 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Severe Acute Respiratory Syndrome --- United States, May 28, 2003CDC continues to work with state and local health departments, the World Health Organization (WHO), and other partners to investigate cases of severe acute respiratory syndrome (SARS). This report updates SARS cases reported worldwide and in the United States and reports a seventh laboratory-confirmed U.S. case. During November 1, 2002--May 28, 2003, a total of 8,240 SARS cases were reported to WHO from 28 countries, including the United States; 745 deaths (case-fatality proportion: 9.0%) have been reported (1). The 363 SARS cases identified in the United States have been reported from 41 states and Puerto Rico, with 297 (82%) cases classified as suspect SARS and 66 (18%) classified as probable SARS (more severe illnesses characterized by the presence of pneumonia or acute respiratory distress syndrome) (Figure, Table) (2). Of the 66 probable SARS patients, 43 (65%) were hospitalized, and two (3%) required mechanical ventilation. No SARS-related deaths have been reported in the United States. Of 66 probable cases, 64 (97%) were attributed to international travel to areas with documented or suspected community transmission of SARS within the 10 days before illness onset; the remaining two (3%) probable cases occurred in a health-care worker who provided care to a SARS patient and a household contact of a SARS patient. Since the last update, new cases of SARS have been reported in Toronto, Canada, and CDC has reissued a travel alert for Toronto (3). Consequently, the surveillance case definition continues to include cases in persons whose illness is consistent with the clinical criteria and began within 10 days of travel to Toronto (2). Serologic testing for antibody to SARS-associated coronavirus (SARS-CoV) has been performed for 32 (48%) probable cases and was positive for seven, six of which have been described previously as laboratory-confirmed cases. For one patient, a reverse transcriptase polymerase chain reaction (RT-PCR) assay detected SARS-CoV ribonucleic acid in a sputum specimen collected 14 days after illness onset (4,5); this patient subsequently had antibody to SARS-CoV. The seventh patient, a household contact of one of the six patients with positive serology, was reported previously as a probable SARS patient on the basis of clinical and epidemiologic criteria (4). Among the seven patients, four had positive serology on or before day 12 after onset of symptoms. The other three had negative serologic tests on day 4, 6, and 14, respectively, and a positive test in the next available serum sample on day 28, 25, and 41, respectively. Serologic testing has been performed for 111 (37%) suspect cases; antibody was not detected for any of those tested. CDC measures SARS-CoV--specific total IgG, IgM, and IgA antibodies by both enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence antibody (IFA) (6). A serum specimen is reported as positive when both tests are positive. Antibodies against other human and nonhuman coronaviruses do not react in these assays, and tests on sera from 384 persons without SARS-CoV infection all were negative. These findings indicate that SARS-CoV has emerged recently within the population and that the serologic methods are specific for detection of antibody against SARS-CoV and have a low false-positive rate. Rapid identification of SARS-CoV as the etiologic agent of SARS and extensive international collaboration has aided in the development of this diagnostic test. Of the 66 probable SARS cases, convalescent serum has been collected for 40 (61%). Testing of convalescent serum is invaluable in confirming infection with SARS-CoV, and every effort should be made to obtain follow-up specimens >21 days after onset of illness. Reported by: State and local health departments. SARS Investigative Team, CDC. References
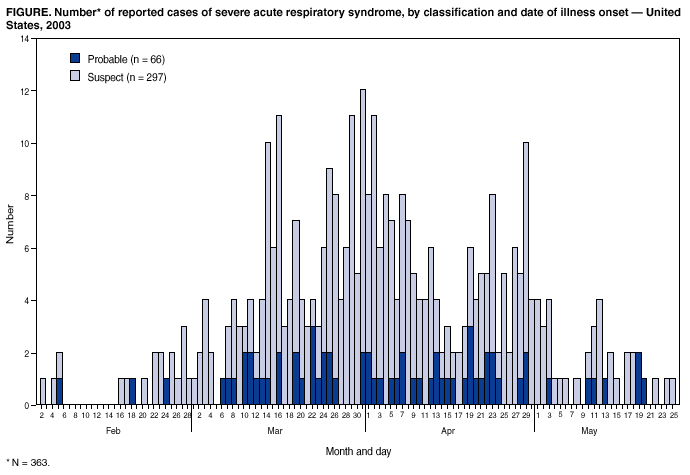 Return to top. Table 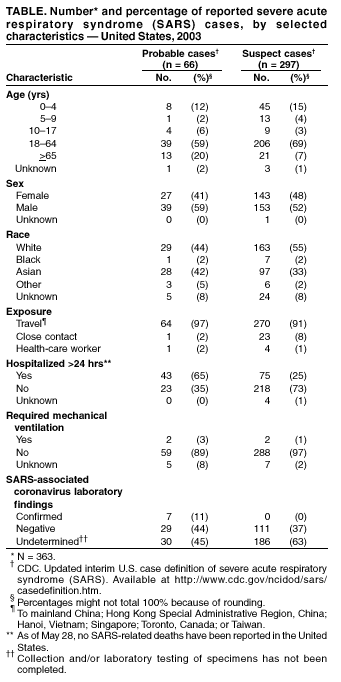 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to [email protected].Page converted: 5/29/2003 |
|||||||||
This page last reviewed 5/29/2003
|