 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Enterovirus Surveillance --- United States, 1970--2005Nino Khetsuriani, MD, Ashley LaMonte-Fowlkes, MPH, M. Steven Oberste, PhD, Mark A. Pallansch, PhD
Corresponding author: Nino Khetsuriani, MD, PhD, National Center for Immunization and Respiratory Diseases (proposed), CDC, 1600 Clifton Road, NE, MS A-34, Atlanta, GA 30333. Telephone: 404-639-0863; Fax: 404-639-1307; E-mail: [email protected]. AbstractProblem: Enteroviruses are common human viruses associated with various clinical syndromes, from minor febrile illness to severe, potentially fatal conditions (e.g., aseptic meningitis, paralysis, myocarditis, and neonatal enteroviral sepsis). Multiple enterovirus serotypes exist. Individual serotypes have different temporal patterns of circulation and often are associated with different clinical manifestations. Changes in circulating serotypes might be accompanied by large-scale outbreaks. Reporting Period Covered: 1970--2005. Description of Surveillance System: The National Enterovirus Surveillance System (NESS) is a voluntary, passive surveillance system that has monitored trends in circulating enteroviruses since 1961. Enterovirus detections by serotype with specimen type, collection date, and demographic information are reported monthly by participating laboratories to CDC, which summarizes the data and disseminates the results. For this analysis, the available data set for 1970--1982 included only information on serotype and state for each report; complete records were available for 1983--2005. Results: During 1970--2005, a total of 52,812 enterovirus detections were reported to NESS (29,772 of them during 1983--2005). Laboratory participation and the numbers of reports declined throughout the 1990s, but they increased again after 2000. The 15 most commonly reported enteroviruses accounted for 83.5% of reports with known serotype, and the five most commonly reported serotypes (echoviruses [E] 9, 11, 30, and 6, and coxsackievirus B5) accounted for 48.1%. Predominant serotypes and ranking of individual enteroviruses changed over time. Long-term circulation patterns for individual serotypes varied but were consistent with epidemic (e.g., E9, E13, E30, and coxsackievirus B5) or endemic patterns (e.g., coxsackieviruses A9, B2, B4, and enterovirus 71). Children aged <1 year accounted for 44.2% of reports with known age. Male predominance was present among patients aged <20 years, but not among those aged >20 years (male/female ratio: 1.4 and 0.9, respectively). Enterovirus detections had prominent summer-fall seasonality, with June--October accounting for 77.9% of reports with known month of specimen collection. Cerebrospinal fluid was the most common specimen type, followed by respiratory and fecal specimens (49.8%, 26.9%, and 13.6%, respectively). Death was reported for 3.3% of detections with known outcome. Infections with coxsackievirus B4 (odds ratio [OR] = 3.3; 95% confidence interval [CI] = 1.7--6.0), and human parechovirus 1 (formerly E22) (OR = 3.7; CI = 1.7--7.6) were associated with higher risk for death, and infections with E9 were associated with lower risk for death (OR = 0.1; CI = 0--0.4) than infections with other enteroviruses. Interpretation: NESS data allowed identification and description of a core group of consistently circulating enteroviruses that probably determine the disease burden associated with enterovirus infections. These data also are helpful in guiding outbreak investigations and identifying targets for development of diagnostic assays and antivirals. Efforts to update the reporting system initiated in the early 2000s (i.e., simplification of reporting forms and transition to electronic reporting) resulted in a substantial increase in reporting compared with the late 1990s. Public Health Action: Efforts to increase laboratory participation in NESS should continue to allow for more complete and accurate surveillance for enteroviruses. Further improvement in the timeliness of feedback through the development of a NESS website to allow access to historic data and to the information on circulating serotypes can provide additional incentives to public health laboratories to participate in NESS. Introduction Enteroviruses (genus Enterovirus, family Picornaviridae) are among the most common viruses infecting humans worldwide. Enteroviruses are associated with diverse clinical syndromes ranging from minor febrile illness to severe, potentially fatal conditions (e.g., aseptic meningitis, encephalitis, paralysis, myocarditis, and neonatal enteroviral sepsis) and could be linked with the development of some chronic diseases (e.g., type 1 diabetes and dilated cardiomyopathy) (1,2). Each year, an estimated 10--15 million symptomatic enterovirus infections occur in the United States (3). Enteroviruses are small (approximately 30 nm), nonenveloped, single-stranded RNA viruses with an icosahedral capsid composed of 60 subunits consisting of four structural proteins (VP1 to VP4). Enterovirus RNA is approximately 7.5 kb long and encodes a polyprotein that is processed to yield the mature structural and nonstructural proteins. The coding region is bounded by nontranslated regions at the 5' and 3' ends (1). Serotypes of human enteroviruses have traditionally been classified into echoviruses, coxsackieviruses group A and B, and polioviruses. This traditional taxonomy was based on the associated disease in humans and animal model systems, sometimes resulting in overlaps between groups and difficulties with classification. As a result, beginning in the 1960s, newly discovered enteroviruses receive a numeric designation (e.g., enterovirus 71) instead of being assigned to one of the traditional groups (1,4). Current taxonomy (4) takes into account molecular and biologic characteristics and divides human enteroviruses into four species (human enterovirus [HEV] A, B, C, and D) but keeps traditional names for individual serotypes. Sixty-eight serotypes are included in the International Committee on Taxonomy of Viruses classification (Table 1). The distribution of enteroviruses by species only partially corresponds to the groups in the traditional classification. Because molecular techniques of enterovirus typing are becoming increasingly available, new enteroviruses continue to be identified, and enteroviruses 79--101 have been recently described (5--13; CDC, unpublished data, 2005). Echoviruses 22 and 23 have been reclassified as a new genus (Parechovirus) in Picornaviridae and are termed human parechoviruses 1 and 2, respectively (4,14). Although they belong to genetically and biologically distinct genera, human parechoviruses and human enteroviruses share many epidemiologic and clinical characteristics (4). The National Enterovirus Surveillance System (NESS), initiated in 1961 and known during its early years as the Enterovirus Surveillance Program (15), is the only data source for nationwide trends in enterovirus infections in the United States. Serotype-based enterovirus surveillance in the United States has five objectives: 1) to help public health practitioners determine long-term patterns of circulation for individual enteroviruses; 2) to help interpret trends in enteroviral diseases (e.g., aseptic meningitis) by associating them with circulating serotypes, which can be helpful for studying the association of enteroviruses with clinical manifestations (e.g., chronic diseases such as diabetes); 3) to guide outbreak investigations by enabling linkage of disease clusters with individual serotypes; 4) to help guide development of new diagnostic tests and therapies; and 5) to monitor poliovirus detections, thereby supplementing poliomyelitis surveillance in the United States. Enterovirus infections, with the exception of paralytic polio, are not nationally reportable in the United States. Therefore, NESS is a voluntary, passive surveillance system. State public health laboratories and certain other diagnostic laboratories willing to participate report monthly all enterovirus detections in their laboratories to CDC, which summarizes the data and disseminates the results. The surveillance is serotype-specific (i.e., requires reporting of individual serotypes). In the original system, which operated during 1961--1968, only total numbers of isolates for each serotype were reported. Beginning in 1969, the unit of reporting changed from the isolate to the patient from whom the virus was isolated, and the states began to report the age, sex, and clinical information for individual source patients (15). By the 1990s, reporting of clinical information had become incomplete as laboratory access to details of patient information became more limited. As a result, in 2002, the NESS reporting form was revised to include only basic demographic data (e.g., age, sex, and state), date of specimen collection, and specimen type. Laboratory methods used for enterovirus identification have changed substantially over time. Initially, enteroviruses were detected exclusively by viral culture (in vitro or in suckling mice) and identified by neutralization reaction using intersecting pools of type-specific antisera (1). Immunofluorescent assays using monoclonal antibodies became available for some enteroviruses in the 1980s (2). In the 1990s, new molecular methods of enterovirus detection and identification were introduced. Beginning in the mid-1990s, the panenterovirus-polymerase chain reaction (PCR) assay, which detects all enteroviruses but does not allow their differentiation, became increasingly available and supplanted viral culture in many diagnostic laboratories (16--19). The molecular method for enterovirus typing based on sequence of the VP1 gene (which encodes important type-specific epitopes and, therefore, correlates with serotype) was developed in the late 1990s and is being used by increasing numbers of laboratories (6--13). To evaluate the impact of the changes in laboratory methods of enterovirus detection on enterovirus surveillance, beginning in 2001, reporting laboratories were asked to provide information about the methods used for enterovirus detection and typing. MethodsThis report presents a descriptive analysis of nonpolio enterovirus infections reported to NESS during 1970--2005 through passive surveillance. Polioviruses, which are rarely reported to NESS because of the eradication of indigenous wild polioviruses in the Americas (20) and cessation of oral poliovaccine use in 2000 (21), are excluded from this analysis. Parechoviruses are included because they continue to be reported to NESS even after their reclassification. References to "enteroviruses" as a group throughout the report include parechoviruses. The available computerized data set for 1970--1982 contained the information on enterovirus serotypes and the reporting state only. The analysis of outcome is limited to 1983--1998 because none of the participating laboratories reported outcome after 1998, probably because of the lack of access to this information. Therefore, the analysis of overall trends includes the entire study period, but the descriptive epidemiologic analysis is limited to 1983--2005, the period for which complete information is available. The numbers presented might be slightly different from the previously reported data because, for this analysis, the identified duplicate reports have been deleted and the records with obvious errors (e.g., use of nonexisting codes for serotype designation) were recoded as "unknown." Analyses were performed using the data available from laboratory reports, which inherently reflect the testing practices of the individual laboratories. Therefore, these data are not representative of population rates nor are they strictly comparable between laboratories. A descriptive analysis was conducted of reported enterovirus isolations for the entire 36-year period and for three 10-year (1970--1979, 1980--1989, and 1990--1999) and one 6-year (2000--2005) period by month of specimen collection; specimen type; patient age, sex, and outcome; and assessed trends in the number of reporting states and the impact of changing laboratory practices. In addition, long-term temporal trends were analyzed to identify patterns of circulation for individual serotypes. Fifteen enteroviruses most commonly reported during the study period and certain less-common viruses of particular interest because of their virologic or epidemiologic characteristics or disease associations (coxsackieviruses A16 and 24, echovirus 13, enteroviruses 68 and 71, and human parechoviruses 1 and 2) are described individually. ResultsDuring 1970--2005, a total of 52,812 nonpolio enterovirus detections were reported to CDC; of these, 29,772 detections were reported during 1983--2005. On average, 1,467 reports were submitted annually. The annual number of reports varied widely, from 388 in 1999 to 3,460 in 1972. The average annual number of reported detections was highest in the 1980s (1,814; range: 1,224--2,221) and lowest during the 1990s (1,012; range: 343--2,072). The annual number of states and territories with enterovirus reports also varied substantially, from 11 in 1999 to 47 in 2004 (Figure 1). The number of reporting laboratories increased from a low of 11 in 1999, to 24 and 27 during 2000 and 2001, but then declined to 18--19 during subsequent years. In 2001, three laboratories used genomic methods for enterovirus identification and typing. By 2005, this number had increased to seven. Enterovirus detections had remarkable seasonality. Cases increased sharply during summer and fall months with a peak in August (5,601 [22.3%] of 25,076 reports with known month during 1983--2005) (Figure 2). For 19,531 (77.9%) reports, specimens were collected during June--October. This summer-fall seasonality was more prominent for enterovirus detections from cerebrospinal fluid (CSF) specimens (81.3% [7,787 of 9,583] reports during June--October) than for reports with fecal (77.6% [2,043 of 2,633 reports]) (Chi-square: p<10-4) or respiratory specimens (69.8% [3,684 of 5,275 reports]) (Chi-square: p<10-6) as the source of detection. Age was indicated for 25,114 (84.4%) cases. Infants aged <1 year accounted for 11,095 cases (44.2% of all reports with known age), children aged 1--4 years for 3,763 cases (15.0%), aged 5--9 years for 2,911 cases (11.6%), and aged 10--19 years for 2,996 cases (11.9%) cases. In 4,349 (17.3%) cases, enteroviruses were detected from persons aged >20 years. Sex was reported in 26,812 cases (90.1% of all reports during 1983--2005). Males accounted for 15,294 reports (57.0% of reports with known sex), and females accounted for 11,518 (43.0%) reports (male/female ratio: 1.3). When stratified by age, the male predominance was present only among persons aged <20 years (male/female ratio: 1.4). The male/female ratio among persons aged >20 years was 0.9. Specimen type was known for 22,579 (75.8%) of 29,772 reports during 1983--2005. CSF was the most common specimen, with 11,248 reports (49.8% of reports with known specimen type), followed by respiratory specimens (6,068 [26.9%]) and stool/rectal swab (3,078 [13.6%]). Other specimen types accounted for 2,185 (9.7%) reports. Outcome was reported for 3,932 (15.9%) of 24,654 cases during 1983--1998 and was not reported after 1998. During this reporting period, 131 (3.3%) patients died. Of the 115 deaths with known age, 77 (67.0%) occurred among children aged <1 year. The proportion of reports with fatal outcome had a bimodal distribution by age, with the peaks for ages <1 year and >45 years (Figure 3). Enterovirus serotype was specified for 49,637 (94.0%) reports. The proportion of reports with unknown serotype ranged from very low (0.2% in 1974) to very high (21.1% in 2002). The proportion of reports with unknown serotype increased substantially during 1997--2002 (range: 15.0%--21.1%) but declined again after 2002 (Figure 1). During 1970--2005, a total of 56 enterovirus serotypes and two parechoviruses were reported (Table 2). Of these, the 15 most commonly reported serotypes accounted for 83.5% of all reports. The five most common serotypes (echoviruses 9, 11, 30, and 6, and coxsackievirus B5) accounted for approximately half of all reports (48.1%). The most commonly reported serotypes changed over time, as did the ranking of individual serotypes (Table 2). Some serotypes appeared consistently among those most commonly reported, and others were reported frequently for a period of time but were less common at other times. Thirty-six serotypes appeared among the 15 most commonly reported enteroviruses for at least 1 year during the study period, 26 serotypes appeared among the top five, and 11 serotypes were reported as the most common enterovirus for a given year. Along with the five most common enteroviruses, the 11 serotypes include coxsackieviruses A9 and B2, and echoviruses 3, 7, 13, and 16. Long-term trends of circulation of individual enterovirus serotypes were consistent with two major patterns: epidemic and endemic. Serotypes with an epidemic pattern of circulation were characterized by substantial fluctuations in circulation levels over time, including large peaks when the serotype was among the most prevalent enteroviruses reported for a given year. Serotypes with an endemic pattern had stable and usually low-levels of circulation with few distinct peaks. The results of the serotype-specific descriptive analysis by sex, age group, specimen type and collection month, and outcome indicate similarities and patterns characteristic of individual enteroviruses (Table 3). Individual SerotypesEchovirus 9 Increases in echovirus 9 activity often are accompanied by widespread, large-scale outbreaks (22--27). In the United States, peaks in nationwide hospitalization rates for aseptic meningitis have been observed for years when echovirus 9 was predominant (28). The most commonly reported clinical syndrome associated with echovirus 9 infection is aseptic meningitis, but paralysis, encephalitis, and severe infections of neonates and immunodeficient persons also have been reported (29--32). Echovirus 9 was the most commonly reported enterovirus in the United States during 1970--2005, accounting for 11.8% of reports with known serotype (Table 2). Echovirus 9 has a distinct epidemic pattern of circulation, with regular sharp increases in reports every 3--5 years (Figure 4). The virus consistently appeared among the most commonly reported serotypes (Table 2) and was the most commonly identified enterovirus six times (1975, 1978, 1984, 1988, 1995, and 2003). Most often, echovirus 9 detections were reported among persons aged 5--19 years. The source of detection in two thirds of reports was CSF. Fatal outcome was rarely reported (0.2%), and persons infected with echovirus 9 were at a significantly lower risk for death than persons infected with other serotypes (odds ratio [OR] = 0.1; 95% confidence interval [CI] = 0--0.4). Echovirus 11 The most serious, potentially fatal clinical presentation associated with echovirus 11 is neonatal systemic illness, presenting as hepatitis-hemorrhage syndrome (33--36). Echovirus 11 has been associated with numerous limited outbreaks in neonatal nurseries (34,36). Aseptic meningitis is a common presentation, but unlike other common enteroviruses, echovirus 11 has rarely been associated with community outbreaks of meningitis (35,37,38). Echovirus 11, the second most commonly identified enterovirus during the study period, accounted for 11.4% of reports with known serotype (Table 2). Echovirus 11 has an epidemic pattern of circulation; increases in reports occur with irregular intervals and usually last for several years (Figure 5). The overall level of echovirus 11 circulation was highest during 1979--1992, when it was the most commonly reported enterovirus six times (1979, 1980, 1982, 1985, 1986, and 1992). The virus has been relatively quiescent since then, with the exception of a sharp increase in 1999, when it was the most commonly reported enterovirus. The majority of echovirus 11 detections during the study period were made in infants aged <1 year, and CSF was the most common specimen. Death of a patient was reported for 4.6% of echovirus 11 detections with known outcome (Table 3). Echovirus 30 Aseptic meningitis is the most commonly reported syndrome associated with echovirus 30 (39--45). Meningoencephalitis and milder illnesses (e.g., respiratory illnesses and herpangina) also occur (43,44). Increases in echovirus 30 activity are characterized by global spread and large-scale aseptic meningitis outbreaks (22,26,37,39--45). Waves of echovirus 30 activity are associated with distinct new genomic lineages, which usually replace previously circulating ones (46--48). Echovirus 30 was the third most commonly identified enterovirus during the study period and accounted for 10.1% of reports with known serotype (Table 2). Echovirus 30 has an epidemic pattern of circulation. During the study period, increases in reported activity occurred with irregular intervals and lasted for few years (Figure 6). During the early years of the study period, echovirus 30 was rarely reported. Four major waves of increased activity occurred after 1975 (1975--1985, 1990--1993, 1997--1998, and 2003--2005). The virus consistently appeared among the most commonly reported enteroviruses (Table 2) and was the most commonly identified enterovirus seven times (1981,1990,1991,1993,1997,1998, and 2004). Echovirus 30 was more commonly detected from older children and adults (66.8%) than from young children, with only 33.2% of reports among persons aged <5 years. CSF was the source for approximately three fourths of echovirus 30 detections (Table 3). Coxsackievirus B5 The major clinical presentations of coxsackievirus B5 infections are similar to those observed for other group B coxsackieviruses and include myopericarditis and neonatal systemic illness (encephalomyocarditis syndrome), aseptic meningitis, meningoencephalitis, and acute flaccid paralysis. Hand, foot, and mouth disease and herpangina also have been reported, and a potential association with development of type 1 diabetes has been suggested (41,49--56). Outbreaks, mostly of aseptic meningitis, are common in epidemic years (50,54,55). Coxsackievirus B5, the fourth most commonly identified enterovirus during the study period, accounted for 8.7% of reports with known serotype during 1970--2005 (Table 2). Coxsackievirus B5 has a distinct epidemic pattern of circulation with regular sharp increases every 3--6 years, which usually last for 1 year (Figure 7). The extent of the increase in activity varied, and smaller peaks sometimes were observed between major ones. Throughout the study period, coxsackievirus B5 consistently appeared among the most commonly reported enteroviruses (Table 2) and was the most commonly identified enterovirus seven times (1972, 1973, 1983, 1989, 1996, 2000, and 2005). Approximately half of all coxsackievirus B5 detections came from young infants, and CSF was the most common source. The summer-fall seasonality in coxsackievirus B5 detections was more prominent than for most other serotypes (Table 3). Echovirus 6 During its high activity periods, echovirus 6 often is associated with outbreaks, such as community outbreaks of aseptic meningitis or nursery outbreaks among neonates (27,21,57--60). Reported clinical presentations include aseptic meningitis, meningoencephalitis, rashes, and gastrointestinal illnesses. Neonatal echovirus 6 infections can be associated with hepatitis and pneumonitis (57--63). Echovirus 6 was the fifth most commonly identified enterovirus during the study period and accounted for 6.2% of reports with known serotype (Table 2). Echovirus 6 has an epidemic pattern of circulation reminiscent of that for echoviruses 11 and 30 (Figure 8), with three major waves of activity (1970--1977, 1985--1990, and 1994--2000) and an increase in 2005. Although echovirus 6 consistently appeared among the most commonly reported enteroviruses (Table 2), it was the most commonly reported serotype only in 1977. Children aged <1 year were the most common source of echovirus 6 detection, CSF was the most common specimen accounting for more than 50% of reports, and 5.6% of detections with known outcome resulted in death (Table 3). Coxsackievirus B2 Clinical illnesses associated with coxsackievirus B2 include aseptic meningitis, myocarditis, and neonatal systemic illness (38,51,64,65). Outbreaks in settings such as football teams and summer camps have been reported (64,66), but widespread community outbreaks are not typical. Coxsackievirus B2 accounted for 5.2% of reports with known serotype during 1970--2005 (Table 2). The virus has an endemic pattern of circulation with year-to-year variability (Figure 9) and has consistently appeared among the top 15 serotypes. Two episodes of unusually high activity of coxsackievirus B2 have been document: in 1976 and 1994, when it became the most commonly reported enterovirus, accounting for >15% of all reported enteroviruses. The proportion of children aged <1 year among reports of coxsackievirus B2 detections exceeded 60%, and CSF was the most common source of detection (Table 3). Coxsackievirus A9 Neurologic illnesses (e.g., aseptic meningitis, meningoencephalitis, and encephalitis), including cases occurring in the context of hand, foot, and mouth disease outbreaks, and epidemic neuropathy predominate among clinical manifestations of coxsackievirus A9 infection (38,67,68). In addition to sporadic detection, a community outbreak has been reported (69). Coxsackievirus A9 accounted for 4.8% of reports with known serotype reported to NESS during 1970--2005 (Table 2). Although coxsackievirus A9 consistently appeared among the most commonly reported enteroviruses, its highest rank during the study period was second in 1973, when it accounted for approximately 12% of all reports. Coxsackievirus A9 is a typical representative of enteroviruses with an endemic pattern of circulation (Figure 10). Children aged <1 year were the most common age group for coxsackievirus A9 detection, and CSF was the most common specimen. Death occurred in 1.7% of reports with known outcome (Table 3). Echovirus 4 Outbreaks of aseptic meningitis caused by echovirus 4 have been described in several countries (26,70--72). A new genetic variant of echovirus 4, which has replaced previously circulating lineages, might have been circulating since 1997 (70). Echovirus 4 accounted for 4.6% of reports with known serotype (Table 2). Echovirus 4 has a cyclical, epidemic pattern of circulation with irregular intervals between periods of high activity. The highest level of activity was observed during 1970--1978 (in 1973, it was the most commonly identified enterovirus, accounting for >30% of all reports), and during 1984--1990, with a minor peak during 2001--2002 (Figure 11). The virus was frequently present among the 15 most common enteroviruses, but its ranking declined from fourth during the 1970s to 19th during the 1990s (Table 2). In 36.1% of reports, echovirus 4 was detected among persons aged 5--19 years. Children aged <5 years accounted for <50% of all echovirus 4 reports. CSF was the source of echovirus 4 in approximately two thirds of reports (Table 3). Coxsackievirus B4 Outbreaks of coxsackievirus B4 are rare. Most common clinical syndromes associated with coxsackievirus B4 include aseptic meningitis, encephalitis, myopericarditis, neonatal infections, febrile rash illnesses, and respiratory manifestations (73--75). Coxsackievirus B4 accounted for 4.2% of reports with known serotypes and has consistently appeared among the 15 most common enteroviruses, but it has never been the predominant serotype (Table 2). Coxsackievirus B4 has an endemic pattern of circulation (Figure 12). Approximately 60% of all coxsackievirus B4 detections came from young infants, and CSF was the most common source. Similar to coxsackievirus B5, the summer-fall seasonality in coxsackievirus B4 detections was more prominent than for the majority of other serotypes. Coxsackievirus B4 had one of the highest proportions of reports with fatal outcome (9.8%) (Table 3), with a significantly higher risk of death when compared with fatal outcomes among persons infected with any other enterovirus serotypes (OR = 3.3; CI = 1.7--6.4). Echovirus 7 Clinical presentations of echovirus 7 infection include aseptic meningitis, meningoencephalitis, paralysis, gastrointestinal manifestations, and neonatal systemic illness. Outbreaks associated with echovirus 7 have been rarely reported (76--80). Echovirus 7 accounted for 4.0% of reports with known serotype during 1970--2005 (Table 2). Echovirus 7 has a cyclical, epidemic pattern of circulation with irregular intervals between periods of high activity. Echovirus 7 commonly appeared among the 15 most common enteroviruses and was the most commonly reported serotype in 2002. The increased activity of echovirus 7 was reported during 1978--1979, 1985--1987, 1991--1997, and 2002 (Figure 13). In approximately 60% of reports, echovirus 7 was detected in young infants. CSF was the most common source of echovirus 7 detections (Table 3). Coxsackievirus B3 Coxsackievirus B3 has been commonly associated with myopericarditis, aseptic meningitis, neonatal systemic illness, meningoencephalitis in immunodeficient persons, herpangina, and rash illnesses (81--83). Outbreaks of neonatal infections and herpangina and a cluster of myocarditis cases in the context of community wide coxsackievirus B3 outbreak have been reported (84--86). Coxsackievirus B3 accounted for 3.9% of reports with known serotype (Table 2). Coxsackievirus B3 has an epidemic pattern of circulation. The increases in its activity are of variable duration and extent and occur after variable periods of quiescence (Figure 14). Coxsackievirus B3 has consistently appeared among the 15 most commonly reported enteroviruses but has never been the most commonly reported virus, with a highest ranking of second in 1980 and 1994. The proportion of children aged <1 year among reports of coxsackievirus B3 detections exceeded 60%, and respiratory specimens were the most common source of detection. Fatal outcome was reported for 5.4% of coxsackievirus B3 detections with known outcome (Table 3). Echovirus 18 Outbreaks associated with echovirus 18 have been reported during the periods of its increased circulation. The most commonly reported clinical syndrome associated with echovirus 18 infection is aseptic meningitis, but paralysis, neonatal sepsis, and other manifestations also have been documented (40,87--89). Echovirus 18 accounted for 2.7% of reports with known serotype (Table 2). The virus has an epidemic pattern of circulation with increased levels of activity after prolonged quiescence. During 1970--2005, two periods of increased activity of echovirus 18 were observed (1986--1987 and 1995--2005) (Figure 15). Activity reached its highest level in 2001. Echovirus 18 appeared among the 15 most common enteroviruses during 18 of 36 years of the study period; it has never been the predominant enterovirus, but ranked second in 1987 and 2001. Children aged <1 year were the most common age group for echovirus 18 detection, and CSF was the most common specimen. Death was reported for 1.8% of echovirus 18 cases with known outcome (Table 3). Coxsackievirus B1 Coxsackievirus B1 has been associated with outbreaks of aseptic meningitis and pleurodynia (35,90--92). Common clinical presentations are similar to other group B coxsackieviruses and include aseptic meningitis, meningoencephalitis, myocarditis, hand, foot, and mouth disease, and pleurodynia. Coxsackievirus B1 also can cause systemic neonatal illness sometimes presenting as fulminant hepatitis with coagulopathy, a syndrome usually associated with echovirus 11 rather than with encephalomyocarditis syndrome typical for coxsackieviruses B2-5 (33,92). Coxsackievirus B1 accounted for 2.3% of reports with known serotype during 1970--2005 (Table 2). Coxsackievirus B1 has an epidemic pattern of circulation, with increased activity occurring with irregular intervals and usually lasting several years (Figure 16). The overall level of coxsackievirus B1 circulation showed a tendency toward increase since the 1990s. Coxsackievirus B1 appeared among the 15 most common enteroviruses during 17 of 36 years of the study period; it has never been the most commonly reported enterovirus but ranked second in 1977. Two thirds of coxsackievirus B1 reports during the study period were from infants aged <1 year, and respiratory specimens were the most common source of its detection. No fatal outcomes were reported for 104 coxsackievirus B1 detections with known outcome. Similar to coxsackieviruses B4 and B5, the summer-fall seasonality in coxsackievirus B1 detections was more prominent than for the majority of other serotypes (Table 3). Reports of coxsackievirus B1 had the least prominent male predominance of all serotypes (52.5%). Echovirus 3 An echovirus 3 aseptic meningitis outbreak was reported in the early 1970s, but few cases were reported in subsequent years (93). Other reported clinical syndromes include meningoencephalitis and paralysis (94,95). Although echovirus 3 remains rare in the United States, an outbreak was reported in South Africa in 2001 (96). Echovirus 3 accounted for 1.9% of reports with known serotype (Table 2). The virus has a distinct epidemic pattern of circulation with regular increases every 4--7 years (Figure 17). Echovirus 3 was the most commonly detected enterovirus in 1970, but since then, the magnitude of periodic increases has been declining. After the mid-1990s, the serotype has become very uncommon. The majority of echovirus 3 reports were identified in infants aged <1 year, and respiratory specimens were the most common source of its detection (Table 3). Echovirus 5 A small outbreak of echovirus 5 aseptic meningitis occurred among adults in Finland in 1985, concurrent with the last reported increase of this virus in the United States (97). During 1970--2005, echovirus 5 accounted for 1.8% of reports with known serotype (Table 2). Echovirus 5 has an epidemic pattern of circulation, with increased activity occurring with irregular intervals and usually lasting several years (Figure 18). Echovirus 5 frequently appeared among the 15 most common enteroviruses with a high rank of fourth in 1982. After 1990, the magnitude of periodic increases in its activity declined considerably. Children aged <1 year were the most common source for echovirus 5 detection, and CSF was the most common specimen. Fatal outcome was reported in 3.8% of echovirus 5 infections with known outcome (Table 3). Echovirus 13 During the approximately 50 years after its initial identification, echovirus 13 remained rare, with no reports of outbreaks associated with this virus. However, a worldwide spread of the virus occurred during 2000--2001 and caused major outbreaks of aseptic meningitis throughout the world (57,98--103). Genetically, the majority of echovirus 13 isolates have diverged considerably from the prototype strain, but all circulating strains are closely related (98). Clinical presentations associated with echovirus 13 include aseptic meningitis, encephalitis, respiratory and gastrointestinal manifestations, and chronic infection of immunodeficient hosts (98,104,105). During 1970--2005, echovirus 13 accounted for 1.2% of reports with known serotype (Table 2). A slight increase occurred in 2000 (12 reports), followed by a sharp peak of 412 reports in 2001, when it became the most commonly identified enterovirus in the United States (Figure 19). Since then, the levels of echovirus 13 activity have declined, but the virus continues to appear among the 15 most common serotypes. Children aged <1 year were the most common age group for echovirus 13 reports, and CSF was the most common specimen. Echovirus 13 had less prominent summer-fall seasonality than other enteroviruses, with only 62.4% of reports during June--October (Table 3). Coxsackievirus A16 The most common illness associated with coxsackievirus A16 infection is hand, foot, and mouth disease. More serious manifestations are rare, but cases of aseptic meningitis, fatal myocarditis, rhabdomyolysis with renal failure, and neonatal febrile illness have been described, and outbreaks have been reported (106--110). During 1970--2005, coxsackievirus A16 accounted for 1.2% of reports with known serotype (Table 2). The virus rarely appeared among the 15 most common enteroviruses and never ranked higher than sixth. Coxsackievirus A16 has an endemic pattern of circulation (Figure 20). The reported overall levels of coxsackievirus A16 activity were higher during the 1970s and 1980s and have declined since 1990. The age group most commonly associated with coxsackievirus A16 detections was children aged 1--4 years, and specimens coded as "other" were the most frequent source, possibly referring to scrapings from hand, foot, and mouth disease lesions. The summer-fall seasonality was present, but to a somewhat lesser extent than for other enteroviruses. Death was reported for 1.1% of coxsackievirus A16 infections in persons with known outcome (Table 3). Coxsackievirus A24 Coxsackievirus A24 variant is one of the two enteroviruses, along with enterovirus 70, associated with massive outbreaks of acute hemorrhagic conjunctivitis, which periodically occur in tropical areas and involve large populations (111,112). Coxsackievirus A24 was rarely reported during the study period (Table 2). Of 55 reports, 45 (81.8%) were associated with a single outbreak of acute hemorrhagic conjunctivitis in Puerto Rico (112). Approximately half of detections were from adults, all but one specimen were coded as "other" (presumably, conjunctival swabs), and nearly all of the reported infections occurred during the summer-fall period. Enterovirus 68 Enterovirus 68 is unique among the enteroviruses because it has properties of both enteroviruses and rhinoviruses (members of another genus in the Picornaviridae family). Strains of enterovirus 68 have been independently identified as rhinovirus 87, but recent genomic sequence data for both viruses confirmed that they are of the same type (113,114). Enterovirus 68 is one of the most rarely reported serotypes, with only 26 reports throughout the 36-year study period (Table 2). The first report of enterovirus 68 to NESS was in 1987, and the highest number of reports in a single year was 11 in 2003. This increase was largely associated with enhanced detection at the site of a study of respiratory viruses among asthma patients (113). In all but one case, enterovirus 68 was detected from respiratory specimens, and in one case in 2005 from CSF of a patient with acute flaccid paralysis. This case, in a young adult, was the first report of CNS infection by enterovirus 68. The most common age group associated with enterovirus 68 was children aged 1--4 years, but approximately one fourth of all reports were in adults aged >20 years. Fifty percent of all reported enterovirus 68 infections occurred outside the typical summer-fall season (Table 3). Enterovirus 71 Enterovirus 71 is associated with serious neurologic manifestations (e.g., aseptic meningitis, polio-like paralysis, and bulbar encephalitis) and mild rashes, including hand, foot, and mouth disease. Outbreaks of paralysis were reported in Europe during the 1960s and 1970s (115). During the late 1990s and early 2000s, outbreaks of fatal bulbar encephalitis among young children occurred in the southeast Asian countries, usually in the context of enterovirus 71-associated outbreaks of hand, foot, and mouth disease (116--119). Localized outbreaks of enterovirus 71-associated illnesses have been documented in the United States since 1973 (115), but no detections were reported to NESS until 1983. During 1983--2005, a total of 270 cases of enterovirus 71 were reported (Table 2). The overall temporal pattern of enterovirus 71 reports was consistent with endemic circulation (Figure 21). An increasing trend in overall level of reported activity was documented, especially after the mid-1990s, but large-scale nationwide activity, similar to the ones in southeast Asia in the late 1990s and early 2000s, has not been observed. Enterovirus 71 was reported during 23 of the 36 study years, and it appeared among the 15 most common serotypes 11 times (1987, 1994, and 1996--2005), with a high ranking of fifth in 1994. Children aged <1 year were the most common source of enterovirus 71 reports; children aged <5 years accounted for approximately three fourths of all reports. Approximately 40% of detections were from respiratory specimens, and 70% of reported infections occurred during the enterovirus season (Table 3). Human Parechoviruses 1 and 2 Clinical manifestations of human parechovirus infections include aseptic meningitis, encephalomyelitis, acute flaccid paralysis, and neonatal sepsis with necrotizing enterocolitis (14,120). A nursery outbreak of human parechovirus 1 infection has been described (120). During 1970--2005, human parechovirus 1 (formerly echovirus 22) accounted for 880 detections (1.8% of reports with known serotype) (Table 2). Although the virus was among the 15 most common serotypes during 26 of 36 years, its highest rank was eighth (1977, 1982, 1985, and 1996), consistent with the endemic pattern of circulation. Overall level of reported activity was highest throughout the 1980s and declined to the current low level after 1996 (Figure 22). Approximately 95% of human parechovirus 1 detections were reported in children aged <5 years and 73% in children aged <1 year. The most commonly reported source was respiratory specimens. The majority (62.9%) of infections occurred during the summer-fall season, but the seasonality was not as prominent as for the majority of enteroviruses. Human parechovirus 1 had the highest reported proportion of fatal outcomes (10.9%) compared with all other serotypes, and persons infected with this virus were at a significantly higher risk for death than those infected with other serotypes (OR = 3.7; CI = 1.7--7.6). Human parechovirus 2 (formerly echovirus 23) was rarely reported, and its characteristics resembled those for human parechovirus 1 (Tables 2 and 3). Other Serotypes Other coxsackie A viruses were rarely reported, and each accounted for a very small proportion of reports; reports of these viruses became extremely rare after the mid-1990s. The exception from this pattern was coxsackievirus A1, with 15 reports in 2004 (compared with nine during 1970--2003). These reports were associated with an outbreak of aseptic meningitis caused by coxsackievirus A1 that was identified by the CDC Enterovirus Laboratory using molecular methods. Reports of coxsackievirus B6 were at a steady low level throughout the study period. Other echovirus serotypes together accounted for approximately 10% of all reports with known serotype, with individual serotype contribution ranging from <0.1% to 1.4%. Reports of several of these serotypes remained stable throughout the study period, and reports of echoviruses 14, 16, 17, 19, 26, 27, 29, 31, and 32 became very rare or absent during the 1990s and 2000s. Only one case of enterovirus 70 was reported. This virus is usually associated with large scale outbreaks of acute hemorrhagic conjunctivitis in tropical countries, but importations into the United States by travelers from affected areas have been reported (121). For the entire study period, no cases of coxsackieviruses A19 and A22, enterovirus 69, and recently identified enteroviruses numbered 73 and higher were reported. DiscussionSerotype-based enterovirus surveillance provides a mechanism for determining patterns of enterovirus circulation and for identifying predominant serotypes for a given season. Monitoring circulating enteroviruses is important because individual serotypes can be associated with different clinical manifestations and outcomes and have different temporal patterns of circulation. Changes in predominant serotypes can be accompanied by large-scale outbreaks of enteroviral illnesses. Although predominant serotypes change over time, the analysis of long-term surveillance data identified a core group of enteroviruses that consistently appear among those most commonly reported. These common serotypes probably determine, to a substantial degree, the overall impact of enterovirus infections in terms of associated disease burden. In addition, the availability of surveillance data for the period spanning nearly four decades allowed identification of long-term temporal trends for individual serotypes missed in reports with shorter observation periods. Although the reasons for differences in long-term patterns of individual serotypes are unclear, both the agent and host factors probably affect enterovirus circulation. For many serotypes (e.g., echoviruses 13 and 30 and coxsackievirus B5), increases in activity have been associated with the emergence of new genetic lineages, which often replace the previously circulating ones (46,48,55,98); for other enteroviruses, multiple lineages might co-exist (122). Reemergence of an old epidemic genotype after being replaced for several years by another genotype also has been described (123). Population immunity to a particular serotype determines, to a great degree, the potential extent of the virus spread and disease. Accumulation of a susceptible population, especially in younger cohorts, during the years of the quiescence of the virus probably is a contributing factor to periodic increases in circulation levels of enteroviruses with a regular epidemic pattern. NESS provided data to determine descriptive epidemiologic characteristics for enteroviruses as a group and for individual serotypes, including relatively rare ones. The analysis in this report demonstrated a higher risk for death associated with coxsackievirus B4 and human parechovirus 1 compared with detections of other enterovirus serotypes and a lower risk for fatal outcome for those infected with echovirus 9, which have implications for patient prognosis. Associations of other serotypes with an increased or reduced risk for fatal outcome might have been missed because outcome was reported in only a small proportion of cases. The factors contributing to the differences in the risk for fatal outcome are unknown. Different serotypes were detected more frequently in certain types of specimens, reflecting variations by serotype in typical clinical presentations. For example, echoviruses 9 and 30, the viruses commonly associated with aseptic meningitis outbreaks, were mostly detected in CSF, and coxsackievirus A16, the leading cause of hand, foot, and mouth disease in the United States, was mostly commonly detected in specimens other than CSF, stool, or respiratory swabs. The summer-fall seasonality of enterovirus infections in temperate climates has been well established (1,2,28). The more pronounced seasonality for enterovirus detection in CSF compared with other specimens probably reflects the highly seasonal occurrence of aseptic meningitis, the most common CNS illness associated with enteroviruses, and the heightened index of suspicion for patients with neurologic symptoms during the traditional enteroviral season. Male predominance among patients with enterovirus infections (1,2) was only present among persons aged <20 years. The lack of this association for adults aged >20 years probably resulted from greater exposure of female caregivers to young children who are the primary source of exposure within a household. The decline in the proportion of reports with known serotype after the mid-1990s was probably caused by decreasing availability of typing reagents and concerns over cost and clinical utility of enterovirus typing. The increase noted after 2001 is associated with increasing use of molecular typing methods by reporting laboratories. Strengths and LimitationsBy allowing identification of temporal trends and patterns of circulation of individual enterovirus serotypes and providing information on circulating serotypes, enterovirus surveillance becomes a useful instrument from both the public health and clinical perspectives. Although NESS is not designed for real-time outbreak detection, the information from this surveillance system allows timely identification of predominant serotypes and helps guide outbreak investigations. The increase in reports of echoviruses 13 and 18 noted by NESS early in the 2001 enterovirus season (124) helped state and local health departments identify these serotypes as the major agents associated with aseptic meningitis outbreaks that year (98). Recognition of the outbreak serotypes was particularly important because specific monoclonal antibodies for echoviruses 13 and 18 are not commercially available, so indirect immunofluorescence could not be used for typing to differentiate outbreak cases from sporadic enterovirus infections. Knowledge of the outbreak serotypes encouraged laboratories that used exclusively monoclonal antibodies for typing to refer isolates to a reference laboratory capable of typing by other methods. Identification of the outbreak serotypes also helped confirm enteroviruses as the predominant etiology of aseptic meningitis during a West Nile virus avian epizootic (125). Similarly, the NESS data indicating an increase in echoviruses 9 and 30 helped focus the investigation of aseptic meningitis outbreaks in 2003 on these serotypes (22). Enterovirus surveillance data can be helpful in identifying targets for diagnostic assay development. Serotype-specific PCR assays for echoviruses 13 and 18 rapidly developed by the CDC Enterovirus Laboratory in 2001 in response to the increases in their reports documented by NESS (124) provided a useful tool for outbreak investigations, allowing timely detection of epidemic serotypes (98). The information about circulating enterovirus serotypes also is important for development of antiviral compounds against enteroviruses because different serotypes have differential sensitivity to at least one candidate antienterovirus drug (pleconaril) (126). In addition, NESS data are useful for interpreting trends in enteroviral illnesses and for studies of enterovirus associations with specific diseases. For example, the increases in nationwide rates for viral meningitis-associated hospitalizations in the United States occurred when certain serotypes predominated (e.g., echovirus 30 in 1991 and 1998 and echovirus 9 in 1999); lower rates of hospitalization were observed during the years when a predominance of certain other serotypes (e.g., echovirus 11 in 1988) was observed (28). Although this report is limited to nonpolio enteroviruses, monitoring poliovirus detection is an important aspect of NESS. Because of the elimination of indigenous wild polioviruses in the Americas and cessation of oral poliovirus vaccine (OPV) use (20,21), no poliovirus reservoir exists in the United States, except for few immunodeficient persons with persistent poliovirus infection, and poliovirus reports to NESS most likely reflect importations. Only one poliovirus (vaccine type) was reported to NESS in 2000, and no other polioviruses were reported until 2005. In 2005, vaccine-derived poliovirus type 1 circulation in a group objecting to vaccination was reported in Minnesota (127). This episode underscores the potential for poliovirus importation as long as wild polioviruses continue to circulate in certain parts of the world and OPV continues to be used by the majority of countries. As a result, NESS will remain a supplemental means of polio surveillance in the United States until the global eradication of wild polioviruses and the cessation of OPV use. NESS is subject to at least three limitations. First, enteroviruses that commonly infect younger patients or that are associated with more severe illnesses might be overrepresented in NESS because clinical specimens from young children and more severely ill patients are submitted for testing more frequently. A potential explanation for a testing bias is that specimens might be more frequently submitted for enterovirus testing among young or more severely ill patients, in whom a specific diagnosis is considered more urgent, compared with older patients with milder disease, in whom ruling out bacterial meningitis might be a sufficient diagnosis. The extent of this testing bias is difficult to measure because population-based data on the incidence of enterovirus infections are not easily available. Aseptic meningitis is the most common severe clinical manifestation associated with enterovirus infection. In one study, population-based estimates of persons hospitalized with viral meningitis in the United States based on the National Hospital Discharge Survey (NHDS) data (28) were compared with NESS data. The proportion of infants aged <1 year among patients reported to NESS was approximately double that from the NHDS study (44.2% and 23.5%, respectively). The proportion of fatal outcomes was approximately eightfold higher among patients reported to NESS (3.3%) than among patients hospitalized with viral meningitis (0.4%) (28). Second, because of the voluntary and passive nature of reporting to NESS, the small number of reports from certain states, and the absence of reports from some others, these results might not be representative of enterovirus circulation in the entire United States. The annual numbers of reports to NESS varied considerably. Consistent with a previous report (3), this year-to-year variability is, in part, the function of variability in the number of reporting laboratories. A general decline in use of viral culture because of its high cost, labor intensiveness, and modest clinical utility and the reduced availability of typing reagents along with the introduction of panenterovirus PCR assays were additional factors leading to the relative decline in enterovirus surveillance reports observed during the 1990s. The increased number of participating sites since 2000 and the widest ever geographic coverage achieved by NESS in 2005 are encouraging and might be attributable to simplified methods for data submission. However, the majority of state public health laboratories did not report to NESS during the last few years, and the available information for these states was received indirectly through private laboratories and the CDC Enterovirus Laboratory testing specimens of patients from these states and reporting to NESS. Because of the higher cost of private laboratory testing, reported enterovirus activity in states from these sources is underrepresented in NESS. Efforts to increase laboratory participation in NESS should continue to allow for more complete and accurate surveillance for enteroviruses in the United States. Finally, serotype distribution in NESS is biased because of the low likelihood of serotypes being reported that cannot be readily detected by available methods. For example, coxsackie A viruses are most likely underrepresented in NESS because many of these viruses grow poorly in cell culture. Traditionally, the primary method for their detection has been isolation by intracerebral inoculation of suckling mice. The use of this technique has declined during the preceding 10--15 years, concurrent with the overall decline in coxsackie A virus reports to NESS. The sharp increase in coxsackievirus A1 reports in 2003 with the use of molecular typing suggests that as molecular methods are adopted more widely, more accurate information on coxsackie A virus epidemiology will become available. Public Health ActionsConcerns about declining laboratory participation in NESS throughout the 1990s led to efforts to update the reporting system and to make it more user-friendly. These efforts, initiated in the early 2000s, included simplification of reporting forms and transition to electronic reporting and resulted in a substantial increase in reporting compared with the late 1990s. Further improvements in the timeliness of feedback could provide additional incentives to public health laboratories to participate in NESS. A NESS website is being developed that will allow access to historic national and state enterovirus surveillance data and information on circulating serotypes. Developing a system for web-based data entry will further streamline the process of reporting to NESS and allow for improved feedback to stakeholders. The system will have sufficient built-in flexibility to adapt to changing laboratory practices and evolving taxonomy. Reporting of newly identified enteroviruses and other picornaviruses could be incorporated as needed. The assistance provided by CDC's Enterovirus Laboratory to state public health laboratories in implementing molecular methods of typing and efforts to increase laboratory participation in NESS should be continued to allow for more complete and accurate surveillance for enteroviruses in the United States. References
Table 1  Return to top. Figure 1 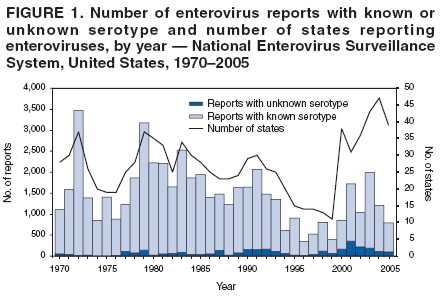 Return to top. Table 2 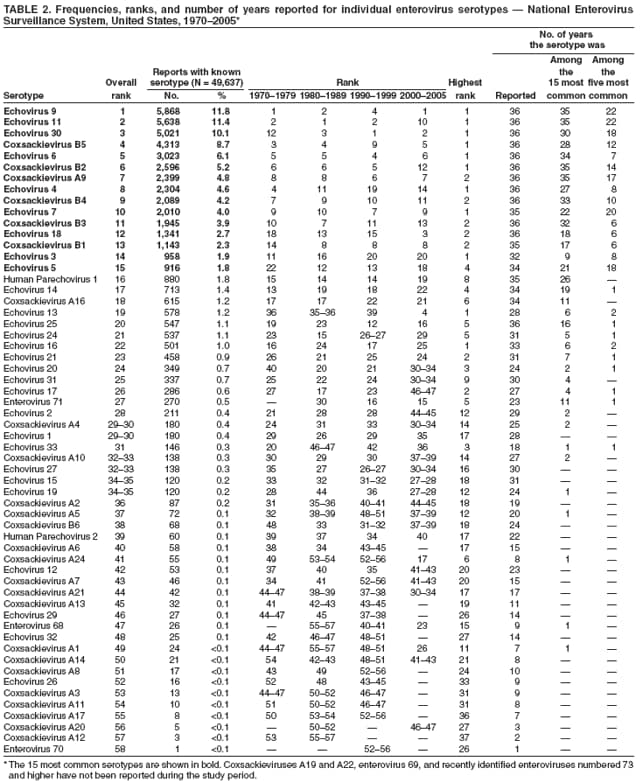 Return to top. Figure 2 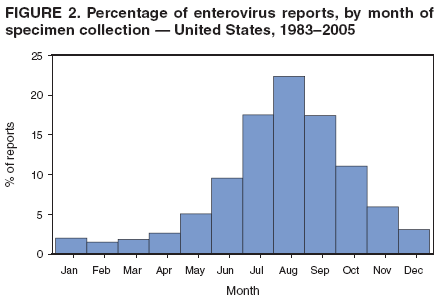 Return to top. Table 3 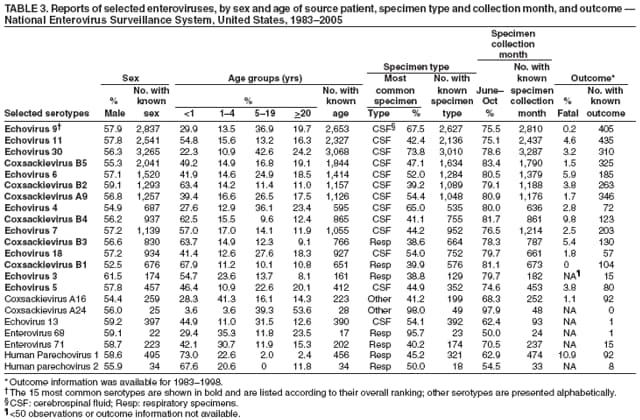 Return to top. Figure 3 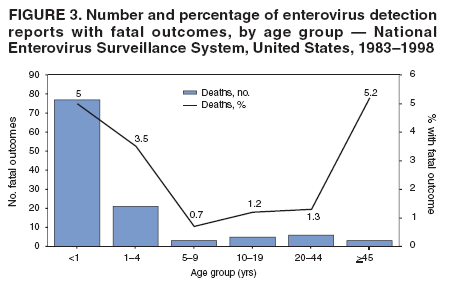 Return to top. Figure 4 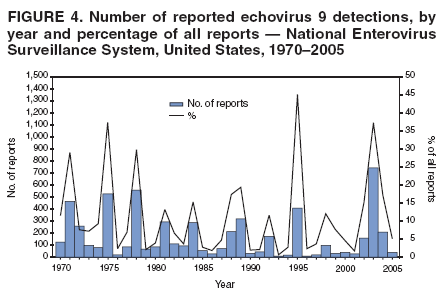 Return to top. Figure 5 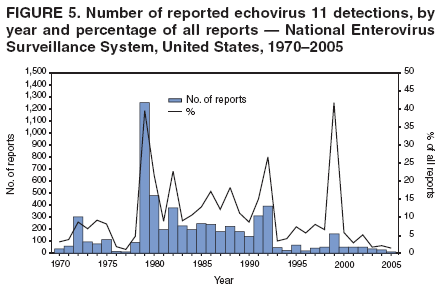 Return to top. Figure 6 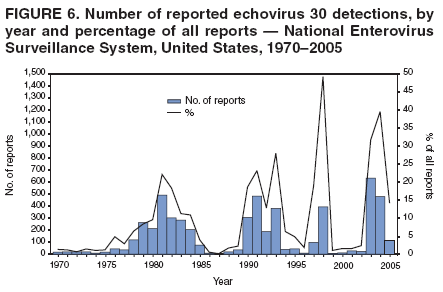 Return to top. Figure 7 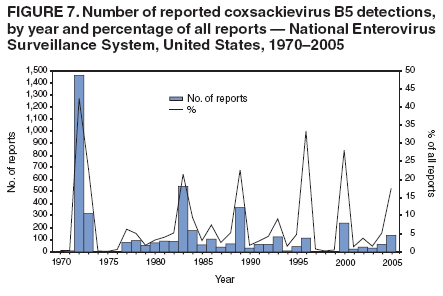 Return to top. Figure 8 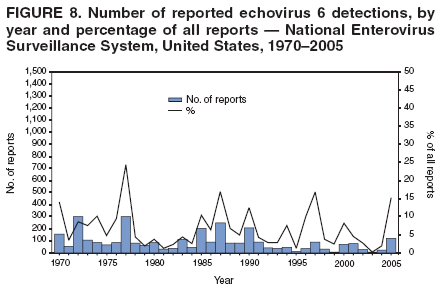 Return to top. Figure 9 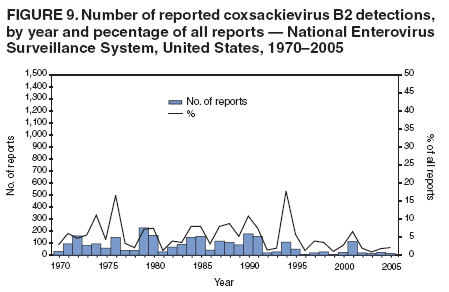 Return to top. Figure 10 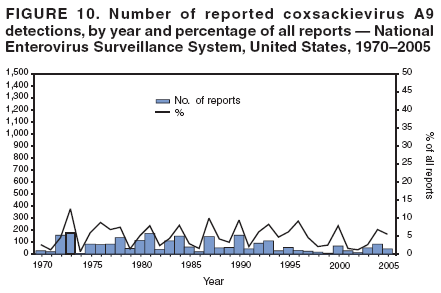 Return to top. Figure 11 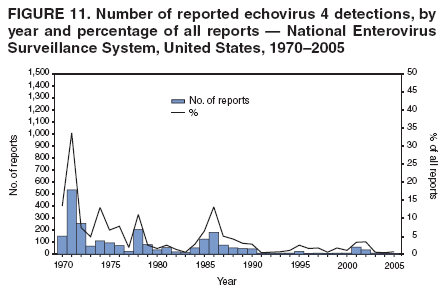 Return to top. Figure 12 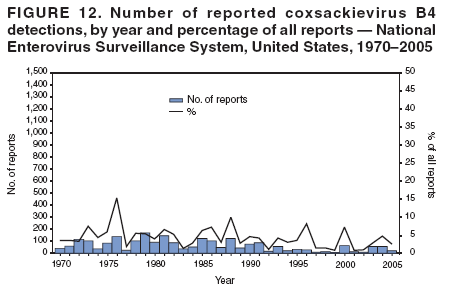 Return to top. Figure 13 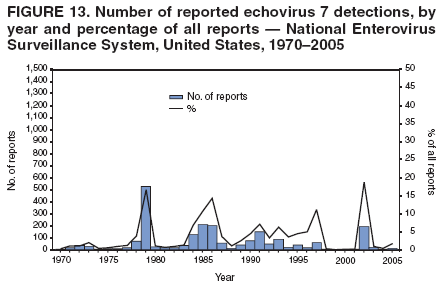 Return to top. Figure 14 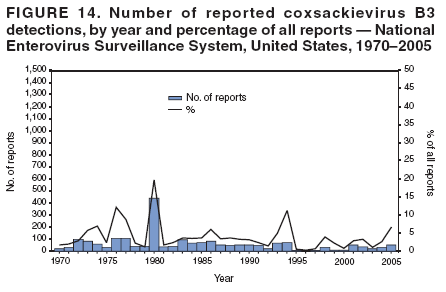 Return to top. Figure 15 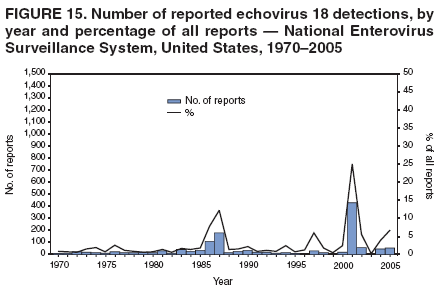 Return to top. Figure 16 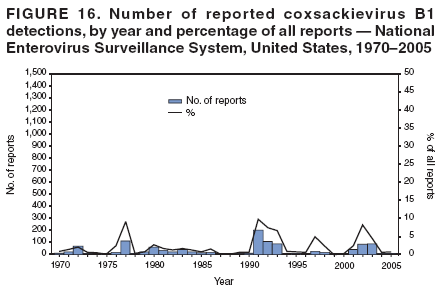 Return to top. Figure 17 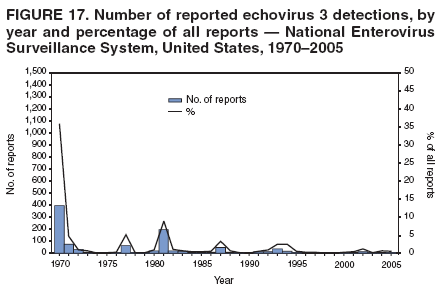 Return to top. Figure 18 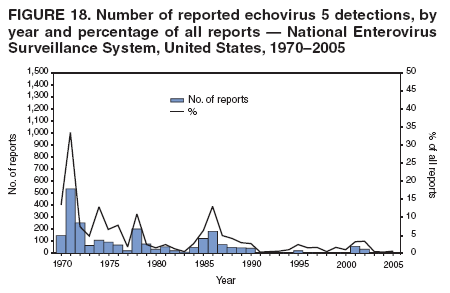 Return to top. Figure 19 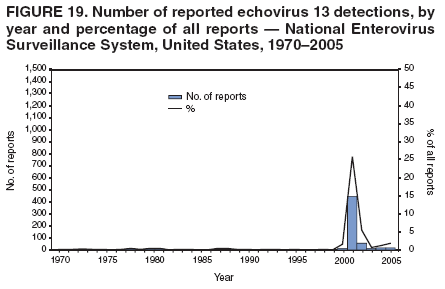 Return to top. Figure 20 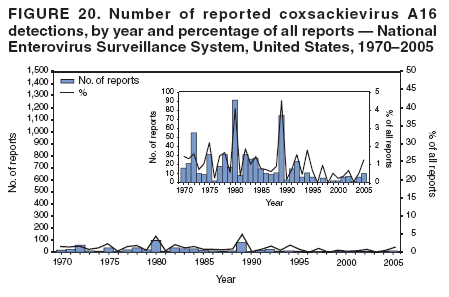 Return to top. Figure 21 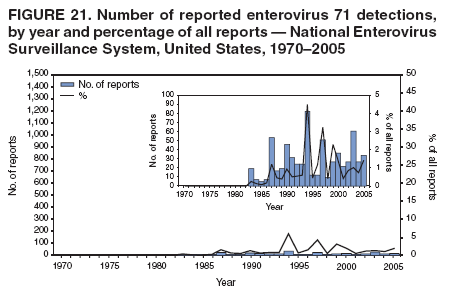 Return to top. Figure 22 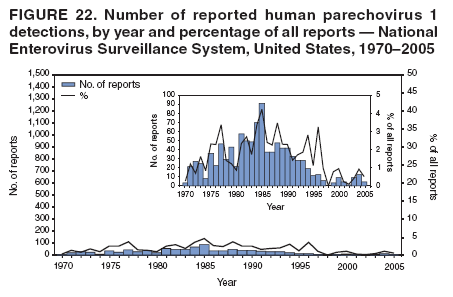 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Date last reviewed: 8/29/2006 |
|||||||||
|