Florida; Lynne M. Mofenson, M.D., National Institutes of Health, Bethesda, Maryland;. John Moye, Jr., M.D., National Institutes of Health, Bethesda, Maryland;. Sharon Nachman, M.D., State University of New York Health Science Center at Stony Brook, New York, New York; James Oleske, M.D., University of Medicine and Dentistry of New Jersey, Newark, New Jersey; Savita Pahwa, M.D., North Shore University Hospital, Manhasset, New York; Paul Palumbo, M.D., University of Medicine and Dentistry of New Jersey, Newark, New Jersey; Mary Paul, M.D., Baylor College of Medicine, Houston, Texas; David Pugatch, M.D., Hasbro Children's Hospital, Providence, Rhode Island; Tamara Rakusan, M.D., Children's Hospital, Washington, DC. Pamela Rothplez-Puglia, M.D., University of Medicine and Dentistry of New Jersey, Newark, New Jersey; Gwendolyn Scott, M.D., University of Miami School of Medicine, Miami, Florida; Leslie Serchuck, M.D., National Institutes of Health, Bethesda, Maryland;. William Shearer, M.D., Baylor College of Medicine, Houston, Texas; Stephen Spector, M.D., University of California at San Diego, La Jolla, California; Deborah Storm, Ph.D., University of Medicine and Dentistry of New Jersey, Newark, New Jersey; Russell Van Dyke, M.D., Tulane University School of Medicine, New Orleans, Loisiana; Geoffrey Weinberg, M.D., University of Rochester School of Medicine, Rochester, New York; Harland Winter, M.D., Massachusetts General Hospital, Boston, Massachusetts; Andrew Wiznia, M.D., Jacobi Medical Center, Bronx, New York; and Ram Yogev, M.D., Children's Memorial Hospital, Chicago, Illinois. Box 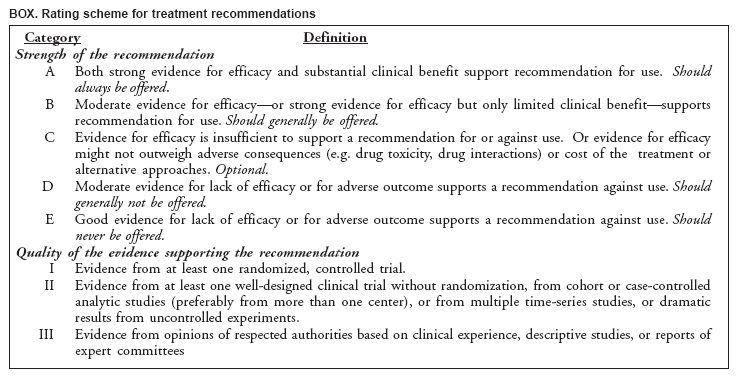 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 11/23/2004 |
|||||||||
This page last reviewed 11/23/2004
|