 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Sexually Transmitted Diseases Treatment Guidelines, 2006Please note: An update has been published for this report. To view the update, please click here.Prepared by
The material in this report originated in National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), Kevin A. Fenton, MD, PhD, Director; and the Division of STD Prevention, John M. Douglas, MD, Director. Corresponding preparer: Kimberly A. Workowski, MD, Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, 10 Corporate Square, Corporate Square Blvd., MS E-02, Atlanta, GA 30333. Telephone: 404-639-1898; Fax: 404-639-8610; E-mail: [email protected]. SummaryThese guidelines for the treatment of persons who have sexually transmitted diseases (STDs) were developed by CDC after consultation with a group of professionals knowledgeable in the field of STDs who met in Atlanta, Georgia, during April 19--21, 2005. The information in this report updates the Sexually Transmitted Diseases Treatment Guidelines, 2002 (MMWR 2002;51[No. RR-6]). Included in these updated guidelines are an expanded diagnostic evaluation for cervicitis and trichomoniasis; new antimicrobial recommendations for trichomoniasis; additional data on the clinical efficacy of azithromycin for chlamydial infections in pregnancy; discussion of the role of Mycoplasma genitalium and trichomoniasis in urethritis/cervicitis and treatment-related implications; emergence of lymphogranuloma venereum proctocolitis among men who have sex with men (MSM); expanded discussion of the criteria for spinal fluid examination to evaluate for neurosyphilis; the emergence of azithromycin- resistant Treponema pallidum; increasing prevalence of quinolone-resistant Neisseria gonorrhoeae in MSM; revised discussion concerning the sexual transmission of hepatitis C; postexposure prophylaxis after sexual assault; and an expanded discussion of STD prevention approaches. IntroductionPhysicians and other health-care providers play a critical role in preventing and treating sexually transmitted diseases (STDs). These guidelines for the treatment of STDs are intended to assist with that effort. Although these guidelines emphasize treatment, prevention strategies and diagnostic recommendations also are discussed. MethodsThis report was produced through a multistage process. Beginning in 2004, CDC personnel and professionals knowledgeable in the field of STDs systematically reviewed evidence, including published abstracts and peer-reviewed journal articles concerning each of the major STDs, focusing on information that had become available since publication of the Sexually Transmitted Diseases Treatment Guidelines, 2002 (1). Background papers were written and tables of evidence were constructed summarizing the type of study (e.g., randomized controlled trial or case series), study population and setting, treatments or other interventions, outcome measures assessed, reported findings, and weaknesses and biases in study design and analysis. A draft document was developed on the basis of the reviews. In April 2005, CDC staff members and invited consultants assembled in Atlanta, Georgia, for a 3-day meeting to present the key questions regarding STD treatment that emerged from the evidence-based reviews and the information available to answer those questions. When relevant, the questions focused on four principal outcomes of STD therapy for each individual disease: 1) microbiologic cure, 2) alleviation of signs and symptoms, 3) prevention of sequelae, and 4) prevention of transmission. Cost-effectiveness and other advantages (e.g., single-dose formulations and directly observed therapy of specific regimens) also were discussed. The consultants then assessed whether the questions identified were relevant, ranked them in order of priority, and attempted to arrive at answers using the available evidence. In addition, the consultants evaluated the quality of evidence supporting the answers on the basis of the number, type, and quality of the studies. In several areas, the process diverged from that previously described. The sections on hepatitis B virus (HBV) and hepatitis A virus (HAV) infections are based on previously or recently approved recommendations (2--4) of the Advisory Committee on Immunization Practices. The recommendations for STD screening during pregnancy were developed after CDC staff reviewed the recommendations from other knowledgeable groups. Throughout this report, the evidence used as the basis for specific recommendations is discussed briefly. More comprehensive, annotated discussions of such evidence will appear in background papers that will be published in a supplement issue of Clinical Infectious Diseases. When more than one therapeutic regimen is recommended, the sequence is in alphabetical order unless the choices for therapy are prioritized based on efficacy, convenience, or cost. For STDs with more than one recommended treatment regimen, it can be assumed that all regimens have similar efficacy and similar rates of intolerance or toxicity, unless otherwise specified. Persons treating STDs should use recommended regimens primarily; alternative regimens can be considered in instances of substantial drug allergy or other contraindications to the recommended regimens. These recommendations were developed in consultation with public and private sector professionals knowledgeable in the treatment of persons with STDs (see Consultants list). The recommendations are applicable to various patient-care settings, including family planning clinics, private physicians' offices, managed care organizations, and other primary-care facilities. These recommendations are meant to serve as a source of clinical guidance: health-care providers should always consider the individual clinical circumstances of each person in the context of local disease prevalence. These guidelines focus on the treatment and counseling of individual persons and do not address other community services and interventions that are important in STD/human immunodeficiency virus (HIV) prevention. Clinical Prevention GuidanceThe prevention and control of STDs are based on the following five major strategies: 1) education and counseling of persons at risk on ways to avoid STDs through changes in sexual behaviors; 2) identification of asymptomatically infected persons and of symptomatic persons unlikely to seek diagnostic and treatment services; 3) effective diagnosis and treatment of infected persons; 4) evaluation, treatment, and counseling of sex partners of persons who are infected with an STD; and 5) preexposure vaccination of persons at risk for vaccine-preventable STD. Primary prevention of STD begins with changing the sexual behaviors that place persons at risk for infection. Health-care providers have a unique opportunity to provide education and counseling to their patients. As part of the clinical interview, health-care providers should routinely and regularly obtain sexual histories from their patients and address management of risk reduction as indicated in this report. Guidance in obtaining a sexual history is available in Contraceptive Technology, 18th edition (5) and in the curriculum provided by CDC's STD/HIV Prevention Training Centers (http://www.stdhivpreventiontraining.org). Counseling skills, characterized by respect, compassion, and a nonjudgmental attitude toward all patients, are essential to obtaining a thorough sexual history and to delivering prevention messages effectively. Key techniques that can be effective in facilitating rapport with patients include the use of 1) open-ended questions (e.g., "Tell me about any new sex partners you've had since your last visit" and "what's your experience with using condoms been like?"), 2) understandable language ("have you ever had a sore or scab on your penis?"), and 3) normalizing language ("some of my patients have difficulty using a condom with every sex act. How is it for you?"). One approach to eliciting information concerning five key areas of interest has been summarized. The Five Ps: Partners, Prevention of Pregnancy, Protection from STDs, Practices, Past History of STDs 1. Partners
For condom answers
Additional questions to identify HIV and hepatitis risk
Patients should be reassured that treatment will be provided regardless of individual circumstances (e.g., ability to pay, citizenship or immigration status, language spoken, or specific sex practices). Many patients seeking treatment or screening for a particular STD should be evaluated for all common STDs; even so, all patients should be informed concerning all the STDs for which they are being tested and if testing for a common STD (e.g., genital herpes) is not being performed. STD/HIV Prevention CounselingEffective delivery of prevention messages requires that providers integrate communication of general risk reduction messages that are relevant to the client (i.e., client-centered counseling) and education regarding specific actions that can reduce the risk for STD/HIV transmission (e.g., abstinence, condom use, limiting the number of sex partners, modifying sexual behaviors, and vaccination). Each of these specific actions is discussed separately in this report.
In addition to individual prevention counseling, some videos and large group presentations provide explicit information concerning how to use condoms correctly. These have been effective in reducing the occurrence of additional STDs among persons at high risk, including STD clinic patients and adolescents. Because the incidence of some STDs, notably syphilis, has increased in HIV-infected persons, the use of client-centered STD counseling for HIV-infected persons has received strong emphasis from public health agencies and organizations. Consensus guidelines issued by CDC, the Health Resources and Services Administration, the HIV Medicine Association of the Infectious Diseases Society of America, and the National Institutes of Health emphasize that STD/HIV risk assessment, STD screening, and client-centered risk reduction counseling should be provided routinely to HIV-infected persons (9). Several specific methods have been designed for the HIV care setting (10--12). Additional information regarding these approaches is available at http://effectiveinterventions.org. Prevention MethodsClient-Initiated Interventions to Reduce Sexual Transmission of STD/HIV and Unintended PregnancyAbstinence and Reduction of Number of Sex PartnersThe most reliable way to avoid transmission of STDs is to abstain from sex (i.e., oral, vaginal, or anal sex) or to be in a long-term, mutually monogamous relationship with an uninfected partner. Counseling that encourages abstinence from sexual intercourse is crucial for persons who are being treated for an STD (or whose partners are undergoing treatment) and for persons who want to avoid the possible consequences of sex completely (e.g., STD/HIV and unintended pregnancy). A more comprehensive discussion of abstinence is available in Contraceptive Technology, 18th edition (5). For persons embarking on a mutually monogamous relationship, screening for common STDs before initiating sex might reduce the risk for future transmission of asymptomatic STDs. Preexposure VaccinationPreexposure vaccination is one of the most effective methods for preventing transmission of some STDs. For example, because HBV infection is frequently sexually transmitted, hepatitis B vaccination is recommended for all unvaccinated, uninfected persons being evaluated for an STD. In addition, hepatitis A vaccine is licensed and is recommended for men who have sex with men (MSM) and illicit drug users (i.e., both injecting and noninjecting). Specific details regarding hepatitis A and B vaccination are available at http://www.cdc.gov/hepatitis. A quadrivalent vaccine against human papillomavirus (HPV types 6, 11, 16, 18) is now available and licensed for females aged 9--26 years. Vaccine trials for other STDs are being conducted. Male CondomsWhen used consistently and correctly, male latex condoms are highly effective in preventing the sexual transmission of HIV infection (i.e., HIV-negative partners in heterosexual serodiscordant relationships in which condoms were consistently used were 80% less likely to become HIV-infected compared with persons in similar relationships in which condoms were not used) and can reduce the risk for other STDs, including chlamydia, gonorrhea, and trichomoniasis, and might reduce the risk of women developing pelvic inflammatory disease (PID) (13,14). Condom use might reduce the risk for transmission of herpes simplex virus-2 (HSV-2), although data for this effect are more limited (15,16). Condom use might reduce the risk for HPV-associated diseases (e.g., genital warts and cervical cancer [17]) and mitigate the adverse consequences of infection with HPV, as their use has been associated with higher rates of regression of cervical intraepithelial neoplasia (CIN) and clearance of HPV infection in women (18), and with regression of HPV-associated penile lesions in men (19). A limited number of prospective studies have demonstrated a protective effect of condoms on the acquisition of genital HPV; one recent prospective study among newly sexually active college women demonstrated that consistent condom use was associated with a 70% reduction in risk for HPV transmission (20). Condoms are regulated as medical devices and are subject to random sampling and testing by the Food and Drug Administration (FDA). Each latex condom manufactured in the United States is tested electronically for holes before packaging. Rates of condom breakage during sexual intercourse and withdrawal are approximately two broken condoms per 100 condoms used in the United States. The failure of condoms to protect against STD transmission or unintended pregnancy usually results from inconsistent or incorrect use rather than condom breakage. Male condoms made of materials other than latex are available in the United States. Although they have had higher breakage and slippage rates when compared with latex condoms and are usually more costly, the pregnancy rates among women whose partners use these condoms are similar to latex condoms. Two general categories of nonlatex condoms exist. The first type is made of polyurethane or other synthetic material and provides protection against STD/HIV and pregnancy equal to that of latex condoms. These can be substituted for persons with latex allergy. The second type is natural membrane condoms (frequently called "natural" condoms or, incorrectly, lambskin condoms). These condoms are usually made from lamb cecum and can have pores up to 1500 nm in diameter. Whereas these pores do not allow the passage of sperm, they are more than 10 times the diameter of HIV and more than 25 times that of HBV. Moreover, laboratory studies demonstrate that viral STD transmission can occur with natural membrane condoms. Using natural membrane condoms for protection against STDs is not recommended. Patients should be advised that condoms must be used consistently and correctly to be effective in preventing STDs, and they should be instructed in the correct use of condoms. The following recommendations ensure the proper use of male condoms:
Female CondomsLaboratory studies indicate that the female condom (Reality™), which consists of a lubricated polyurethane sheath with a ring on each end that is inserted into the vagina, is an effective mechanical barrier to viruses, including HIV, and to semen (21). A limited number of clinical studies have evaluated the efficacy of female condoms in providing protection from STDs, including HIV (22). If used consistently and correctly, the female condom might substantially reduce the risk for STDs. When a male condom cannot be used properly, sex partners should consider using a female condom. Female condoms are costly compared with male condoms. The female condom also has been used for STD/HIV protection during receptive anal intercourse (23). Whereas it might provide some protection in this setting, its efficacy is undefined. Vaginal Spermicides and DiaphragmsVaginal spermicides containing nonoxynol-9 (N-9) are not effective in preventing cervical gonorrhea, chlamydia, or HIV infection (24). Furthermore, frequent use of spermicides containing N-9 has been associated with disruption of the genital epithelium, which might be associated with an increased risk for HIV transmission. Therefore, N-9 is not recommended for STD/HIV prevention. In case-control and cross-sectional studies, diaphragm use has been demonstrated to protect against cervical gonorrhea, chlamydia, and trichomoniasis; a randomized controlled trial will be conducted. On the basis of all available evidence, diaphragms should not be relied on as the sole source of protection against HIV infection. Diaphragm and spermicide use have been associated with an increased risk for bacterial urinary tract infections in women. Condoms and N-9 Vaginal SpermicidesCondoms lubricated with spermicides are no more effective than other lubricated condoms in protecting against the transmission of HIV and other STDs, and those that are lubricated with N-9 pose the concerns that have been previously discussed. Use of condoms lubricated with N-9 is not recommended for STD/HIV prevention because spermicide-coated condoms cost more, have a shorter shelf-life than other lubricated condoms, and have been associated with urinary tract infection in young women. Rectal Use of N-9 SpermicidesRecent studies indicate that N-9 might increase the risk for HIV transmission during vaginal intercourse (24). Although similar studies have not been conducted among men who use N-9 spermicide during anal intercourse with other men, N-9 can damage the cells lining the rectum, which might provide a portal of entry for HIV and other sexually transmissible agents. Therefore, N-9 should not be used as a microbicide or lubricant during anal intercourse. Nonbarrier Contraception, Surgical Sterilization, and HysterectomySexually active women who are not at risk for pregnancy might incorrectly perceive themselves to be at no risk for STDs, including HIV infection. Contraceptive methods that are not mechanical barriers offer no protection against HIV or other STDs. Women who use hormonal contraception (e.g., oral contraceptives, Norplant™, and Depo-Provera™), have intrauterine devices (IUD), have been surgically sterilized, or have had hysterectomies should be counseled regarding the use of condoms and the risk for STDs, including HIV infection. Emergency Contraception (EC)Emergency use of oral contraceptive pills containing levonorgesterol alone reduces the risk for pregnancy after unprotected intercourse by 89%. Pills containing a combination of ethinyl estradiol and either norgestrel or levonorgestrel can be used and reduce the risk for pregnancy by 75%. Emergency insertion of a copper IUD also is highly effective, reducing the risk by as much as 99%. EC with oral contraceptive pills should be initiated as soon as possible after unprotected intercourse and definitely within 120 hours (i.e., 5 days). The only medical contraindication to provision of EC is current pregnancy. Providers who manage persons at risk for STDs should counsel women concerning the option for EC, if indicated, and provide it in a timely fashion if desired by the woman. Plan B (two 750 mcg levonorgestrel tablets) has been approved by FDA and is available in the United States for the prevention of unintended pregnancy. Additional information on EC is available in Contraceptive Technology, 18th edition (5), or at http://www.arhp.org/healthcareproviders/resources/contraceptionresources. Postexposure Prophylaxis (PEP) for HIVGuidelines for the use of PEP aimed at preventing HIV acquisition as a result of sexual exposure are available and are discussed in this report (see Sexual Assault and STDs). Partner ManagementPartner notification, previously referred to as "contact tracing" but recently included in the broader category of partner services, is the process by which providers or public health authorities learn from persons with STDs about their sex partners and help to arrange for the evaluation and treatment of sex partners. Providers can seek this information and help to arrange for evaluation and treatment of sex partners, either directly or with assistance from state and local health departments. The intensity of partner services and the specific STDs for which they are offered vary among providers, agencies, and geographic areas. Ideally, such services should be accompanied by health counseling and might include referral of patients and their partners for other services, whenever appropriate. In general, whether partner notification effectively decreases exposure to STDs and whether it changes the incidence and prevalence of STDs in a community are uncertain. The paucity of supporting evidence regarding the effectiveness of partner notification has spurred the exploration of alternative approaches. One such approach is to place partner notification in a larger context by making interventions in the sexual and social networks in which persons are exposed to STDs. Prospective evaluations incorporating assessment of venues, community structure, and social and sexual, contacts in conjunction with partner notification of efforts are promising in terms of increasing case-finding and warrant further exploration. The scope of such efforts probably precludes individual clinician efforts to use network-based approaches, but STD-control programs might find them useful. Many persons individually benefit from partner notification. When partners are treated, index patients have reduced risk for reinfection. At a population level, partner notification can disrupt networks of STD transmission and reduce disease incidence. Therefore, providers should encourage their patients with STDs to notify their sex partners and urge them to seek medical evaluation and treatment, regardless of whether assistance is available from health agencies. When medical evaluation, counseling, and treatment of partners cannot be done because of the particular circumstances of a patient or partner or because of resource limitations, other partner management options can be considered. One option is patient-delivered therapy, a form of expedited partner therapy (EPT) in which partners of infected patients are treated without previous medical evaluation or prevention counseling (http://www.cdc.gov/std/treatment/EPTFinalReport2006.pdf). The evidence supporting patient-delivered therapy is based on three clinical trials that included heterosexual men and women with chlamydia or gonorrhea. The strength of the supporting evidence differed by STD and by the sex of the index case when reinfection of the index case was the measured outcome (25--27). Despite this variation, patient-delivered therapy (i.e., via medications or prescriptions) can prevent reinfection of index case and has been associated with a higher likelihood of partner notification, compared with unassisted patient referral of partners. Medications and prescriptions for patient-delivered therapy should be accompanied by treatment instructions, appropriate warnings about taking medications if pregnant, general health counseling, and advice that partners should seek personal medical evaluations, particularly women with symptoms of STDs or PID. Existing data suggest that EPT has a limited role in partner management for trichomoniasis (28). No data support its use in the routine management of syphilis. There is no experience with expedited partner therapy for gonorrhea or chlamydia infection among MSM. Currently, EPT is not feasible in many settings because of operational barriers, including the lack of clear legal status of EPT in some states. Reporting and ConfidentialityThe accurate and timely reporting of STDs is integrally important for assessing morbidity trends, targeting limited resources, and assisting local health authorities in partner notification and treatment. STD/HIV and acquired immunodeficiency syndrome (AIDS) cases should be reported in accordance with state and local statutory requirements. Syphilis, gonorrhea, chlamydia, chanroid, HIV infection, and AIDS are reportable diseases in every state. The requirements for reporting other STDs differ by state, and clinicians should be familiar with state and local reporting requirements. Reporting can be provider- and/or laboratory-based. Clinicians who are unsure of state and local reporting requirements should seek advice from state or local health departments or STD programs. STD and HIV reports are kept strictly confidential. In the majority of jurisdictions, such reports are protected by statute from subpoena. Before public health representatives conduct a follow-up of a positive STD-test result, they should consult the patient's health-care provider to verify the diagnosis and treatment. Special PopulationsPregnant WomenIntrauterine or perinatally transmitted STDs can have severely debilitating effects on pregnant women, their partners, and their fetuses. All pregnant women and their sex partners should be asked about STDs, counseled about the possibility of perinatal infections, and ensured access to treatment, if needed. Recommended Screening Tests
Other Concerns
For a more detailed discussion of STD testing and treatment among pregnant women and other infections not transmitted sexually, refer to the following references: Guide to Clinical Preventive Services (29); Guidelines for Perinatal Care (30); ACOG Practice Bulletin: Prophylatic Antibiotics in Labor and Delivery (31); ACOG Committee Opinion: Primary and Preventive Care: Periodic Assessments (32); Recommendations for the Prevention and Management of Chlamydia trachomatis Infections (33); Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States---Recommendations of the Immunization Practices Advisory Committee (ACIP) (2,4); Mother-To-Infant Transmission of Hepatitis C Virus (34); Hepatitis C: Screening in Pregnancy (35); American College of Obstetricians and Gynecologists (ACOG) Educational Bulletin: Viral Hepatitis in Pregnancy (36); Revised Public Health Service Recommendations for HIV Screening of Pregnant Women (37); Prenatal and Perinatal Human Immunodeficiency Virus Testing: Expanded Recommendations (38); US Preventative Task Force HIV Screening Guidelines (39); Rapid HIV Antibody Testing During Labor and Delivery for Women of Unknown HIV Status: A Practical Guide and Model Protocol (40); and Sexually Transmitted Diseases in Adolescents (41). These sources are not entirely consistent in their recommendations. For example, the Guide to Clinical Preventive Services recommends screening of patients at high risk for chlamydia but indicates that the optimal timing for screening is uncertain. The Guidelines for Perinatal Care recommends that pregnant women at high risk for chlamydia be screened for infection during the first prenatal care visit and during the third trimester. Recommendations to screen pregnant women for STDs are based on disease severity and sequelae, prevalence in the population, costs, medicolegal considerations (e.g., state laws), and other factors. The screening recommendations in this report are broader (i.e., if followed, more women will be screened for more STDs than would be screened by following other recommendations) and are compatible with other CDC guidelines. AdolescentsThe rates of many STDs are highest among adolescents. For example, the reported rates of chlamydia and gonorrhea are highest among females aged 15--19 years, and many persons acquire HPV infection during their adolescent years. Among adolescents with acute HBV infection, the most commonly reported risk factors are having sexual contact with a chronically infected person or with multiple sex partners, or reporting their sexual preference as homosexual. As part of a comprehensive strategy to eliminate HBV transmission in the United States, ACIP has recommended that all children and adolescents be administered HBV vaccine (2). Younger adolescents (i.e., persons aged <15 years) who are sexually active are at particular risk for STDs, especially youth in detention facilities, STD clinic patients, male homosexuals, and injecting-drug users (IDUs). Adolescents are at higher risk for STDs because they frequently have unprotected intercourse, are biologically more susceptible to infection, are engaged in sexual partnerships frequently of limited duration, and face multiple obstacles to using health care. Several of these issues can be addressed by clinicians who provide services to adolescents. Clinicians can address adolescents' lack of knowledge and awareness regarding the risks and consequences of STDs by offering guidance concerning healthy sexual behavior and, therefore, prevent the establishment of patterns of behavior that can undermine sexual health. With a few exceptions, all adolescents in the United States can legally consent to the confidential diagnosis and treatment of STDs. In all 50 states and the District of Columbia, medical care for STDs can be provided to adolescents without parental consent or knowledge. In addition, in the majority of states, adolescents can consent to HIV counseling and testing. Consent laws for vaccination of adolescents differ by state. Several states consider provision of vaccine similar to treatment of STDs and provide vaccination services without parental consent. Because of the crucial importance of confidentially, health-care providers should follow policies that provide confidentiality and comply with state laws for STD services. Despite the prevalence of STDs among adolescents, providers frequently fail to inquire about sexual behavior, assess risk for STDs, provide counseling on risk reduction, and screen for asymptomatic infection during clinical encounters. The style and content of counseling and health education on these sensitive subjects should be adapted for adolescents. Discussions should be appropriate for the patient's developmental level and should be aimed at identifying risky behaviors (e.g., sex and drug-use behaviors). Careful, nonjudgmental, and thorough counseling are particularly vital for adolescents who might not acknowledge that they engage in high-risk behaviors. ChildrenManagement of children who have STDs requires close cooperation between clinicians, laboratorians, and child-protection authorities. Official investigations, when indicated, should be initiated promptly. Some diseases (e.g., gonorrhea, syphilis, and chlamydia), if acquired after the neonatal period, are virtually 100% indicative of sexual contact. For other diseases (e.g., HPV infection and vaginitis), the association with sexual contact is not as clear (see Sexual Assault and STDs). MSMSome MSM are at high risk for HIV infection and other viral and bacterial STDs. The frequency of unsafe sexual practices and the reported rates of bacterial STDs and incident HIV infection have declined substantially in MSM from the 1980s through the mid-1990s. However, during the previous 10 years, increased rates of infectious syphilis, gonorrhea, and chlamydial infection and of higher rates of unsafe sexual behaviors have been documented among MSM in the United States and virtually all industrialized countries. The effect of these behavioral changes on HIV transmission has not been ascertained, but preliminary data suggest that the incidence of HIV infection might be increasing among some MSM. These adverse trends probably are related to changing attitudes concerning HIV infection because of the effects of improved HIV/AIDS therapy on quality of life and survival, changing patterns of substance abuse, demographic shifts in MSM populations, and changes in sex partner networks resulting from new venues for partner acquisition. Clinicians should assess the risks of STDs for all male patients, including a routine inquiry about the sex of patients' sex partners. MSM, including those with HIV infection, should routinely undergo nonjudgmental STD/HIV risk assessment and client-centered prevention counseling to reduce the likelihood of acquiring or transmitting HIV or other STDs. Clinicians should be familiar with local community resources available to assist MSM at high risk in facilitating behavioral change. Clinicians also should routinely ask sexually active MSM about symptoms consistent with common STDs, including urethral discharge, dysuria, genital and perianal ulcers, regional lymphadenopathy, skin rash, and anorectal symptoms consistent with proctitis. Clinicians also should maintain a low threshold for diagnostic testing of symptomatic patients. Routine laboratory screening for common STDs is indicated for all sexually active MSM. The following screening recommendations are based on preliminary data (42,43). These tests should be performed at least annually for sexually active MSM, including men with or without established HIV infection:
In addition, some specialists would consider type-specific serologic tests for HSV-2, if infection status is unknown. Routine testing for anal cytologic abnormalities or anal HPV infection is not recommended until more data are available on the reliability of screening methods, the safety of and response to treatment, and programmatic considerations. More frequent STD screening (i.e., at 3--6 month intervals) is indicated for MSM who have multiple or anonymous partners, have sex in conjunction with illicit drug use, use methamphetamine, or whose sex partners participate in these activities. Vaccination against hepatitis A and B is recommended for all MSM in whom previous infection or immunization cannot be documented. Preimmunization serologic testing might be considered to reduce the cost of vaccinating MSM who are already immune to these infections, but this testing should not be delay vaccination. Vaccinating persons who are immune to HAV or HBV infection because of previous infection or vaccination does not increase the risk for vaccine-related adverse events (see Hepatitis B, Prevaccination Antibody Screening). Women Who Have Sex with Women (WSW)Few data are available on the risk of STDs conferred by sex between women, but transmission risk probably varies by the specific STD and sexual practice (e.g., oral-genital sex, vaginal or anal sex using hands, fingers, or penetrative sex items, and oral-anal sex) (44,45). Practices involving digital-vaginal or digital-anal contact, particularly with shared penetrative sex items, present a possible means for transmission of infected cervicovaginal secretions. This possibility is most directly supported by reports of metronidazole-resistant trichomoniasis and genotype-concordant HIV transmitted sexually between women who reported these behaviors and by the high prevalence of BV among monogamous WSW. Transmission of HPV can occur with skin-to-skin or skin-to-mucosa contact, which can occur during sex between women. HPV deoxyribonucleic acid (DNA) has been detected through polymerase chain reaction (PCR)-based methods from the cervix, vagina, and vulva in 13%--30% of WSW, and high- and low-grade squamous intraepithelial lesions (SIL) have been detected on Pap tests in WSW who reported no previous sex with men (46). However, the majority of self-identified WSW (53%--99%) have had sex with men and might continue this practice (47). Therefore, all women should undergo Pap test screening using current national guidelines, regardless of sexual preference or sexual practices. HSV-2 genital transmission between female sex partners is probably inefficient, but the relatively frequent practice of orogenital sex among WSW might place them at higher risk for genital infection with HSV-1. This hypothesis is supported by the recognized association between HSV-1 seropositivity and previous number of female partners among WSW. Transmission of syphilis between female sex partners, probably through oral sex, has been reported. Although the rate of transmission of C. trachomatis between women is unknown, WSW who also have sex with men are at risk and should undergo routine screening according to guidelines. HIV Infection: Detection, Counseling, and ReferralInfection with HIV produces a spectrum of disease that progresses from a clinically latent or asymptomatic state to AIDS as a late manifestation. The pace of disease progression varies. In untreated patients, the time between infection with HIV and the development of AIDS ranges from a few months to as long as 17 years (median: 10 years). The majority of adults and adolescents infected with HIV remain symptom-free for extended periods, but viral replication is active during all stages of infection and increases substantially as the immune system deteriorates. In the absence of treatment, AIDS will develop eventually in nearly all HIV-infected persons. Improvements in antiretroviral therapy and increasing awareness among both patients and health-care providers of the risk factors associated with HIV transmission have led to more testing for HIV and earlier diagnosis, frequently before symptoms develop. However, the conditions of nearly 40% of persons who acquire HIV infection continue to be diagnosed late, within 1 year of acquiring AIDS. Prompt diagnosis of HIV infection is essential for multiple reasons. Treatments are available that slow the decline of immune system function; use of these therapies has been associated with substantial declines in HIV-associated morbidity and mortality in recent years. HIV-infected persons who have altered immune function are at increased risk for infections for which preventive measures are available (e.g., Pneumocystis jiroveci pneumonia, toxoplasma encephalitis [TE], disseminated Mycobacterium avium complex [MAC] disease, tuberculosis [TB], and bacterial pneumonia). Because of its effect on the immune system, HIV affects the diagnosis, evaluation, treatment, and follow-up of multiple other diseases and might affect the efficacy of antimicrobial therapy for some STDs. Finally, the early diagnosis of HIV enables health-care providers to counsel infected patients, refer them to various support services, and help prevent HIV transmission to others. Acutely infected persons might have elevated HIV viral loads and, therefore, might be more likely to transmit HIV to their partners (48,49). Proper management of HIV infection involves a complex array of behavioral, psychosocial, and medical services. Although some services might not be available in STD treatment facilities. Therefore, referral to a health-care provider or facility experienced in caring for HIV-infected patients is advised. Providers working in STD-treatment facilities should be knowledgeable about the options for referral available in their communities. While receiving care in STD-treatment facilities, HIV-infected patients should be educated about HIV infection and the various options available for support services and HIV care. A detailed discussion of the multiple, complex services required for management of HIV infection is beyond the scope of this section; however, this information is available in other published resources (6,9,50,51). In subsequent sections, this report provides information regarding diagnostic testing for HIV infection, counseling patients who have HIV infection, referral of patients for support services, including medical care, and the management of sex and injecting-drug partners in STD-treatment facilities. In addition, the report discusses HIV infection during pregnancy and in infants and children. Detection of HIV Infection: Screening and Diagnostic TestingAll persons who seek evaluation and treatment for STDs should be screened for HIV infection. Screening should be routine, regardless of whether the patient is known or suspected to have specific behavioral risks for HIV infection. Consent and Pretest Information HIV screening should be voluntary and conducted only with the patient's knowledge and understanding that testing is planned. Persons should be informed orally or in writing that HIV testing will be performed unless they decline (i.e., opt-out screening). Oral or written communications should include an explanation of positive and negative test results, and patients should be offered an opportunity to ask questions and to decline testing. Prevention Counseling Prevention counseling does not need to be explicitly linked to the HIV-testing process. However, some patients might be more likely to think about HIV and consider their risks when undergoing an HIV test. HIV testing might present an ideal opportunity to provide or arrange for prevention counseling to assist with behavior changes that can reduce risk for acquiring HIV infection. Prevention counseling should be offered and encouraged in all health-care facilities serving patients at high risk and in those (e.g., STD clinics) where information on HIV-risk behaviors is routinely elicited. Diagnostic Testing HIV infection usually is diagnosed by tests for antibodies against HIV-1. Some combination tests also detect antibodies against HIV-2 (i.e., HIV-1/2). Antibody testing begins with a sensitive screening test (e.g., the enzyme immunoassay [EIA] or rapid test). The advent of HIV rapid testing has enabled clinicians to make a substantially accurate presumptive diagnosis of HIV-1 infection within half an hour. This testing can facilitate the identification of the more than 250,000 persons living with undiagnosed HIV in the United States. Reactive screening tests must be confirmed by a supplemental test (e.g., the Western blot [WB]) or an immunofluorescence assay (IFA) (52). If confirmed by a supplemental test, a positive antibody test result indicates that a person is infected with HIV and is capable of transmitting the virus to others. HIV antibody is detectable in at least 95% of patients within 3 months after infection. Although a negative antibody test result usually indicates that a person is not infected, antibody tests cannot exclude recent infection. The majority of HIV infections in the United States are caused by HIV-1. However, HIV-2 infection should be suspected in persons who have epidemiologic risk factors, including being from West Africa (where HIV-2 is endemic) or have sex partners from endemic areas, have sex partners known to be infected with HIV-2, or have received a blood transfusion or nonsterile injection in a West African country. HIV-2 testing also is indicated when clinical evidence of HIV exists but tests for HIV-1 antibodies or HIV-1 viral load are not positive, or when HIV-1 WB results include the unusual indeterminate pattern of gag (p55, p24, p17) plus pol (p66, p51, p31) bands in the absence of env (gp160, gp120, gp41) bands. Health-care providers should be knowledgeable about the symptoms and signs of acute retroviral syndrome, which is characterized by fever, malaise, lymphadenopathy, and skin rash. This syndrome frequently occurs in the first few weeks after HIV infection, before antibody test results become positive. Suspicion of acute retroviral syndrome should prompt nucleic acid testing (HIV plasma ribonucleic acid [RNA]) to detect the presence of HIV, although not all nucleic acid tests are approved for diagnostic purposes; a positive HIV nucleic acid test should be confirmed by subsequent antibody testing to document seroconversion (using standard methods, EIA, and WB). Acutely infected patients might be highly contagious because of increased plasma and genital HIV RNA concentrations and might be continuing to engage in risky behaviors (48,49). Current guidelines suggest that persons with recently acquired HIV infection might benefit from antiretroviral drugs and be candidates for clinical trials (53,54). Therefore, patients with acute HIV infection should be referred immediately to an HIV clinical care provider. Diagnosis of HIV infection should prompt efforts to reduce the risk behavior that resulted in HIV infection and could result in transmission of HIV to others (55). Early counseling and education are particularly important for persons with recently acquired infection because HIV plasma RNA levels are characteristically high during this phase of infection and probably constitute an increased risk for HIV transmission. The following are specific recommendations for diagnostic testing for HIV infection:
Counseling for Patients with HIV Infection and Referral to Support ServicesPersons can be expected to be distressed when first informed of a positive HIV test result. Such persons face multiple major adaptive challenges, including 1) accepting the possibility of a shortened life span, 2) coping with the reactions of others to a stigmatizing illness, 3) developing and adopting strategies for maintaining physical and emotional health, and 4) initiating changes in behavior to prevent HIV transmission to others. Many persons will require assistance with making reproductive choices, gaining access to health services, confronting possible employment or housing discrimination, and coping with changes in personal relationships. Therefore, behavioral and psychosocial services are an integral part of health care for HIV-infected persons. Such services should be available on site or through referral when HIV infection is diagnosed. A comprehensive discussion of specific recommendations is available in the Guidelines for HIV Counseling, Testing, and Referral and Revised Recommendations for HIV Screening of Pregnant Women (6). Innovative and successful interventions to decrease risk taking by HIV-infected patients have been developed for diverse populations (12). Practice settings for offering HIV care differ depending on local resources and needs. Primary care providers and outpatient facilities should ensure that appropriate resources are available for each patient to avoid fragmentation of care. Although a single source that is capable of providing comprehensive care for all stages of HIV infection is preferred, the limited availability of such resources frequently results in the need to coordinate care among medical and social service providers in different locations. Providers should avoid long delays between diagnosis of HIV infection and access to additional medical and psychosocial services. The use of HIV rapid testing can help avoid unnecessary delays. Recently identified HIV infection might not have been recently acquired. Persons newly diagnosed with HIV might be at any stage of infection. Therefore, health-care providers should be alert for symptoms or signs that suggest advanced HIV infection (e.g., fever, weight loss, diarrhea, cough, shortness of breath, and oral candidiasis). The presence of any of these symptoms should prompt urgent referral for specialty medical care. Similarly, providers should be alert for signs of psychologic distress and be prepared to refer patients accordingly. Diagnosis of HIV infection reinforces the need to counsel patients regarding high-risk behaviors because the consequences of such behaviors include the risk for acquiring additional STDs and for transmitting HIV (and other STDs) to other persons. Such attention to behaviors in HIV-infected persons is consistent with national strategies for HIV prevention (55). Providers should refer patients for prevention counseling and risk-reduction support concerning high-risk behaviors (e.g., substance abuse and high-risk sexual behaviors). In multiple recent studies, researchers have developed successful prevention interventions for different HIV-infected populations that can be adapted to individuals (56,57). Persons with newly diagnosed HIV infection who receive care in the STD treatment setting should be educated concerning what to expect as they enter medical care for HIV infection (51). In nonemergent situations, the initial evaluation of HIV-positive patients usually includes the following:
Some specialists recommend type-specific testing for HSV-2 if herpes infection status is unknown. A first dose of hepatitis A and/or hepatitis B vaccination for previously unvaccinated persons for whom vaccine is recommended (see Hepatitis A and Hepatitis B) should be administered at this first visit. In subsequent visits, when the results of laboratory and skin tests are available, antiretroviral therapy may be offered, if indicated, after initial antiretroviral resistance testing is performed (53) and specific prophylactic medications are administered to reduce the incidence of opportunistic infections (e.g., Pneumocystis jiroveci pneumonia, TE, disseminated MAC infection, and TB) (50). The vaccination series for hepatitis A and/or B should be offered for those in whom vaccination is recommended. Influenza vaccination should be offered annually, and pneumococcal vaccination should be given if it has not been administered in the previous 5 years (50,51). Providers should be alert to the possibility of new or recurrent STDs and should treat such conditions aggressively. The occurrence of an STD in an HIV-infected person is an indication of high-risk behavior and should prompt referral for counseling. Because many STDs are asymptomatic, routine screening for curable STDs (e.g., syphilis, gonorrhea, and chlamydia) should be performed at least yearly for sexually active persons. Women should be screened for cervical cancer precursor lesions by annual Pap smears. More frequent STD screening might be appropriate depending on individual risk behaviors, the local epidemiology of STDs, and whether incident STDs are detected by screening or by the presence of symptoms. Newly diagnosed HIV-infected persons should receive or be referred for a thorough psychosocial evaluation, including ascertainment of behavioral factors indicating risk for transmitting HIV. Patients might require referral for specific behavioral intervention (e.g., a substance abuse program), mental health disorders (e.g., depression), or emotional distress. They might require assistance with securing and maintaining employment and housing. Women should be counseled or appropriately referred regarding reproductive choices and contraceptive options. Patients with multiple psychosocial problems might be candidates for comprehensive risk-reduction counseling and services (8). The following are specific recommendations for HIV counseling and referral:
Several innovative interventions for HIV prevention have been developed for diverse at-risk populations, and these can be locally replicated or adapted (11,12). Involvement of nongovernment organizations and community-based organizations might complement such efforts in the clinical setting. Management of Sex Partners and Injecting-Drug Partners Clinicians evaluating HIV-infected persons should collect information to determine whether any partners should be notified concerning possible exposure to HIV (6). When referring to persons who are infected with HIV, the term "partner" includes not only sex partners but also IDUs who share syringes or other injection equipment. The rationale for partner notification is that the early diagnosis and treatment of HIV infection in these partners might reduce morbidity and provides the opportunity to encourage risk-reducing behaviors. Partner notification for HIV infection should be confidential and depends on the voluntary cooperation of the patient. Specific guidance regarding spousal notification may vary by jurisdiction. Two complementary notification processes, patient referral and provider referral, can be used to identify partners. With patient referral, patients directly inform their partners of their exposure to HIV infection. With provider referral, trained health department personnel locate partners on the basis of the names, descriptions, and addresses provided by the patient. During the notification process, the confidentiality of patients is protected; their names are not revealed to partners who are notified. Many state and local health departments provide these services. The following are specific recommendations for implementing partner-notification procedures:
Special ConsiderationsPregnancy. All pregnant women in the United States should be tested for HIV infection as early during pregnancy as possible. Testing should occur after the patient is notified that she will be tested for HIV as part of the routine panel of prenatal tests, unless she declines (i.e., opt-out screening) (30--32). For women who decline, providers should continue to strongly encourage testing and address concerns that pose obstacles to testing. Women who decline testing because they have had a previous negative HIV test should be informed of the importance of retesting during each pregnancy. Testing pregnant women is particularly important, not only to maintain the health of the patient, but also because interventions (i.e., antiretroviral and obstetrical) can reduce the risk of perinatal transmission of HIV. After pregnant women have been identified as being HIV-infected, they should be educated about the risk of perinatal infection. Evidence indicates that, in the absence of antiretroviral and other interventions, 15%--25% of infants born to HIV-infected mothers will become infected with HIV; such evidence also indicates that an additional 12%--14% will become infected during breastfeeding where HIV-infected women breastfeed their infants into the second year of life (59,60). The risk of perinatal HIV transmission can be reduced substantially to <2% through the use of antiretroviral regimens and obstetrical interventions (i.e., zidovudine or nevirapine and elective cesarean section at 38 weeks of pregnancy) and by avoiding breastfeeding (61). Pregnant women who are HIV infected should be counseled concerning their options (either on-site or by referral), given appropriate antenatal treatment, and advised not to breastfeed their infants (for women living in the United States, where infant formula is readily available and can be safely prepared). HIV Infection Among Infants and Children. Diagnosis of HIV infection in a pregnant woman indicates the need to consider whether other children of the woman might be infected. Infants and young children with HIV infection differ from adults and adolescents with respect to the diagnosis, clinical presentation, and management of HIV disease. For example, because maternal HIV antibody passes through the placenta, antibody tests for HIV are expected to be positive in the sera of both infected and uninfected infants born to seropositive mothers. A definitive determination of HIV infection for an infant aged <18 months is usually based on HIV nucleic acid testing. Management of infants, children, and adolescents who are known or suspected to be infected with HIV requires referral to physicians familiar with the manifestations and treatment of pediatric HIV infection (50,51,62). Diseases Characterized by Genital UlcersManagement of Patients Who Have Genital UlcersIn the United States, the majority of young, sexually active patients who have genital ulcers have either genital herpes, syphilis, or chancroid. The frequency of each condition differs by geographic area and patient population; however, genital herpes is the most prevalent of these diseases. More than one of these diseases can be present in a patient who has genital ulcers. All three of these diseases has been associated with an increased risk for HIV infection. Not all genital ulcers are caused by sexually transmitted infections. A diagnosis based only on the patient's medical history and physical examination frequently is inaccurate. Therefore, all patients who have genital ulcers should be evaluated with a serologic test for syphilis and a diagnostic evaluation for genital herpes; in settings where chancroid is prevalent, a test for Haemophilus ducreyi should also be performed. Specific tests for evaluation of genital ulcers include 1) syphilis serology and either darkfield examination or direct immunofluorescence test for T. pallidum; 2) culture or antigen test for HSV; and 3) culture for H. ducreyi. No FDA-cleared PCR test for these organisms is available in the United States; however, such testing can be performed by clinical laboratories that have developed their own tests and conducted a Clinical Laboratory Improvement Amendment (CLIA) verification study. Type-specific serology for HSV-2 might be helpful in identifying persons with genital herpes (see Genital Herpes, Type-Specific Serologic Tests). Biopsy of genital ulcers might be helpful in identifying the cause of ulcers that are unusual or that do not respond to initial therapy. HIV testing should be performed on all patients who have genital ulcers caused by T. pallidum or H. ducreyi, and should be strongly considered for those who have genital ulcers caused by HSV (see Diagnostic Considerations, sections, Syphilis, Chancroid, and Genital Herpes Simplex Virus). Health-care providers frequently must treat patients before test results are available because early treatment decreases the possibility of ongoing transmission and because successful treatment of genital herpes depends on prompt initiation of therapy. The clinician should treat for the diagnosis considered most likely, on the basis of clinical presentation and epidemiologic circumstances. In some instances, treatment must be initiated for additional conditions because of diagnostic uncertainty. Even after complete diagnostic evaluation, at least 25% of patients who have genital ulcers have no laboratory-confirmed diagnosis. ChancroidIn the United States, chancroid usually occurs in discrete outbreaks, although the disease is endemic in some areas. Chancroid is a cofactor for HIV transmission, as are genital herpes and syphilis; high rates of HIV infection among patients who have chancroid occur in the United States and other countries. Approximately 10% of persons who have chancroid that was acquired in the United States are coinfected with T. pallidum or HSV; this percentage is higher in persons who have acquired chancroid outside the United States. A definitive diagnosis of chancroid requires the identification of H. ducreyi on special culture media that is not widely available from commercial sources; even when these media are used, sensitivity is <80%. No FDA-cleared PCR test for H. ducreyi is available in the United States, but such testing can be performed by clinical laboratories that have developed their own PCR test and conducted a CLIA verification study. The combination of a painful genital ulcer and tender suppurative inguinal adenopathy suggests the diagnosis of chancroid. A probable diagnosis of chancroid, for both clinical and surveillance purposes, can be made if all of the following criteria are met: 1) the patient has one or more painful genital ulcers; 2) the patient has no evidence of T. pallidum infection by darkfield examination of ulcer exudate or by a serologic test for syphilis performed at least 7 days after onset of ulcers; 3) the clinical presentation, appearance of genital ulcers and, if present, regional lymphadenopathy are typical for chancroid; and 4) a test for HSV performed on the ulcer exudate is negative. Treatment Successful treatment for chancroid cures the infection, resolves the clinical symptoms, and prevents transmission to others. In advanced cases, scarring can result, despite successful therapy. Recommended Regimens*
* Ciprofloxacin is contraindicated for pregnant and lactating women. Azithromycin and ceftriaxone offer the advantage of single-dose therapy. Worldwide, several isolates with intermediate resistance to either ciprofloxacin or erythromycin have been reported. Other Management Considerations Male patients who are uncircumcised and patients with HIV infection do not respond as well to treatment as those who are circumcised or HIV negative. Patients should be tested for HIV infection at the time chancroid is diagnosed. Patients should be retested for syphilis and HIV 3 months after the diagnosis of chancroid, if the initial test results were negative. Follow-Up Patients should be reexamined 3--7 days after initiation of therapy. If treatment is successful, ulcers usually improve symptomatically within 3 days and objectively within 7 days after therapy. If no clinical improvement is evident, the clinician must consider whether 1) the diagnosis is correct, 2) the patient is coinfected with another STD, 3) the patient is infected with HIV, 4) the treatment was not used as instructed, or 5) the H. ducreyi strain causing the infection is resistant to the prescribed antimicrobial. The time required for complete healing depends on the size of the ulcer; large ulcers might require >2 weeks. In addition, healing is slower for some uncircumcised men who have ulcers under the foreskin. Clinical resolution of fluctuant lymphadenopathy is slower than resolution for ulcers and might require needle aspiration or incision and drainage. Although needle aspiration of chancroid buboes is a simple procedure, incision and drainage might be preferred because of a reduced need for repeat drainage procedures. Management of Sex Partners Sex partners of patients who have chancroid should be examined and treated, regardless of whether symptoms of the disease are present, if they had sexual contact with the patient during the 10 days preceding the patient's onset of symptoms. Special Considerations PregnancyThe safety and efficacy of azithromycin for pregnant and lactating women have not been established. Ciprofloxacin is contraindicated during pregnancy and lactation. No adverse effects of chancroid on pregnancy outcome have been reported. HIV InfectionHIV-infected patients who have chancroid should be monitored closely because, as a group, these patients are more likely to experience treatment failure and to have ulcers that heal more slowly. HIV-infected patients might require longer courses of therapy than those recommended for HIV-negative patients, and treatment failures can occur with any regimen. Because evidence is limited concerning the therapeutic efficacy of the recommended ceftriaxone and azithromycin regimens in HIV-infected patients, these regimens should be used for such patients only if follow-up can be ensured. Some specialists prefer the erythromycin 7-day regimen for treating HIV-infected persons. Genital HSV InfectionsGenital herpes is a chronic, life-long viral infection. Two types of HSV have been identified, HSV-1 and HSV-2. The majority of cases of recurrent genital herpes are caused by HSV-2 although HSV-1 might become more common as a cause of first episode genital herpes. At least 50 million persons in the United States have genital HSV infection. The majority of persons infected with HSV-2 have not been diagnosed with genital herpes. Many such persons have mild or unrecognized infections but shed virus intermittently in the genital tract. The majority of genital herpes infections are transmitted by persons unaware that they have the infection or who are asymptomatic when transmission occurs. Diagnosis of HSV Infection The clinical diagnosis of genital herpes is both insensitive and nonspecific. The classical painful multiple vesicular or ulcerative lesions are absent in many infected persons. Up to 50% of first-episode cases of genital herpes are caused by HSV-1 (63), but recurrences and subclinical shedding are much less frequent for genital HSV-1 infection than genital HSV-2 infection (64,65). Therefore, whether genital herpes is caused by HSV-1 or HSV-2 influences prognosis and counseling. Therefore, the clinical diagnosis of genital herpes should be confirmed by laboratory testing (66). Both virologic and type-specific serologic tests for HSV should be available in clinical settings that provide care for patients with STDs or those at risk for STDs. Virologic Tests Isolation of HSV in cell culture is the preferred virologic test for patients who seek medical treatment for genital ulcers or other mucocutaneous lesions. However, the sensitivity of culture is low, especially for recurrent lesions, and declines rapidly as lesions begin to heal. PCR assays for HSV DNA are more sensitive and have been used instead of viral culture (67,68); however, PCR tests are not FDA-cleared for testing of genital specimens. PCR is the test of choice for detecting HSV in spinal fluid for diagnosis of HSV infection of the central nervous system (CNS). Viral culture isolates should be typed to determine if HSV-1 or HSV-2 is the cause of the infection. Lack of HSV detection (i.e., culture or PCR) does not indicate a lack of HSV infection, as viral shedding is intermittent. The use of cytologic detection of cellular changes of HSV infection is an insensitive and nonspecific method of diagnosis, both for genital lesions (i.e., Tzanck preparation) and for cervical Pap smears and should not be relied upon. Type-Specific Serologic Tests Both type-specific and nontype-specific antibodies to HSV develop during the first several weeks after infection and persist indefinitely. Accurate type-specific HSV serologic assays are based on the HSV-specific glycoprotein G2 (HSV-2) and glycoprotein G1 (HSV-1). Such assays first became commercially available in 1999, but older assays that do not accurately distinguish HSV-1 from HSV-2 antibody (despite claims to the contrary) remain on the market. Therefore, the serologic type-specific glycoprotein G (gG)-based assays should be specifically requested when serology is performed (69--71). The FDA-cleared glycoprotein G-based type-specific assays include the laboratory-based assays HerpeSelect™-1 enzyme-linked immunosorbent assay (ELISA) immunoglobulin G (IgG) or HerpeSelect™-2 ELISA IgG and HerpeSelect™ 1 and 2 Immunoblot IgG (Focus Technology, Inc., Herndon, Virginia), and HSV-2 ELISA (Trinity Biotech USA, Berkeley Heights, New Jersey). Two other assays, Biokit HSV-2 and SureVue HSV-2 (Biokit USA, Lexington, Massachusetts, and Fisher Scientific, Pittsburgh, Pennsylvania, respectively), are point-of-care tests that provide results for HSV-2 antibodies from capillary blood or serum during a clinic visit. The sensitivities of these glycoprotein G type-specific tests for the detection of HSV-2 antibody vary from 80%--98%, and false-negative results might be more frequent at early stages of infection. The specificities of these assays are >96%. False-positive results can occur, especially in patients with a low likelihood of HSV infection. Repeat or confirmatory testing might be indicated in some settings, especially if recent acquisition of genital herpes is suspected. Because nearly all HSV-2 infections are sexually acquired, the presence of type-specific HSV-2 antibody implies anogenital infection and education and counseling appropriate for persons with genital herpes should be provided. The presence of HSV-1 antibody alone is more difficult to interpret. The majority of persons with HSV-1 antibody have oral HSV infection acquired during childhood, which might be asymptomatic. However, acquisition of genital HSV-1 appears to be increasing, and genital HSV-1 also might be asymptomatic. Lack of symptoms in an HSV-1 seropositive person does not distinguish anogenital from orolabial or cutaneous infection. Persons with HSV-1 infection, regardless of site of infection, remain at risk for HSV-2 acquisition. Type-specific HSV serologic assays might be useful in the following scenarios: 1) recurrent genital symptoms or atypical symptoms with negative HSV cultures; 2) a clinical diagnosis of genital herpes without laboratory confirmation; and 3) a partner with genital herpes. Some specialists believe that HSV serologic testing should be included in a comprehensive evaluation for STDs among persons with multiple sex partners, HIV infection, and among MSM at increased risk for HIV acquisition. Screening for HSV-1 or HSV-2 in the general population is not indicated. Principles of Management of Genital Herpes Antiviral chemotherapy offers clinical benefits to the majority of symptomatic patients and is the mainstay of management. Counseling regarding the natural history of genital herpes, sexual and perinatal transmission, and methods to reduce transmission is integral to clinical management. Systemic antiviral drugs can partially control the signs and symptoms of herpes episodes when used to treat first clinical and recurrent episodes, or when used as daily suppressive therapy. However, these drugs neither eradicate latent virus nor affect the risk, frequency, or severity of recurrences after the drug is discontinued. Randomized trials have indicated that three antiviral medications provide clinical benefit for genital herpes: acyclovir, valacyclovir, and famciclovir (72--80). Valacyclovir is the valine ester of acyclovir and has enhanced absorption after oral administration. Famciclovir also has high oral bioavailability. Topical therapy with antiviral drugs offers minimal clinical benefit, and its use is discouraged. First Clinical Episode of Genital HerpesMany persons with first-episode herpes have mild clinical manifestations but later develop severe or prolonged symptoms. Therefore, patients with initial genital herpes should receive antiviral therapy. Recommended Regimens*
* Treatment might be extended if healing is incomplete after 10 days of therapy. Established HSV-2 infection The majority of patients with symptomatic, first-episode genital HSV-2 infection subsequently experience recurrent episodes of genital lesions; recurrences are less frequent after initial genital HSV-1 infection. Intermittent asymptomatic shedding occurs in persons with genital HSV-2 infection, even in those with longstanding or clinically silent infection. Antiviral therapy for recurrent genital herpes can be administered either episodically to ameliorate or shorten the duration of lesions or continuously as suppressive therapy to reduce the frequency of recurrences. Many persons, including those with mild or infrequent recurrent outbreaks, benefit from antiviral therapy; therefore, options for treatment should be discussed. Some persons might prefer suppressive therapy, which has the additional advantage of decreasing the risk of genital HSV-2 transmission to susceptible partners (81). Suppressive Therapy for Recurrent Genital HerpesSuppressive therapy reduces the frequency of genital herpes recurrences by 70%--80% in patients who have frequent recurrences (i.e., >6 recurrences per year), and many patients report no symptomatic outbreaks. Treatment also is effective in patients with less frequent recurrences. Safety and efficacy have been documented among patients receiving daily therapy with acyclovir for as long as 6 years and with valacyclovir or famciclovir for 1 year. Quality of life frequently is improved in patients with frequent recurrences who receive suppressive therapy, compared with episodic treatment. The frequency of recurrent genital herpes outbreaks diminishes over time in many patients, and the patient's psychological adjustment to the disease might change. Therefore, periodically during suppressive treatment (e.g., once a year), providers should discuss the need to continue therapy with the patient. Daily treatment with valacyclovir 500 mg daily decreases the rate of HSV-2 transmission in discordant, heterosexual couples in which the source partner has a history of genital HSV-2 infection (82). Such couples should be encouraged to consider suppressive antiviral therapy as part of a strategy to prevent transmission, in addition to consistent condom use and avoidance of sexual activity during recurrences. Suppressive antiviral therapy probably reduces transmission when used by persons who have multiple partners (including MSM) and by those who are HSV-2 seropositive without a history of genital herpes.
Recommended Regimens
Valacyclovir 500 mg once a day might be less effective than other valacyclovir or acyclovir dosing regimens in patients who have very frequent recurrences (i.e., >10 episodes per year). Several studies have compared valacyclovir or famciclovir with acyclovir. The results of these studies suggest that valacyclovir and famciclovir are comparable to acyclovir in clinical outcome (74,78,79,83). Ease of administration and cost also are important considerations for prolonged treatment.
Episodic Therapy for Recurrent Genital HerpesEffective episodic treatment of recurrent herpes requires initiation of therapy within 1 day of lesion onset or during the prodrome that precedes some outbreaks. The patient should be provided with a supply of drug or a prescription for the medication with instructions to initiate treatment immediately when symptoms begin. Recommended Regimens
Severe Disease Intravenous (IV) acyclovir therapy should be provided for patients who have severe HSV disease or complications that necessitate hospitalization (e.g., disseminated infection, pneumonitis, or hepatitis) or CNS complications (e.g., meningitis or encephalitis). The recommended regimen is acyclovir 5--10 mg/kg body weight IV every 8 hours for 2--7 days or until clinical improvement is observed, followed by oral antiviral therapy to complete at least 10 days of total therapy. Counseling Counseling of infected persons and their sex partners is critical to the management of genital herpes. The goal of counseling is to 1) help patients cope with the infection and 2) prevent sexual and perinatal transmission (8). Although initial counseling can be provided at the first visit, many patients benefit from learning about the chronic aspects of the disease after the acute illness subsides. Multiple resources, including websites (http://www.ashastd.org and http://www.ihmf.org) and printed materials are available to assist patients, their partners, and clinicians in counseling. HSV-infected persons might express anxiety concerning genital herpes that does not reflect the actual clinical severity of their disease; the psychological effect of HSV infection frequently is substantial. Common concerns regarding genital herpes include the severity of initial clinical manifestations, recurrent episodes, sexual relationships and transmission to sex partners, and ability to bear healthy children. The misconception that HSV causes cancer should be dispelled. The psychological effect of a serologic diagnosis of HSV-2 infection in a person with asymptomatic or unrecognized genital herpes appears small and transient (84). The following recommendations apply to counseling of persons with HSV infection:
Management of Sex Partners The sex partners of patients who have genital herpes can benefit from evaluation and counseling. Symptomatic sex partners should be evaluated and treated in the same manner as patients who have genital lesions. Asymptomatic sex partners of patients who have genital herpes should be questioned concerning histories of genital lesions and offered type-specific serologic testing for HSV infection. Special Considerations Allergy, Intolerance, and Adverse ReactionsAllergic and other adverse reactions to acyclovir, valacyclovir, and famciclovir are rare. Desensitization to acyclovir has been described (85). HIV InfectionImmunocompromised patients might have prolonged or severe episodes of genital, perianal, or oral herpes. Lesions caused by HSV are common among HIV-infected patients and might be severe, painful, and atypical. HSV shedding is increased in HIV-infected persons. Whereas antiretroviral therapy reduces the severity and frequency of symptomatic genital herpes, frequent subclinical shedding still occurs (86). Suppressive or episodic therapy with oral antiviral agents is effective in decreasing the clinical manifestations of HSV among HIV-positive persons (87--89). HIV-infected persons are likely to be more contagious for HSV; the extent to which suppressive antiviral therapy will decrease HSV transmission from this population is unknown. Some specialists suggest that HSV type-specific serologies be offered to HIV-positive persons during their initial evaluation, and that suppressive antiviral therapy be considered in those who have HSV-2 infection. Recommended Regimens for Daily Suppressive Therapy in Persons Infected with HIV
Recommended Regimens for Episodic Infection in Persons Infected with HIV
Acyclovir, valacyclovir, and famciclovir are safe for use in immunocompromised patients in the doses recommended for treatment of genital herpes. For severe HSV disease, initiating therapy with acyclovir 5--10 mg/kg body weight IV every 8 hours might be necessary. If lesions persist or recur in a patient receiving antiviral treatment, HSV resistance should be suspected and a viral isolate should be obtained for sensitivity testing (90). Such patients should be managed in consultation with an HIV specialist, and alternate therapy should be administered. All acyclovir-resistant strains are resistant to valacyclovir, and the majority are resistant to famciclovir. Foscarnet, 40 mg/kg body weight IV every 8 hours until clinical resolution is attained, is frequently effective for treatment of acyclovir-resistant genital herpes. Topical cidofovir gel 1% applied to the lesions once daily for 5 consecutive days also might be effective. This preparation is not commercially available and must be compounded at a pharmacy. Genital Herpes in Pregnancy The majority of mothers of infants who acquire neonatal herpes lack histories of clinically evident genital herpes. The risk for transmission to the neonate from an infected mother is high (30%--50%) among women who acquire genital herpes near the time of delivery and is low (<1%) among women with histories of recurrent herpes at term or who acquire genital HSV during the first half of pregnancy. However, because recurrent genital herpes is much more common than initial HSV infection during pregnancy, the proportion of neonatal HSV infections acquired from mothers with recurrent herpes is substantial. Prevention of neonatal herpes depends both on preventing acquisition of genital HSV infection during late pregnancy and avoiding exposure of the infant to herpetic lesions during delivery. Women without known genital herpes should be counseled to avoid intercourse during the third trimester with partners known or suspected of having genital herpes. In addition, pregnant women without known orolabial herpes should be advised to avoid receptive oral sex during the third trimester with partners known or suspected to have orolabial herpes. Some specialists believe that type-specific serologic tests are useful to identify pregnant women at risk for HSV infection and to guide counseling regarding the risk for acquiring genital herpes during pregnancy. Such testing should be offered to women without genital herpes whose sex partner has HSV infection. The effectiveness of antiviral therapy to decrease the risk for HSV transmission to pregnant women has not been studied. All pregnant women should be asked whether they have a history of genital herpes. At the onset of labor, all women should be questioned carefully about symptoms of genital herpes, including prodromal symptoms, and all women should be examined carefully for herpetic lesions. Women without symptoms or signs of genital herpes or its prodrome can deliver vaginally. The majority of specialists recommend that women with recurrent genital herpetic lesions at the onset of labor deliver by cesarean section to prevent neonatal herpes. However, cesarean section does not completely eliminate the risk for HSV transmission to the infant. The safety of systemic acyclovir, valacyclovir, and famciclovir therapy in pregnant women has not been definitively established. Available data do not indicate an increased risk for major birth defects compared with the general population in women treated with acyclovir during the first trimester (91). These findings provide some assurance to women who have had prenatal exposure to acyclovir. The experience with prenatal exposure to valacyclovir and famciclovir is too limited to provide useful information on pregnancy outcomes. Acyclovir may be administered orally to pregnant women with first episode genital herpes or severe recurrent herpes and should be administered IV to pregnant women with severe HSV infection. Acyclovir treatment late in pregnancy reduces the frequency of cesarean sections among women who have recurrent genital herpes by diminishing the frequency of recurrences at term, and many specialists recommend such treatment (92--94). No data support the use of antiviral therapy among HSV seropositive women without a history of genital herpes. The risk for herpes is high in infants of women who acquire genital HSV during late pregnancy; such women should be managed in consultation with an infectious diseases specialist. Some specialists recommend acyclovir therapy in this circumstance, some recommend routine cesarean section to reduce the risk for neonatal herpes, and others recommend both. Neonatal Herpes Infants exposed to HSV during birth, as documented by maternal virologic testing or presumed by observation of maternal lesions, should be followed carefully in consultation with a specialist. Some specialists recommend that such infants undergo surveillance cultures of mucosal surfaces to detect HSV infection before development of clinical signs of neonatal herpes. In addition, some specialists recommend the use of acyclovir for infants born to women who acquired HSV near term because the risk for neonatal herpes is high for these infants. All infants who have neonatal herpes should be promptly evaluated and treated with systemic acyclovir. The recommended regimen for infants treated for known or suspected neonatal herpes is acyclovir 20 mg/kg body weight IV every 8 hours for 21 days for disseminated and CNS disease or for 14 days for disease limited to the skin and mucous membranes. Granuloma Inguinale (Donovanosis)Granuloma inguinale is a genital ulcerative disease caused by the intracellular gram-negative bacterium Klebsiella granulomatis (formerly known as Calymmatobacterium granulomatis). The disease occurs rarely in the United States, although it is endemic in some tropical and developing areas, including India; Papua, New Guinea; central Australia; and southern Africa. Clinically, the disease is commonly characterized as painless, progressive ulcerative lesions without regional lymphadenopathy. The lesions are highly vascular (i.e., beefy red appearance) and bleed easily on contact. However, the clinical presentation also can include hypertrophic, necrotic, or sclerotic variants. The causative organism is difficult to culture, and diagnosis requires visualization of dark-staining Donovan bodies on tissue crush preparation or biopsy. No FDA-cleared PCR tests for the detection of K. granulomatis DNA exist, but such an assay might be useful if a CLIA verification study has been conducted. The lesions might develop secondary bacterial infection or can coexist with other sexually transmitted pathogens. Treatment A limited number of studies on Donovanosis treatment have been published. Treatment halts progression of lesions, although prolonged therapy is usually required to permit granulation and reepithelialization of the ulcers. Healing typically proceeds inward from the ulcer margins. Relapse can occur 6--18 months after apparently effective therapy. Several antimicrobial regimens have been effective, but a limited number of controlled trials have been published (95). Recommended Regimen
Alternative Regimens
Therapy should be continued at least 3 weeks and until all lesions have completely healed. Some specialists recommend the addition of an aminoglycoside (e.g., gentamicin 1 mg/kg IV every 8 hours) to these regimens if improvement is not evident within the first few days of therapy. Follow-Up Patients should be followed clinically until signs and symptoms have resolved. Management of Sex Partners Persons who have had sexual contact with a patient who has granuloma inguinale within the 60 days before onset of the patient's symptoms should be examined and offered therapy. However, the value of empiric therapy in the absence of clinical signs and symptoms has not been established. Special Considerations PregnancyPregnancy is a relative contraindication to the use of sulfonamides. Pregnant and lactating women should be treated with the erythromycin regimen, and consideration should be given to the addition of a parenteral aminoglycoside (e.g., gentamicin). Azithromycin might prove useful for treating granuloma inguinale during pregnancy, but published data are lacking. Doxycycline and ciprofloxacin are contraindicated in pregnant women.
HIV InfectionPersons with both granuloma inguinale and HIV infection should receive the same regimens as those who are HIV negative. Consideration should be given to the addition of a parenteral aminoglycoside (e.g., gentamicin). Lymphogranuloma VenereumLymphogranuloma venereum (LGV) is caused by C. trachomatis serovars L1, L2, or L3 (96). The most common clinical manifestation of LGV among heterosexuals is tender inguinal and/or femoral lymphadenopathy that is typically unilateral. A self-limited genital ulcer or papule sometimes occurs at the site of inoculation. However, by the time patients seek care, the lesions might have disappeared. Rectal exposure in women or MSM might result in proctocolitis (including mucoid and/or hemorrhagic rectal discharge, anal pain, constipation, fever, and/or tenesmus). LGV is an invasive, systemic infection, and if it is not treated early, LGV proctocolitis might lead to chronic, colorectal fistulas and strictures. Genital and colorectal LGV lesions might also develop secondary bacterial infection or might be coinfected with other sexually and nonsexually transmitted pathogens. Diagnosis is based on clinical suspicion, epidemiologic information, and the exclusion of other etiologies (of proctocolitis, inguinal lymphadenopathy, or genital or rectal ulcers), along with C. trachomatis testing, if available. Genital and lymph node specimens (i.e., lesion swab or bubo aspirate) may be tested for C. trachomatis by culture, direct immunofluorescence, or nucleic acid detection. Nucleic acid amplification tests (NAAT) for C. trachomatis are not FDA-cleared for testing rectal specimens. Additional procedures (e.g., genotyping) are required for differentiating LGV from non-LGV C. trachomatis but are not widely available. Chlamydia serology (complement fixation titers >1:64) can support the diagnosis in the appropriate clinical context. Comparative data between types of serologic tests are lacking, and the diagnostic utility of serologic methods other than complement fixation and some microimmunofluorescence procedures has not been established. Serologic test interpretation for LGV is not standardized, tests have not been validated for clinical proctitis presentations, and C. trachomatis serovar-specific serologic tests are not widely available. In the absence of specific LGV diagnostic testing, patients with a clinical syndrome consistent with LGV, including proctocolitis or genital ulcer disease with lymphadenopathy, should be treated for LGV as described in this report.
Treatment Treatment cures infection and prevents ongoing tissue damage, although tissue reaction to the injection can result in scarring. Buboes might require aspiration through intact skin or incision and drainage to prevent the formation of inguinal/femoral ulcerations. Doxycycline is the preferred treatment. Recommended Regimen
Alternative Regimen
Some STD specialists believe that azithromycin 1.0 g orally once weekly for 3 weeks is probably effective, although clinical data are lacking. Follow-Up Patients should be followed clinically until signs and symptoms have resolved. Management of Sex Partners Persons who have had sexual contact with a patient who has LGV within the 60 days before onset of the patient's symptoms should be examined, tested for urethral or cervical chlamydial infection, and treated with a standard chlamydia regimen (azithromycin 1 gm orally x 1 or doxycycline 100 mg orally twice a day for 7 days). The optimum contact interval is unknown; some specialists use longer contact intervals. Special Considerations PregnancyPregnant and lactating women should be treated with erythromycin. Azithromycin might prove useful for treatment of LGV in pregnancy, but no published data are available regarding its safety and efficacy. Doxycycline is contraindicated in pregnant women. HIV InfectionPersons with both LGV and HIV infection should receive the same regimens as those who are HIV negative. Prolonged therapy might be required, and delay in resolution of symptoms might occur. SyphilisGeneral Principles BackgroundSyphilis is a systemic disease caused by T. pallidum. Patients who have syphilis might seek treatment for signs or symptoms of primary infection (i.e., ulcer or chancre at the infection site), secondary infection (i.e., manifestations that include, but are not limited to, skin rash, mucocutaneous lesions, and lymphadenopathy), or tertiary infection (e.g., cardiac or ophthalmic manifestations, auditory abnormalities, or gummatous lesions). Latent infections (i.e., those lacking clinical manifestations) are detected by serologic testing. Latent syphilis acquired within the preceding year is referred to as early latent syphilis; all other cases of latent syphilis are either late latent syphilis or latent syphilis of unknown duration. Treatment for both late latent syphilis and tertiary syphilis theoretically might require a longer duration of therapy because organisms are dividing more slowly; however, the validity of this concept has not been assessed. Diagnostic Considerations and Use of Serologic TestsDarkfield examinations and direct fluorescent antibody (DFA) tests of lesion exudate or tissue are the definitive methods for diagnosing early syphilis. A presumptive diagnosis is possible with the use of two types of serologic tests: 1) nontreponemal tests (e.g., Venereal Disease Research Laboratory [VDRL] and RPR) and 2) treponemal tests (e.g., fluorescent treponemal antibody absorbed [FTA-ABS] and T. pallidum particle agglutination [TP-PA]). The use of only one type of serologic test is insufficient for diagnosis because false-positive nontreponemal test results are sometimes associated with various medical conditions unrelated to syphilis. Nontreponemal test antibody titers usually correlate with disease activity, and results should be reported quantitatively. A fourfold change in titer, equivalent to a change of two dilutions (e.g., from 1:16--1:4 or from 1:8--1:32), is considered necessary to demonstrate a clinically significant difference between two nontreponemal test results that were obtained using the same serologic test. Sequential serologic tests in individual patients should be performed by using the same testing method (e.g., VDRL or RPR), preferably by the same laboratory. The VDRL and RPR are equally valid assays, but quantitative results from the two tests cannot be compared directly because RPR titers frequently are slightly higher than VDRL titers. Nontreponemal tests usually become nonreactive with time after treatment; however, in some patients, nontreponemal antibodies can persist at a low titer for a long period of time, sometimes for the life of the patient. This response is referred to as the serofast reaction. The majority of patients who have reactive treponemal tests will have reactive tests for the remainder of their lives, regardless of treatment or disease activity. However, 15%--25% of patients treated during the primary stage revert to being serologically nonreactive after 2--3 years (97). Treponemal test antibody titers do not correlate with disease activity and should not be used to assess treatment response. Some clinical laboratories and blood banks have begun to screen samples using treponemal EIA tests (98). This strategy will identify both persons with previous treatment and persons with untreated or incompletely treated syphilis. False-positive results can occur, particularly among populations with a low prevalence of syphilis. Persons with a positive treponemal screening test should have a standard nontreponemal test with titer to guide patient management decisions. If the nontreponemal test is negative, then a different treponemal test should be performed to confirm the results of the initial test. If a second trepomenal test is positive, treatment decisions should be discussed in consultation with a specialist. Some HIV-infected patients can have atypical serologic test results (i.e., unusually high, unusually low, or fluctuating titers). For such patients, when serologic tests do not correspond with clinical syndromes suggestive of early syphilis, use of other tests (e.g., biopsy and direct microscopy) should be considered. However, for the majority of HIV-infected patients, serologic tests are accurate and reliable for the diagnosis of syphilis and for following the response to treatment. No single test can be used to diagnose neurosyphilis. The VDRL-cerebrospinal fluid (CSF) is highly specific, but it is insensitive. The majority of other tests are both insensitive and nonspecific and must be interpreted in relation to other test results and the clinical assessment. Therefore, the diagnosis of neurosyphilis usually depends on various combinations of reactive serologic test results, CSF cell count or protein, or a reactive VDRL-CSF with or without clinical manifestations. The CSF leukocyte count usually is elevated (>5 white blood cell count [WBC]/mm3) in patients with neurosyphilis; this count also is a sensitive measure of the effectiveness of therapy. The VDRL-CSF is the standard serologic test for CSF, and when reactive in the absence of substantial contamination of CSF with blood, it is considered diagnostic of neurosyphilis. However, the VDRL-CSF might be nonreactive even when neurosyphilis is present. Some specialists recommend performing an FTA-ABS test on CSF. The CSF FTA-ABS is less specific (i.e., yields more false-positive results) for neurosyphilis than the VDRL-CSF, but the test is highly sensitive. Therefore, some specialists believe that a negative CSF FTA-ABS test excludes neurosyphilis. TreatmentPenicillin G, administered parenterally, is the preferred drug for treatment of all stages of syphilis. The preparation(s) used (i.e., benzathine, aqueous procaine, or aqueous crystalline), the dosage, and the length of treatment depend on the stage and clinical manifestations of the disease. However, neither combinations of benzathine penicillin and procaine penicillin nor oral penicillin preparations are considered appropriate for the treatment of syphilis. Reports have indicated that inappropriate use of combination benzathine-procaine penicillin (Bicillin C-R®) instead of the standard benzathine penicillin product widely used in the United States (Bicillin L-A®) has occurred. Practitioners, pharmacists, and purchasing agents should be aware of the similar names of these two products and avoid use of the inappropriate combination therapy agent for treating syphilis (99). The efficacy of penicillin for the treatment of syphilis was well established through clinical experience even before the value of randomized controlled clinical trials was recognized. Therefore, nearly all the recommendations for the treatment of syphilis are based on the opinions of persons knowledgeable about STDs and are reinforced by case series, clinical trials, and 50 years of clinical experience. Parenteral penicillin G is the only therapy with documented efficacy for syphilis during pregnancy. Pregnant women with syphilis in any stage who report penicillin allergy should be desensitized and treated with penicillin. Skin testing for penicillin allergy might be useful in pregnant women; such testing also is useful in other patients (see Management of Patients Who Have a History of Penicillin Allergy). The Jarisch-Herxheimer reaction is an acute febrile reaction frequently accompanied by headache, myalgia, and other symptoms that usually occur within the first 24 hours after any therapy for syphilis. Patients should be informed about this possible adverse reaction. The Jarisch-Herxheimer reaction occurs most frequently among patients who have early syphilis. Antipyretics may be used, but they have not been proven to prevent this reaction. The Jarisch-Herxheimer reaction might induce early labor or cause fetal distress in pregnant women, but this should not prevent or delay therapy (see Syphilis During Pregnancy). Management of Sex PartnersSexual transmission of T. pallidum occurs only when mucocutaneous syphilitic lesions are present; such manifestations are uncommon after the first year of infection. However, persons exposed sexually to a patient who has syphilis in any stage should be evaluated clinically and serologically and treated with a recommended regimen, according to the following recommendations:
For identification of at-risk sexual partners, the periods before treatment are 1) 3 months plus duration of symptoms for primary syphilis, 2) 6 months plus duration of symptoms for secondary syphilis, and 3) 1 year for early latent syphilis. Primary and Secondary Syphilis TreatmentParenteral penicillin G has been used effectively for more than 50 years to achieve clinical resolution (i.e., healing of lesions and prevention of sexual transmission) and to prevent late sequelae. However, no comparative trials have been adequately conducted to guide the selection of an optimal penicillin regimen (i.e., the dose, duration, and preparation). Substantially fewer data are available for nonpenicillin regimens. Recommended Regimen for Adults*
* Recommendations for treating HIV-infected persons and pregnant women for syphilis have been discussed in this report (see Syphilis, Special considerations and Syphilis in Pregnancy). Recommended Regimen for ChildrenAfter the newborn period (aged >1 month), children with syphilis should have a CSF examination to detect asymptomatic neurosyphilis, and birth and maternal medical records should be reviewed to assess whether such children have congenital or acquired syphilis (see Congenital Syphilis). Children with acquired primary or secondary syphilis should be evaluated (e.g., through consultation with child-protection services) (see Sexual Assault or Abuse of Children) and treated by using the following pediatric regimen.
Benzathine penicillin G 50,000 units/kg IM, up to the adult dose of 2.4 million units in a single dose Other Management ConsiderationsAll patients who have syphilis should be tested for HIV infection. In geographic areas in which the prevalence of HIV is high, patients who have primary syphilis should be retested for HIV after 3 months if the first HIV test result was negative. Patients who have syphilis and symptoms or signs suggesting neurologic disease (e.g., meningitis) or ophthalmic disease (e.g., uveitis, iritis, neuroretinitis, or optic neuritis) should have an evaluation that includes CSF analysis and ocular slit-lamp examination. Treatment should be guided by the results of this evaluation. Invasion of CSF by T. pallidum accompanied by CSF abnormalities is common among adults who have primary or secondary syphilis. However, neurosyphilis develops in only a limited number of patients after treatment with the penicillin regimens recommended for primary and secondary syphilis. Therefore, unless clinical signs or symptoms of neurologic or ophthalmic involvement are present, CSF analysis is not recommended for routine evaluation of patients who have primary or secondary syphilis. Follow-UpTreatment failure can occur with any regimen. However, assessing response to treatment frequently is difficult, and definitive criteria for cure or failure have not been established. Nontreponemal test titers might decline more slowly for persons who previously had syphilis. Patients should be reexamined clinically and serologically 6 months and 12 months after treatment; more frequent evaluation might be prudent if follow-up is uncertain. Patients who have signs or symptoms that persist or recur or who have a sustained fourfold increase in nontreponemal test titer (i.e., compared with the maximum or baseline titer at the time of treatment) probably failed treatment or were reinfected. These patients should be retreated and reevaluated for HIV infection. Because treatment failure usually cannot be reliably distinguished from reinfection with T. pallidum, a CSF analysis also should be performed. Clinical trial data have demonstrated that 15% of patients with early syphilis treated with the recommended therapy will not achieve a two dilution decline in nontreponemal titer used to define response at 1 year after treatment (100). Failure of nontreponemal test titers to decline fourfold within 6 months after therapy for primary or secondary syphilis might be indicative of probable treatment failure. Persons for whom titers remain serofast should be reevaluated for HIV infection. Optimal management of such patients is unclear. At a minimum, these patients should receive additional clinical and serologic follow-up. HIV-infected patients should be evaluated more frequently (i.e., at 3-month intervals instead of 6-month intervals). If additional follow-up cannot be ensured, re-treatment is recommended. Because treatment failure might be the result of unrecognized CNS infection, many specialists recommend CSF examination in such situations. For retreatment, the majority of STD specialists recommend administering weekly injections of benzathine penicillin G 2.4 million units IM for 3 weeks, unless CSF examination indicates that neurosyphilis is present. In rare instances, serologic titers do not decline despite a negative CSF examination and a repeated course of therapy. Additional therapy or repeated CSF examinations are not warranted in these circumstances. Management of Sex PartnersSee General Principles, Management of Sex Partners. Special ConsiderationsPenicillin Allergy. Data to support the use of alternatives to penicillin in the treatment of early syphilis are limited. However, several therapies might be effective in nonpregnant, penicillin-allergic patients who have primary or secondary syphilis. Doxycycline (100 mg orally twice daily for 14 days) and tetracycline (500 mg four times daily for 14 days) are regimens that have been used for many years. Compliance is likely to be better with doxycycline than tetracycline because tetracycline can cause gastrointestinal side effects. Although limited clinical studies, along with biologic and pharmacologic evidence, suggest that ceftriaxone is effective for treating early syphilis, the optimal dose and duration of ceftriaxone therapy have not been defined. Some specialists recommend 1 g daily either IM or IV for 8--10 days. Some patients who are allergic to penicillin also might be allergic to ceftriaxone; in these circumstances, use of an alternative agent might be required. Preliminary data suggest that azithromycin might be effective as a single oral dose of 2 g (101,102). However, several cases of azithromycin treatment failure have been reported, and resistance to azithromycin has been documented in several geographic areas (103). Close follow-up of persons receiving alternative therapies is essential. The use of any of these therapies in HIV-infected persons has not been well-studied; therefore, the use of doxycycline, ceftriaxone, and azithromycin among such persons must be undertaken with caution. Patients with penicillin allergy whose compliance with therapy or follow-up cannot be ensured should be desensitized and treated with benzathine penicillin. Skin testing for penicillin allergy might be useful in some circumstances in which the reagents and expertise are available to perform the test adequately (see Management of Patients Who Have a History of Penicillin Allergy). Pregnancy. Pregnant patients who are allergic to penicillin should be desensitized and treated with penicillin (see Management of Patients Who Have a History of Penicillin Allergy and Syphilis During Pregnancy). HIV Infection. See Syphilis Among HIV-Infected Persons. Latent Syphilis Latent syphilis is defined as syphilis characterized by seroreactivity without other evidence of disease. Patients who have latent syphilis and who acquired syphilis within the preceding year are classified as having early latent syphilis. Patients' conditions can be diagnosed as early latent syphilis if, within the year preceding the evaluation, they had 1) a documented seroconversion or fourfold or greater increase in titer of a nontreponemal test; 2) unequivocal symptoms of primary or secondary syphilis; 3) a sex partner documented to have primary, secondary, or early latent syphilis; or 4) reactive nontreponemal and treponemal tests from a person whose only possible exposure occurred within the previous 12 months. Nontreponemal serologic titers usually are higher during early latent syphilis than late latent syphilis. However, early latent syphilis cannot be reliably distinguished from late latent syphilis solely on the basis of nontreponemal titers. All patients with latent syphilis should have careful examination of all accessible mucosal surfaces (i.e., the oral cavity, the perineum in women, and perianal area, underneath the foreskin in uncircumcised men) to evaluate for internal mucosal lesions. All patients who have syphilis should be tested for HIV infection. TreatmentTreatment of latent syphilis usually does not affect transmission and is intended to prevent late complications. Although clinical experience supports the effectiveness of penicillin in achieving this goal, limited evidence is available for guidance in choosing specific regimens. The following regimens are recommended for penicillin nonallergic patients who have normal CSF examinations (if performed). Recommended Regimens for Adults Early Latent Syphilis
Late Latent Syphilis or Latent Syphilis of Unknown Duration
After the newborn period, children with syphilis should have a CSF examination to exclude neurosyphilis. In addition, birth and maternal medical records should be reviewed to assess whether children have congenital or acquired syphilis (see Congenital Syphilis). Older children with acquired latent syphilis should be evaluated as described for adults and treated using the following pediatric regimens (see Sexual Assault or Abuse of Children). These regimens are for penicillin nonallergic children who have acquired syphilis and who have normal CSF examination results. Recommended Regimens for Children Early Latent Syphilis
Late Latent Syphilis or Latent Syphilis of Unknown Duration
Other Management ConsiderationsAll persons who have latent syphilis should be evaluated clinically for evidence of tertiary disease (e.g., aortitis and gumma) and syphilitic ocular disease (e.g., iritis and uveitis). Patients who have syphilis and who demonstrate any of the following criteria should have a prompt CSF examination:
If dictated by circumstances and patient preferences, a CSF examination may be performed for patients who do not meet these criteria. Some specialists recommend performing a CSF examination on all patients who have latent syphilis and a nontreponemal serologic test of >1:32 or if the patient is HIV-infected with a serum CD4 count <350 (104). However, the likelihood of neurosyphilis in this circumstance is unknown. If a CSF examination is performed and the results indicate abnormalities consistent with neurosyphilis, the patient should be treated for neurosyphilis (see Neurosyphilis). If a patient misses a dose of penicillin in a course of weekly therapy for late syphilis, the appropriate course of action is unclear. Pharmacologic considerations suggest that an interval of 10--14 days between doses of benzathine penicillin for late syphilis or latent syphilis of unknown duration might be acceptable before restarting the sequence of injections. Missed doses are not acceptable for pregnant patients receiving therapy for late latent syphilis; pregnant women who miss any dose of therapy must repeat the full course of therapy. Follow-Up. Quantitative nontreponemal serologic tests should be repeated at 6, 12, and 24 months. Patients with a normal CSF examination should be re-treated for latent syphilis if 1) titers increase fourfold, 2) an initially high titer (>1:32) fails to decline at least fourfold (i.e., two dilutions) within 12--24 months of therapy, or 3) signs or symptoms attributable to syphilis develop. In rare instances, despite a negative CSF examination and a repeated course of therapy, serologic titers might still not decline. In these circumstances, the need for additional therapy or repeated CSF examinations is unclear. Management of Sex Partners. See General Principles, Management of Sex Partners. Special ConsiderationsPenicillin Allergy. The effectiveness of alternatives to penicillin in the treatment of latent syphilis has not been well-documented. Nonpregnant patients allergic to penicillin who have clearly defined early latent syphilis should respond to therapies recommended as alternatives to penicillin for the treatment of primary and secondary syphilis (see Primary and Secondary Syphilis, Treatment). The only acceptable alternatives for the treatment of late latent syphilis or latent syphilis of unknown duration are doxycycline (100 mg orally twice daily) or tetracycline (500 mg orally four times daily), both for 28 days. These therapies should be used only in conjunction with close serologic and clinical follow-up. Limited clinical studies, along with biologic and pharmacologic evidence, suggest that ceftriaxone might be effective for treating late latent syphilis or syphilis of unknown duration (105). However, the optimal dose and duration of ceftriaxone therapy have not been defined, and treatment decisions should be discussed in consultation with a specialist. Some patients who are allergic to penicillin also might be allergic to ceftriaxone; in these circumstances, use of an alternative agent might be required. The efficacy of these alternative regimens in HIV-infected persons has not been well-studied and, therefore, must be considered with caution. Pregnancy. Pregnant patients who are allergic to penicillin should be desensitized and treated with penicillin (see Management of Patients Who Have a History of Penicillin Allergy and Syphilis During Pregnancy). HIV Infection. See Syphilis Among HIV-Infected Persons. Tertiary Syphilis Tertiary syphilis refers to gumma and cardiovascular syphilis but not to all neurosyphilis. Patients who are not allergic to penicillin and have no evidence of neurosyphilis should be treated with the following regimen. Recommended Regimen
Other Management ConsiderationsPatients who have symptomatic late syphilis should be given a CSF examination before therapy is initiated. Some providers treat all patients who have cardiovascular syphilis with a neurosyphilis regimen. The complete management of patients who have cardiovascular or gummatous syphilis is beyond the scope of these guidelines. These patients should be managed in consultation with an infectious diseases specialist. Follow-Up. Limited information is available concerning clinical response and follow-up of patients who have tertiary syphilis. Management of Sex Partners. See General Principles, Management of Sex Partners. Special ConsiderationsPenicillin Allergy. Patients allergic to penicillin should be treated according to treatment regimens recommended for late latent syphilis. Pregnancy. Pregnant patients who are allergic to penicillin should be desensitized, if necessary, and treated with penicillin (see Management of Patients Who Have a History of Penicillin Allergy and Syphilis During Pregnancy). HIV Infection. See Syphilis Among HIV-Infected Persons. NeurosyphilisTreatmentCNS involvement can occur during any stage of syphilis. A patient who has clinical evidence of neurologic involvement with syphilis (e.g., cognitive dysfunction, motor or sensory deficits, ophthalmic or auditory symptoms, cranial nerve palsies, and symptoms or signs of meningitis) should have a CSF examination. Syphilitic uveitis or other ocular manifestations frequently are associated with neurosyphilis; patients with these symptoms should be treated according to the recommendations for patients with neurosyphilis. A CSF examination should be performed for all such patients to identify those with abnormalities that require follow-up CSF examinations to assess treatment response. Patients who have neurosyphilis or syphilitic eye disease (e.g., uveitis, neuroretinitis, and optic neuritis) should be treated with the following regimen. Recommended Regimen
If compliance with therapy can be ensured, patients may be treated with the following alternative regimen. Alternative Regimen
The durations of the recommended and alternative regimens for neurosyphilis are shorter than that of the regimen used for late syphilis in the absence of neurosyphilis. Therefore, some specialists administer benzathine penicillin, 2.4 million units IM once per week for up to 3 weeks after completion of these neurosyphilis treatment regimens to provide a comparable total duration of therapy. Other Management ConsiderationsOther considerations in the management of patients who have neurosyphilis are as follows:
Follow-Up. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. Follow-up CSF examinations also can be used to evaluate changes in the VDRL-CSF or CSF protein after therapy; however, changes in these two parameters occur more slowly than cell counts, and persistent abnormalities might be less important. If the cell count has not decreased after 6 months or if the CSF is not normal after 2 years, re-treatment should be considered. Recent data on HIV-infected persons with neurosyphilis suggest that CSF abnormalities might persist for extended periods in these persons, and close clinical follow-up is warranted (105,106). Management of Sex Partners. See General Principles, Management of Sex Partners. Special ConsiderationsPenicillin Allergy. Ceftriaxone can be used as an alternative treatment for patients with neurosyphilis, although the possibility of cross-reactivity between this agent and penicillin exists. Some specialists recommend ceftriaxone 2 g daily either IM or IV for 10--14 days. Other regimens have not been adequately evaluated for treatment of neurosyphilis. Therefore, if concern exists regarding the safety of ceftriaxone for a patient with neurosyphilis, the patient should obtain skin testing to confirm penicillin allergy and, if necessary, be desensitized and managed in consultation with a specialist. Pregnancy. Pregnant patients who are allergic to penicillin should be desensitized, if necessary, and treated with penicillin (see Syphilis During Pregnancy). HIV Infection. See Syphilis Among HIV-Infected Patients. Syphilis Among HIV-Infected PersonsDiagnostic ConsiderationsUnusual serologic responses have been observed among HIV-infected persons who have syphilis. The majority of reports have involved serologic titers that were higher than expected, but false-negative serologic test results and delayed appearance of seroreactivity also have been reported. However, unusual serologic responses are uncommon, and the majority of specialists believe that both treponemal and nontreponemal serologic tests for syphilis can be interpreted in the usual manner for the majority of patients who are coinfected with T. pallidum and HIV. When clinical findings are suggestive of syphilis but serologic tests are nonreactive or their interpretation is unclear, alternative tests (e.g., biopsy of a lesion, darkfield examination, or DFA staining of lesion material) might be useful for diagnosis. Neurosyphilis should be considered in the differential diagnosis of neurologic disease in HIV-infected persons. TreatmentCompared with HIV-negative patients, HIV-positive patients who have early syphilis might be at increased risk for neurologic complications and might have higher rates of treatment failure with currently recommended regimens. The magnitude of these risks is not defined precisely but is likely minimal. No treatment regimens for syphilis have been demonstrated to be more effective in preventing neurosyphilis in HIV-infected patients than the syphilis regimens recommended for HIV-negative patients (100). Careful follow-up after therapy is essential. Primary and Secondary Syphilis Among HIV-Infected Persons TreatmentTreatment with benzathine penicillin G, 2.4 million units IM in a single dose is recommended. Some specialists recommend additional treatments (e.g., benzathine penicillin G administered at 1-week intervals for 3 weeks, as recommended for late syphilis) in addition to benzathine penicillin G 2.4 million units IM. Other Management ConsiderationsBecause CSF abnormalities (e.g., mononuclear pleocytosis and elevated protein levels) are common in patients with early syphilis and in persons with HIV infection, the clinical and prognostic significance of such CSF abnormalities in HIV- infected persons with primary or secondary syphilis is unknown. Although the majority of HIV-infected persons respond appropriately to standard benzathine penicillin therapy, some specialists recommend intensified therapy when CNS syphilis is suspected in these persons. Therefore, some specialists recommend CSF examination before treatment of HIV-infected persons with early syphilis, with follow-up CSF examination conducted after treatment in persons with initial abnormalities. Follow-Up. HIV-infected persons should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy. Although of unproven benefit, some specialists recommend a CSF examination 6 months after therapy. HIV-infected persons who meet the criteria for treatment failure (i.e., signs or symptoms that persist or recur or persons who have fourfold increase in nontreponemal test titer) should be managed in the same manner as HIV-negative patients (i.e., a CSF examination and re-treatment). CSF examination and re-treatment also should be strongly considered for persons whose nontreponemal test titers do not decrease fourfold within 6--12 months of therapy. The majority of specialists would re-treat patients with benzathine penicillin G administered as 3 doses of 2.4 million units IM each at weekly intervals, if CSF examinations are normal. Special ConsiderationsPenicillin Allergy. Penicillin-allergic patients who have primary or secondary syphilis and HIV infection should be managed according to the recommendations for penicillin-allergic, HIV-negative patients. The use of alternatives to penicillin has not been well studied in HIV-infected patients. Latent Syphilis Among HIV-Infected PersonsDiagnostic ConsiderationsHIV-infected patients who have early latent syphilis should be managed and treated according to the recommendations for HIV-negative patients who have primary and secondary syphilis. HIV-infected patients who have either late latent syphilis or syphilis of unknown duration should have a CSF examination before treatment. TreatmentPatients with late latent syphilis or syphilis of unknown duration and a normal CSF examination can be treated with benzathine penicillin G, at weekly doses of 2.4 million units for 3 weeks. Patients who have CSF consistent with neurosyphilis should be treated and managed as patients who have neurosyphilis (see Neurosyphilis). Follow-Up. Patients should be evaluated clinically and serologically at 6, 12, 18, and 24 months after therapy. If, at any time, clinical symptoms develop or nontreponemal titers rise fourfold, a repeat CSF examination should be performed and treatment administered accordingly. If during 12--24 months the nontreponemal titer does not decline fourfold, the CSF examination should be repeated and treatment administered accordingly. Special ConsiderationsPenicillin Allergy. The efficacy of alternative nonpenicillin regimens in HIV-infected persons has not been well studied. Patients with penicillin allergy whose compliance with therapy or follow-up cannot be ensured should be desensitized and treated with penicillin (see Management of Patients Who Have a History of Penicillin Allergy). These therapies should be used only in conjunction with close serologic and clinical follow-up. Limited clinical studies, along with biologic and pharmacologic evidence, suggest that ceftriaxone might be effective (105). However, optimal dose and duration of ceftriaxone therapy have not been defined. Syphilis During PregnancyAll women should be screened serologically for syphilis during the early stages of pregnancy. The majority of states mandate screening at the first prenatal visit for all women. Antepartum screening by nontreponemal antibody testing is typical, but in some settings, treponemal antibody testing is being used. Pregnant women with reactive treponemal screening tests should have confirmatory testing with nontreponemal tests with titers. In populations in which use of prenatal care is not optimal, RPR-card test screening and treatment (i.e., if the RPR-card test is reactive) should be performed at the time a pregnancy is diagnosed. For communities and populations in which the prevalence of syphilis is high or for patients at high risk, serologic testing should be performed twice during the third trimester, at 28 to 32 weeks' gestation and at delivery. Any woman who delivers a stillborn infant after 20 weeks' gestation should be tested for syphilis. No infant should leave the hospital without the maternal serologic status having been determined at least once during pregnancy. Diagnostic ConsiderationsSeropositive pregnant women should be considered infected unless an adequate treatment history is documented clearly in the medical records and sequential serologic antibody titers have declined. Serofast low antibody titers might not require treatment; however, persistent higher titer antibody tests might indicate reinfection and require treatment. TreatmentPenicillin is effective for preventing maternal transmission to the fetus and for treating fetal infection. Evidence is insufficient to determine specific, recommended penicillin regimens that are optimal (107). Recommended Regimen
Other Management ConsiderationsSome specialists recommend additional therapy for pregnant women in some settings (e.g., a second dose of benzathine penicillin 2.4 million units IM administered 1 week after the initial dose for women who have primary, secondary, or early latent syphilis). During the second half of pregnancy, syphilis management may be facilitated by a sonographic fetal evaluation for congenital syphilis, but this evaluation should not delay therapy. Sonographic signs of fetal or placental syphilis (i.e., hepatomegaly, ascites, hydrops, or a thickened placenta) indicate a greater risk for fetal treatment failure (108); such cases should be managed in consultation with obstetric specialists. Evidence is insufficient to recommend specific regimens for these situations. Women treated for syphilis during the second half of pregnancy are at risk for premature labor and/or fetal distress, if the treatment precipitates the Jarisch-Herxheimer reaction. These women should be advised to seek obstetric attention after treatment, if they notice any contractions or decrease in fetal movements. Stillbirth is a rare complication of treatment, but concern for this complication should not delay necessary treatment. All patients who have syphilis should be offered testing for HIV infection. Follow-Up. Coordinated prenatal care and treatment follow-up are vital. Serologic titers should be repeated at 28--32 weeks' gestation, at delivery, and following the recommendations for the stage of disease. Serologic titers can be checked monthly in women at high risk for reinfection or in geographic areas in which the prevalence of syphilis is high. The clinical and antibody response should be appropriate for the stage of disease. The majority of women will deliver before their serologic response to treatment can be assessed definitively. Inadequate maternal treatment is likely if delivery occurs within 30 days of therapy, if clinical signs of infection are present at delivery, or if the maternal antibody titer is fourfold higher than the pretreatment titer. Management of Sex Partners. See General Principles, Management of Sex Partners. Special ConsiderationsPenicillin Allergy. For treatment of syphilis during pregnancy, no proven alternatives to penicillin exist. Pregnant women who have a history of penicillin allergy should be desensitized and treated with penicillin. Skin testing might be helpful (see Management of Patients Who Have a History of Penicillin Allergy). Tetracycline and doxycycline usually are not used during pregnancy. Erythromycin should not be used because it does not reliably cure an infected fetus. Data are insufficient to recommend azithromycin or ceftriaxone for treatment of maternal infection and prevention of congenital syphilis. HIV Infection. Placental inflammation from congenital infection might increase the risk for perinatal transmission of HIV. All HIV-infected women should be evaluated for infectious syphilis and treated. Data are insufficient to recommend a specific regimen (see Syphilis Among HIV-Infected Patients). Congenital SyphilisEffective prevention and detection of congenital syphilis depends on the identification of syphilis in pregnant women and, therefore, on the routine serologic screening of pregnant women during the first prenatal visit. In communities and populations in which the risk for congenital syphilis is high, serologic testing and a sexual history also should be obtained at 28 weeks' gestation and at delivery. Moreover, as part of the management of pregnant women who have syphilis, information concerning treatment of sex partners should be obtained to assess the risk for reinfection. All pregnant women who have syphilis should be tested for HIV infection. Routine screening of newborn sera or umbilical cord blood is not recommended. Serologic testing of the mother's serum is preferred rather than testing of the infant's serum because the serologic tests performed on infant serum can be nonreactive if the mother's serologic test result is of low titer or was infected late in pregnancy (see Diagnostic Considerations and Use of Serologic Tests). No infant or mother should leave the hospital unless the maternal serologic status has been documented at least once during pregnancy, and at delivery in communities and populations in which the risk for congenital syphilis is high. Evaluation and Treatment of Infants During the First Month of LifeThe diagnosis of congenital syphilis is complicated by the transplacental transfer of maternal nontreponemal and treponemal IgG antibodies to the fetus. This transfer of antibodies makes the interpretation of reactive serologic tests for syphilis in infants difficult. Treatment decisions frequently must be made on the basis of 1) identification of syphilis in the mother; 2) adequacy of maternal treatment; 3) presence of clinical, laboratory, or radiographic evidence of syphilis in the infant; and 4) comparison of maternal (at delivery) and infant nontreponemal serologic titers by using the same test and preferably the same laboratory. All infants born to mothers who have reactive nontreponemal and treponemal test results should be evaluated with a quantitative nontreponemal serologic test (RPR or VDRL) performed on infant serum because umbilical cord blood can become contaminated with maternal blood and could yield a false-positive result. Conducting a treponemal test (i.e., TP-PA or FTA-ABS) on a newborn's serum is not necessary. No commercially available immunoglobulin (IgM) test can be recommended. All infants born to women who have reactive serologic tests for syphilis should be examined thoroughly for evidence of congenital syphilis (e.g., nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and/or pseudoparalysis of an extremity). Pathologic examination of the placenta or umbilical cord by using specific fluorescent antitreponemal antibody staining is suggested. Darkfield microscopic examination or DFA staining of suspicious lesions or body fluids (e.g., nasal discharge) also should be performed. The following scenarios describe the evaluation and treatment of infants for congenital syphilis: Scenario 1. Infants with proven or highly probable disease and
Recommended Evaluation
Recommended Regimens
If >1 day of therapy is missed, the entire course should be restarted. Data are insufficient regarding the use of other antimicrobial agents (e.g., ampicillin). When possible, a full 10-day course of penicillin is preferred, even if ampicillin was initially provided for possible sepsis. The use of agents other than penicillin requires close serologic follow-up to assess adequacy of therapy. In all other situations, the maternal history of infection with T. pallidum and treatment for syphilis must be considered when evaluating and treating the infant. Scenario 2. Infants who have a normal physical examination and a serum quantitive nontreponemal serologic titer the same or less than fourfold the maternal titer and the
Recommended Evaluation
A complete evaluation is not necessary if 10 days of parenteral therapy is administered. However, such evaluations might be useful; a lumbar puncture might document CSF abnormalities that would prompt close follow-up. Other tests (e.g., CBC, platelet count, and bone radiographs) may be performed to further support a diagnosis of congenital syphilis. If a single dose of benzathine penicillin G is used, then the infant must be fully evaluated (i.e., through CSF examination, long-bone radiographs, and CBC with platelets), the full evaluation must be normal, and follow-up must be certain. If any part of the infant's evaluation is abnormal or not performed or if the CSF analysis is rendered uninterpretable because of contamination with blood, then a 10-day course of penicillin is required.†† Recommended Regimens
Some specialists prefer the 10 days of parenteral therapy if the mother has untreated early syphilis at delivery. Scenario 3. Infants who have a normal physical examination and a serum quantitative nontreponemal serologic titer the same or less than fourfold the maternal titer and the
Recommended Evaluation No evaluation is required. Recommended Regimen
Scenario 4. Infants who have a normal physical examination and a serum quantitative nontreponemal serologic titer the same or less than fourfold the maternal titer and the
Recommended Evaluation No evaluation is required. Recommended Regimen
Evaluation and Treatment of Older Infants and ChildrenChildren who are identified as having reactive serologic tests for syphilis after the neonatal period (i.e., aged >1 month) should have maternal serology and records reviewed to assess whether the child has congenital or acquired syphilis (see Primary and Secondary Syphilis and Latent Syphilis, Sexual Assault or Abuse of Children). Any child at risk for congenital syphilis should receive a full evaluation and testing for HIV infection. Recommended Evaluation
Recommended Regimen
If the child has no clinical manifestations of disease, the CSF examination is normal, and the CSF VDRL test result is negative, some specialists would treat with up to 3 weekly doses of benzathine penicillin G, 50,000 U/kg IM. Any child who is suspected of having congenital syphilis or who has neurologic involvement should be treated with aqueous penicillin G. Some specialists also suggest giving these patients a single dose of benzathine penicillin G, 50,000 units/kg IM after the 10-day course of IV aqueous penicillin. This treatment also would be adequate for children who might have other treponemal infections. Follow-Up All seroreactive infants (or infants whose mothers were seroreactive at delivery) should receive careful follow-up examinations and serologic testing (i.e., a nontreponemal test) every 2--3 months until the test becomes nonreactive or the titer has decreased fourfold. Nontreponemal antibody titers should decline by age 3 months and should be nonreactive by age 6 months if the infant was not infected (i.e., if the reactive test result was caused by passive transfer of maternal IgG antibody) or was infected but adequately treated. The serologic response after therapy might be slower for infants treated after the neonatal period. If these titers are stable or increase after age 6--12 months, the child should be evaluated (e.g., given a CSF examination) and treated with a 10-day course of parenteral penicillin G. Treponemal tests should not be used to evaluate treatment response because the results for an infected child can remain positive despite effective therapy. Passively transferred maternal treponemal antibodies can be present in an infant until age 15 months. A reactive treponemal test after age 18 months is diagnostic of congenital syphilis. If the nontreponemal test is nonreactive at this time, no further evaluation or treatment is necessary. If the nontreponemal test is reactive at age 18 months, the infant should be fully (re)evaluated and treated for congenital syphilis. Infants whose initial CSF evaluations are abnormal should undergo a repeat lumbar puncture approximately every 6 months until the results are normal. A reactive CSF VDRL test or abnormal CSF indices that cannot be attributed to other ongoing illness requires re-treatment for possible neurosyphilis. Follow-up of children treated for congenital syphilis after the newborn period should be conducted as is recommended for neonates. Special Considerations Penicillin AllergyInfants and children who require treatment for syphilis but who have a history of penicillin allergy or develop an allergic reaction presumed secondary to penicillin should be desensitized, if necessary, and then treated with penicillin (see Management of Patients With a History of Penicillin Allergy). Data are insufficient regarding the use of other antimicrobial agents (e.g., ceftriaxone); if a nonpenicillin agent is used, close serologic and CSF follow-up are indicated. HIV InfectionEvidence is insufficient to determine whether infants who have congenital syphilis and whose mothers are coinfected with HIV require different evaluation, therapy, or follow-up for syphilis than is recommended for all infants. Penicillin ShortageDuring periods when the availability of penicillin is compromised, the following is recommended (see http://www.cdc.gov/nchstp/dstd/penicillinG.htm):
If aqueous or procaine penicillin G is not available, ceftriaxone (in doses according to age and weight) may be considered with careful clinical and serologic follow-up. Ceftriaxone must be used with caution in infants with jaundice. For infants aged >30 days, use 75 mg/kg IV/IM a day in a single daily dose for 10--14 days; however, dose adjustment might be necessary based on birthweight. For older infants, the dose should be 100 mg/kg a day in a single daily dose. Studies that strongly support ceftriaxone for the treatment of congenital syphilis have not been conducted. Therefore, ceftriaxone should be used in consultation with a specialist in the treatment of infants with congenital syphilis. Management may include a repeat CSF examination at age 6 months if the initial examination was abnormal.
If any part of the evaluation for congenital syphilis is abnormal, CSF examination is not interpretable, CSF examination was not performed, or follow-up is uncertain, Procaine penicillin G is recommended. A single dose of ceftriaxone is inadequate therapy.
Management of Patients Who Have a History of Penicillin AllergyNo proven alternatives to penicillin are available for treating neurosyphilis, congenital syphilis, or syphilis in pregnant women. Penicillin also is recommended for use, whenever possible, in HIV-infected patients. Of the adult U.S. population, 3%--10% have experienced an immunoglobulin E (IgE) mediated allergic response to penicillin such as urticaria, angioedema, or anaphylaxis (i.e., upper airway obstruction, bronchospasm, or hypotension). Re-administration of penicillin to these patients can cause severe, immediate reactions. Because anaphylactic reactions to penicillin can be fatal, every effort should be made to avoid administering penicillin to penicillin-allergic patients, unless they undergo acute desensitization to eliminate anaphylactic sensitivity. An estimated 10% of persons who report a history of severe allergic reactions to penicillin remain allergic. With the passage of time, the majority of persons who have had a severe reaction to penicillin stop expressing penicillin-specific IgE. These persons can be treated safely with penicillin. The results of many investigations indicate that skin testing with the major and minor determinants of penicillin can reliably identify persons at high risk for penicillin reactions. Although these reagents are easily generated and have been available for >30 years, only benzylpenicilloyl poly-L-lysine (Pre-Pen® [i.e., the major determinant]) and penicillin G have been available commercially. Testing with only the major determinant and penicillin G identifies an estimated 90%--97% of the currently allergic patients. However, because skin testing without the minor determinants would still miss 3%--10% of allergic patients and because serious or fatal reactions can occur among these minor-determinant--positive patients, specialists suggest exercising caution when the full battery of skin-test reagents is not available (Box 1). An additional challenge has occurred with the recent unavailability of Pre-Pen®; however, plans for future availability of this product have been made, as well as a companion minor determinant mixture. RecommendationsIf the full battery of skin-test reagents is available, including the major and minor determinants (see Penicillin Allergy Skin Testing), patients who report a history of penicillin reaction and who are skin-test negative can receive conventional penicillin therapy. Skin-test--positive patients should be desensitized. If the full battery of skin-test reagents, including the minor determinants, is not available, the patient should be skin tested using benzylpenicilloyl poly-L-lysine (i.e., the major determinant) and penicillin G. Patients who have positive test results should be desensitized. Some specialists suggest that persons who have negative test results should be regarded as probably allergic and should be desensitized. Others suggest that those with negative skin-test results can be test-dosed gradually with oral penicillin in a monitored setting in which treatment for anaphylactic reaction can be provided. If the major determinant (Pre-Pen®) is not available for skin testing, all patients with a history suggesting IgE mediated reactions (anaphylaxis, angioedema, bronchospasm, or urticaria) to penicillin should be desensitized in a hospital setting. In patients with reactions not likely to be IgE mediated, outpatient oral desensitization or monitored test doses may be considered. Penicillin Allergy Skin TestingPatients at high risk for anaphylaxis, including those who 1) have a history of penicillin-related anaphylaxis, asthma, or other diseases that would make anaphylaxis more dangerous or 2) are being treated with beta-adrenergic blocking agents should be tested with 100-fold dilutions of the full-strength skin-test reagents before being tested with full-strength reagents. In these situations, patients should be tested in a monitored setting in which treatment for an anaphylactic reaction is available. If possible, the patient should not have taken antihistamines recently (e.g., chlorpheniramine maleate or terfenadine during the preceding 24 hours, diphenhydramine HCl or hydroxyzine during the preceding 4 days, or astemizole during the preceding 3 weeks). Procedures Dilute the antigens either 1) 100-fold for preliminary testing if the patient has had a life-threatening reaction to penicillin or 2) 10-fold if the patient has had another type of immediate, generalized reaction to penicillin within the preceding year. Epicutaneous (Prick) TestsDuplicate drops of skin-test reagent are placed on the volar surface of the forearm. The underlying epidermis is pierced with a 26-gauge needle without drawing blood. An epicutaneous test is positive if the average wheal diameter after 15 minutes is 4 mm larger than that of negative controls; otherwise, the test is negative. The histamine controls should be positive to ensure that results are not falsely negative because of the effect of antihistaminic drugs. Intradermal TestIf epicutaneous tests are negative, duplicate 0.02-mL intradermal injections of negative control and antigen solutions are made into the volar surface of the forearm by using a 26- or 27-gauge needle on a syringe. The diameters of the wheals induced by the injections should be recorded. An intradermal test is positive if the average wheal diameter 15 minutes after injection is >2 mm larger than the initial wheal size and also is >2 mm larger than the negative controls. Otherwise, the tests are negative. Desensitization Patients who have a positive skin test to one of the penicillin determinants can be desensitized (Table 1). This is a straightforward, relatively safe procedure that can be performed orally or IV. Although the two approaches have not been compared, oral desensitization is regarded as safer and easier to perform. Patients should be desensitized in a hospital setting because serious IgE-mediated allergic reactions can occur. Desensitization usually can be completed in approximately 4 hours, after which the first dose of penicillin is administered. After desensitization, patients must be maintained on penicillin continuously for the duration of the course of therapy. Diseases Characterized by Urethritis and CervicitisManagement of Male Patients Who Have UrethritisUrethritis, as characterized by urethral inflammation, can result from infectious and noninfectious conditions. Symptoms, if present, include discharge of mucopurulent or purulent material, dysuria, or urethral pruritus. Asymptomatic infections are common. N. gonorrhoeae and C. trachomatis are clinically important infectious causes of urethritis. If clinic-based diagnostic tools (Gram stain microscopy) are not available, patients should be treated for both gonorrhea and chlamydia. Further testing to determine the specific etiology is recommended because both chlamydia and gonorrhea are reportable to state health departments, and a specific diagnosis might enhance partner notification and improve compliance with treatment, especially in exposed partners. Culture, nucleic acid hybridization tests, and nucleic acid amplification tests are available for the detection of both N. gonorrhoeae and C. trachomatis. Culture and hybridization tests require urethral swab specimens, whereas amplification tests can be performed on urine specimens. Because of their higher sensitivity, amplification tests are preferred for the detection of C. trachomatis. Etiology Several organisms can cause infectious urethritis. The presence of Gram-negative intracellular diplococci (GNID) on urethral smear is indicative of gonorrhea infection, which is frequently accompanied by chlamydial infection. Nongonoccocal urethritis (NGU) is diagnosed when microscopy indicates inflammation without GNID. C. trachomatis is a frequent cause of NGU (i.e., 15%--55% of cases); however, the prevalence varies by age group, with lower prevalence among older men. The proportion of NGU cases caused by chlamydia has been declining gradually. Complications of NGU among men infected with C. trachomatis include epididymitis, prostatitis, and Reiter's syndrome. Documentation of chlamydia infection is essential because of the need for partner referral for evaluation and treatment. The etiology of the majority of cases of nonchlamydial NGU is unknown. Ureaplasma urealyticum and Mycoplasma genitalium have been implicated as etiologic agents of NGU in some studies; however, detection of these organisms is frequently difficult (109--111). T. vaginalis, HSV, and adenovirus might also cause NGU (112--114). Diagnostic and treatment procedures for these organisms are reserved for situations in which these infections are suspected (e.g., contact with trichomoniasis and genital lesions or severe dysuria and meatitis, which might suggest genital herpes) or when NGU is not responsive to therapy. Enteric bacteria have been identified as an uncommon cause of NGU and might be associated with insertive anal sex. Confirmed Urethritis Clinicians should document that urethritis is present. Urethritis can be documented on the basis of any of the following signs or laboratory tests:
If none of these criteria are present, treatment should be deferred, and the patient should be tested for N. gonorrhoeae and C. trachomatis and followed closely if test results are negative. If the results demonstrate infection with either N. gonorrhoeae or C. trachomatis, the appropriate treatment should be given and sex partners referred for evaluation and treatment. Empiric treatment of symptoms without documentation of urethritis is recommended only for patients at high risk for infection who are unlikely to return for a follow-up evaluation. Such patients should be treated for gonorrhea and chlamydia. Partners of patients treated empirically should be evaluated and treated. Management of Patients Who Have Nongonococcal UrethritisDiagnosis All patients who have confirmed or suspected urethritis should be tested for gonorrhea and chlamydia. Testing for chlamydia is strongly recommended because of the increased utility and availability of highly sensitive and specific testing methods and because a specific diagnosis might enhance partner notification and improve compliance with treatment, especially in the exposed partner. Treatment Treatment should be initiated as soon as possible after diagnosis. Azithromycin and doxycycline are highly effective for chlamydial urethritis; however, infections with M. genitalium may respond better to azithromycin (115). Single-dose regimens have the advantage of improved compliance and directly observed treatment. To improve compliance, ideally the medication should be provided in the clinic or health-care provider's office. Recommended Regimens
Alternative Regimens
Follow-Up for Patients Who Have Urethritis Patients should be instructed to return for evaluation if symptoms persist or recur after completion of therapy. Symptoms alone, without documentation of signs or laboratory evidence of urethral inflammation, are not a sufficient basis for re-treatment. Patients should be instructed to abstain from sexual intercourse until 7 days after therapy is initiated, provided their symptoms have resolved and their sex partners have been adequately treated. Persistence of pain, discomfort, and irritative voiding symptoms beyond 3 months should alert the clinician to the possibility of chronic prostatitis/chronic pelvic pain syndrome in men. Persons whose conditions have been diagnosed as a new STD should receive testing for other STDs, including syphilis and HIV. Partner Referral Persons with NGU should refer for evaluation and treatment all sex partners within the preceding 60 days. Because a specific diagnosis might facilitate partner referral, testing for gonorrhea and chlamydia is encouraged. Recurrent and Persistent Urethritis Objective signs of urethritis should be present before initiation of antimicrobial therapy. In persons who have persistent symptoms after treatment without objective signs of urethritis, the value of extending the duration of antimicrobials has not been demonstrated. Persons who have persistent or recurrent urethritis can be re-treated with the initial regimen if they did not comply with the treatment regimen or if they were reexposed to an untreated sex partner. Otherwise, a T. vaginalis culture should be performed using an intraurethral swab or a first-void urine specimen (112). Some cases of recurrent urethritis after doxycycline treatment might be caused by tetracycline-resistant U. urealyticum. Urologic examinations usually do not reveal a specific etiology. Approximately 50% of men with chronic nonbacterial prostatitis/chronic pelvic pain syndrome have evidence of urethral inflammation without any identifiable microbial pathogens. If the patient was compliant with the initial regimen and reexposure can be excluded, the following regimen is recommended. Recommended Regimens
Special Considerations HIV Infection Gonococcal urethritis, chlamydial urethritis, and nongonococcal, nonchlamydial urethritis might facilitate HIV transmission. Patients who have NGU and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. Management of Patients Who Have CervicitisTwo major diagnostic signs characterize cervicitis: 1) a purulent or mucopurulent endocervical exudate visible in the endocervical canal or on an endocervical swab specimen (commonly referred to as "mucopurulent cervicitis" or cervicitis), and 2) sustained endocervical bleeding easily induced by gentle passage of a cotton swab through the cervical os. Either or both signs might be present. Cervicitis frequently is asymptomatic, but some women complain of an abnormal vaginal discharge and intermenstrual vaginal bleeding (e.g., after sexual intercourse). A finding of leukorrhea (>10 WBC per high power field on microscopic examination of vaginal fluid) has been associated with chlamydial and gonococcal infection of the cervix. In the absence of inflammatory vaginitis, leukorrhea might be a sensitive indicator of cervical inflammation with a high negative predictive value (116). Although some specialists consider an increased number of polymorphonuclear leukocytes on endocervical Gram stain as being useful in the diagnosis of cervicitis, this criterion has not been standardized. In addition, it has a low positive-predictive value (PPV) for infection with C. trachomatis and N. gonorrhoeae and is not available in the majority of clinical settings. Finally, although the presence of GNID on Gram stain of endocervical fluid is specific for the diagnosis of gonococcal cervical infection, it is insensitive because it is observed in only 50% of women with this infection. Etiology When an etiologic organism is isolated in the setting of cervicitis, it is typically C. trachomatis or N. gonorrhoeae. Cervicitis also can accompany trichomoniasis and genital herpes (especially primary HSV-2 infection). However, in the majority of cases of cervicitis, no organism is isolated, especially in women at relatively low risk for recent acquisition of these STDs (for example, women aged >30 years). Limited data indicate that infection with M. genitalium and BV as well as frequent douching might cause cervicitis (117--119). For reasons that are unclear, cervicitis can persist despite repeated courses of antimicrobial therapy. Because the majority of persistent cases of cervicitis are not caused by relapse or reinfection with C. trachomatis or N. gonorrhoeae, other determinants (e.g., persistent abnormality of vaginal flora, douching or exposure to chemical irritants, or idiopathic inflammation in the zone of ectopy) might be involved. Diagnosis Because cervicitis might be a sign of upper genital tract infection (endometritis), women who seek medical treatment for a new episode of cervicitis should be assessed for signs of PID and should be tested for C. trachomatis and for N. gonorrhoeae with the most sensitive and specific test available, NAAT. Women with cervicitis also should be evaluated for the presence of BV and trichomoniasis, and these conditions should be treated, if present. Because the sensitivity of microscopy to detect T. vaginalis is relatively low (approximately 50%), symptomatic women with cervicitis and negative microscopy for trichomonads should receive further testing (i.e., culture or antigen-based detection). Although HSV-2 infection has been associated with cervicitis, the utility of specific testing (i.e., culture or serologic testing) for HSV-2 in this setting is unclear. Standardized diagnostic tests for M. genitalium are not commercially available. NAAT for C. trachomatis and N. gonorrhoeae are preferred for the diagnostic evaluation of cervicitis and can be performed on either cervical or urine samples. A finding of >10 WBC in vaginal fluid, in the absence of trichomoniasis, might indicate endocervical inflammation caused specifically by C. trachomatis or N. gonorrhoeae (116,120,121). Treatment Several factors should affect the decision to provide presumptive therapy for cervicitis or to await the results of diagnostic tests. Treatment with antibiotics for C. trachomatis should be provided in women at increased risk for this common STD (age <25 years, new or multiple sex partners, and unprotected sex), especially if follow-up cannot be ensured and if a relatively insensitive diagnostic test (not a NAAT) is used. Concurrent therapy for N. gonorrhoeae is indicated if the prevalence of this infection is high (>5%) in the patient population (young age and facility prevalence). Concomitant trichomoniasis or symptomatic BV should also be treated if detected. For women in whom any component of (or all) presumptive therapy is deferred, the results of sensitive tests for C. trachomatis and N. gonorrhoeae (e.g., nucleic acid amplification tests) should determine the need for treatment subsequent to the initial evaluation. Recommended Regimens for Presumptive Treatment*
* Consider concurrent treatment for gonococcal infection if prevalence of gonorrhea is high in the patient population under assessment. Recurrent and Persistent Cervicitis Women with persistent cervicitis should be reevaluated for possible reexposure to an STD, and her vaginal flora should be reassessed. If relapse and/or reinfection with a specific STD has been excluded, BV is not present, and sex partners have been evaluated and treated, management options for persistent cervicitis are undefined. For such women, the value of repeated or prolonged administration of antibiotic therapy for persistent symptomatic cervicitis is unknown. Women who receive such a course should return after treatment so that a determination can be made regarding whether cervicitis has resolved. In women with persistent symptoms that are clearly attributable to cervicitis, ablative therapy may be considered by a gynecologic specialist. Follow-Up Follow-up should be conducted as recommended for the infections for which a woman is treated. If symptoms persist, women should be instructed to return for reevaluation. Management of Sex Partners Management of sex partners of women treated for cervicitis should be appropriate for the identified or suspected STD. Partners should be notified and examined if chlamydia, gonorrhea, or trichomoniasis was identified or suspected in the index patient and treated for the STDs for which the index patient received treatment. To avoid re-infection, patients and their sex partners should abstain from sexual intercourse until therapy is completed (i.e., 7 days after a single-dose regimen or after completion of a 7-day regimen). Special Considerations HIV InfectionPatients who have cervicitis and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. Treatment of cervicitis in HIV-infected women is vital because cervicitis increases cervical HIV shedding. Treatment of cervicitis in HIV-infected women reduces HIV shedding from the cervix and might reduce HIV transmission to susceptible sex partners (122). Chlamydial InfectionsChlamydial Infections in Adolescents and Adults In the United States, chlamydial genital infection is the most frequently reported infectious disease, and the prevalence is highest in persons aged <25 years (123). Several important sequelae can result from C. trachomatis infection in women; the most serious of these include PID, ectopic pregnancy, and infertility. Some women who have uncomplicated cervical infection already have subclinical upper reproductive tract infection. Asymptomatic infection is common among both men and women, and to detect chlamydial infections health-care providers frequently rely on screening tests. Annual screening of all sexually active women aged <25 years is recommended (124), as is screening of older women with risk factors (e.g., those who have a new sex partner or multiple sex partners). The benefits of C. trachomatis screening in women have been demonstrated in areas where screening programs have reduced both the prevalence of infection and rates of PID (125,126). Evidence is insufficient to recommend routine screening for C. trachomatis in sexually active young men, based on feasibility, efficacy, and cost-effectiveness. However, screening of sexually active young men should be considered in clinical settings with a high prevalence of chlamydia (e.g., adolescent clinics, correctional facilities, and STD clinics). An appropriate sexual risk assessment should be conducted for all persons and might indicate more frequent screening for some women or certain men. Diagnostic Considerations C. trachomatis urogenital infection in women can be diagnosed by testing urine or swab specimens collected from the endocervix or vagina. Diagnosis of C. trachomatis urethral infection in men can be made by testing a urethral swab or urine specimen. Rectal C. trachomatis infections in persons that engage in receptive anal intercourse can be diagnosed by testing a rectal swab specimen. Culture, direct immunofluorescence, EIA, nucleic acid hybridization tests, and NAATs are available for the detection of C. trachomatis on endocervical and male urethral swab specimens (127). NAATs are the most sensitive tests for these specimens and are FDA-cleared for use with urine, and some tests are cleared for use with vaginal swab specimens. The majority of tests, including NAAT and nucleic acid hybridization tests, are not FDA-cleared for use with rectal swab specimens, and chlamydia culture is not widely available for this purpose. Some noncommercial laboratories have initiated NAAT of rectal swab specimens after establishing the performance of the test to meet CLIA requirements. Patients' whose condition has been diagnosed as chlamydia also should be tested for other STDs. TreatmentTreating infected patients prevents transmission to sex partners. In addition, treating pregnant women usually prevents transmission of C. trachomatis to infants during birth. Treatment of sex partners helps to prevent reinfection of the index patient and infection of other partners. Coinfection with C. trachomatis frequently occurs among patients who have gonococcal infection; therefore, presumptive treatment of such patients for chlamydia is appropriate (see Gonococcal Infection, Dual Therapy for Gonococcal and Chlamydial Infections). The following recommended treatment regimens and alternative regimens cure infection and usually relieve symptoms. Recommended Regimens
Alternative Regimens
A recent meta-analysis of 12 randomized clinical trials of azithromycin versus doxycycline for the treatment of genital chlamydial infection demonstrated that the treatments were equally efficacious, with microbial cure rates of 97% and 98%, respectively (128). These studies were conducted primarily in populations in which follow-up was encouraged, adherence to a 7-day regimen was effective, and culture or EIA (rather than the more sensitive NAAT was used for determining microbiological outcome. Azithromycin should always be available to treat patients for whom compliance with multiday dosing is in question. In populations that have erratic health-care--seeking behavior, poor treatment compliance, or unpredictable follow-up, azithromycin might be more cost-effective because it enables the provision of a single-dose of directly observed therapy. However, doxycycline costs less than azithromycin and has no higher risk for adverse events (128). Erythromycin might be less efficacious than either azithromycin or doxycycline, mainly because of the frequent occurrence of gastrointestinal side effects that discourage compliance. Ofloxacin and levofloxacin are effective treatment alternatives but are more expensive and offer no advantage in the dosage regimen. Other quinolones either are not reliably effective against chlamydial infection or have not been evaluated adequately. To maximize compliance with recommended therapies, medications for chlamydial infections should be dispensed on site, and the first dose should be directly observed. To minimize transmission, persons treated for chlamydia should be instructed to abstain from sexual intercourse for 7 days after single-dose therapy or until completion of a 7-day regimen. To minimize the risk for reinfection, patients also should be instructed to abstain from sexual intercourse until all of their sex partners are treated. Follow-upExcept in pregnant women, test-of-cure (repeat testing 3--4 weeks after completing therapy) is not recommended for persons treated with the recommended or alterative regimens, unless therapeutic compliance is in question, symptoms persist, or reinfection is suspected. Moreover, the validity of chlamydial diagnostic testing at <3 weeks after completion of therapy (to identify patients who did not respond to therapy) has not been established. False-negative results might occur because of persistent infections involving limited numbers of chlamydial organisms. In addition, NAAT conducted at <3 weeks after completion of therapy in persons who were treated successfully could yield false-positive results because of the continued presence of dead organisms. A high prevalence of C. trachomatis infection is observed in women who were treated for chlamydial infection in the preceding several months (129,130). The majority of posttreatment infections result from reinfection, frequently occurring because the patient's sex partners were not treated or because the patient resumed sex with a new partner infected with C. trachomatis. Repeat infections confer an elevated risk for PID and other complications when compared with the initial infection. Therefore, recently infected women are a major priority for repeat testing for C. trachomatis. Clinicians and health-care agencies should consider advising all women with chlamydial infection to be retested approximately 3 months after treatment. Providers also are strongly encouraged to retest all women treated for chlamydial infection whenever they next seek medical care within the following 3--12 months, regardless of whether the patient believes that her sex partners were treated. Recognizing that retesting is distinct from a test-of-cure, as discussed in this report, is vital. Limited evidence is available on the benefit of retesting for chlamydia in men previously infected; however, some specialists suggest retesting men approximately 3 months after treatment. Management of Sex PartnersPatients should be instructed to refer their sex partners for evaluation, testing, and treatment. The following recommendations on exposure intervals are based on limited evaluation. Sex partners should be evaluated, tested, and treated if they had sexual contact with the patient during the 60 days preceding onset of symptoms in the patient or diagnosis of chlamydia. The most recent sex partner should be evaluated and treated, even if the time of the last sexual contact was >60 days before symptom onset or diagnosis. If concerns exist that sex partners will not seek evaluation and treatment, or if other management strategies are impractical or unsuccessful, then delivery of antibiotic therapy (either a prescription or medication) by heterosexual male or female patients to their partners might be an option (see Partner Management). Limited studies to date have demonstrated a trend toward a decrease in rates of persistent or recurrent chlamydia with this approach compared with standard partner referral (25,27). Male patients must inform female partners of their infection and be given accompanying written materials about the importance of seeking evaluation for PID (especially if symptomatic). Patient-delivered partner therapy is not routinely recommended for MSM because of a high risk for coexisting infections, especially undiagnosed HIV infection, in their partners. Patients should be instructed to abstain from sexual intercourse until they and their sex partners have completed treatment. Abstinence should be continued until 7 days after a single-dose regimen or after completion of a 7-day regimen. Timely treatment of sex partners is essential for decreasing the risk for reinfecting the index patient. Special ConsiderationsPregnancy. Doxycycline, ofloxacin, and levofloxacin are contraindicated in pregnant women. However, clinical experience and studies suggest that azithromycin is safe and effective (131--133). Repeat testing (preferably by NAAT) 3 weeks after completion of therapy with the following regimens is recommended for all pregnant women to ensure therapeutic cure, considering the sequelae that might occur in the mother and neonate if the infection persists. The frequent gastrointestinal side effects associated with erythromycin might discourage patient compliance with the alternative regimens. Recommended Regimens
Alternative Regimens
Erythromycin estolate is contraindicated during pregnancy because of drug-related hepatotoxicity. The lower dose 14-day erythromycin regimens may be considered if gastrointestinal tolerance is a concern. HIV Infection. Patients who have chlamydial infection and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. Chlamydial Infections Among InfantsPrenatal screening of pregnant women can prevent chlamydial infection among neonates. Pregnant women aged <25 years are at high risk for infection. Local or regional prevalence surveys of chlamydial infection can be conducted to confirm the utility of using these recommendations in particular settings. C. trachomatis infection of neonates results from perinatal exposure to the mother's infected cervix. Neonatal ocular prophylaxis with silver nitrate solution or antibiotic ointments does not prevent perinatal transmission of C. trachomatis from mother to infant. However, ocular prophylaxis with those agents does prevent gonococcal ophthalmia and, therefore, should be continued (see Ophthalmia Neonatorum Prophylaxis). Initial C. trachomatis perinatal infection involves the mucous membranes of the eye, oropharynx, urogenital tract, and rectum and might be asymptomatic in these locations. C. trachomatis infection in neonates is most frequently recognized by conjunctivitis that develops 5--12 days after birth. C. trachomatis also can cause a subacute, afebrile pneumonia with onset at ages 1--3 months. C. trachomatis has been the most frequent identifiable infectious cause of ophthalmia neonatorum, but perinatal chlamydial infections, including opthalmia and pneumonia, are detected less frequently because of the institution of widespread prenatal screening and treatment of pregnant women. Ophthalmia Neonatorum Caused by C. trachomatis A chlamydial etiology should be considered for all infants aged <30 days who have conjunctivitis, especially if the mother has a history of untreated chlamydia infection. Diagnostic ConsiderationsSensitive and specific methods used to diagnose chlamydial ophthalmia in the neonate include both tissue culture and nonculture tests (e.g., DFA tests, EIA, and NAAT). The majority of nonculture tests are not FDA-cleared for the detection of chlamydia from conjunctival swabs, and clinical laboratories must verify the procedure according to CLIA regulations. Specimens must contain conjunctival cells, not exudate alone. Specimens for culture isolation and nonculture tests should be obtained from the everted eyelid using a dacron-tipped swab or the swab specified by the manufacturer's test kit. A specific diagnosis of C. trachomatis infection confirms the need for treatment not only for the neonate but also for the mother and her sex partner(s). Ocular exudate from infants being evaluated for chlamydial conjunctivitis also should be tested for N. gonorrhoeae. Recommended Regimen
Topical antibiotic therapy alone is inadequate for treatment of chlamydial infection and is unnecessary when systemic treatment is administered. Follow-UpThe efficacy of erythromycin treatment is approximately 80%; a second course of therapy might be required and, therefore, follow-up of infants is recommended to determine whether initial treatment was effective. The possibility of concomitant chlamydial pneumonia should be considered. Management of Mothers and Their Sex Partners The mothers of infants who have chlamydial infection and the sex partners of these women should be evaluated and treated (see Chlamydial Infection in Adolescents and Adults). Infant Pneumonia Caused by C. trachomatis Characteristic signs of chlamydial pneumonia in infants include 1) a repetitive staccato cough with tachypnea and 2) hyperinflation and bilateral diffuse infiltrates on a chest radiograph. Wheezing is rare, and infants are typically afebrile. Peripheral eosinophilia (>400 cells/mm3) occurs frequently. Because clinical presentations differ, initial treatment and diagnostic tests should include C. trachomatis for all infants aged 1--3 months who possibly have pneumonia (especially with untreated maternal chlamydial infection). Diagnostic ConsiderationsSpecimens for chlamydial testing should be collected from the nasopharynx. Tissue culture is the definitive standard for chlamydial pneumonia. Nonculture tests (e.g., EIA, DFA, and NAAT) can be used, although nonculture tests of nasopharyngeal specimens have a lower sensitivity and specificity than nonculture tests of ocular specimens. DFA is the only FDA-cleared test for the detection of C. trachomatis from nasopharyngeal specimens. Tracheal aspirates and lung biopsy specimens, if collected, should be tested for C. trachomatis. Because of the delay in obtaining test results for chlamydia, the decision to provide treatment for C. trachomatis pneumonia must frequently be based on clinical and radiologic findings. The results of tests for chlamydial infection assist in the management of an infant's illness and determine the need for treating the mother and her sex partner(s). Recommended Regimen
Follow-UpThe effectiveness of erythromycin in treating pneumonia caused by C. trachomatis is approximately 80%; a second course of therapy might be required. Follow-up of infants is recommended to determine whether the pneumonia has resolved. Some infants with chlamydial pneumonia have abnormal pulmonary function tests later in childhood. Management of Mothers and Their Sex Partners Mothers of infants who have chlamydia pneumonia and the sex partners of these women should be evaluated and treated according to the recommended treatment of adults for chlamydial infections (see Chlamydial Infection in Adolescents and Adults). Infants Born to Mothers Who Have Chlamydial Infection Infants born to mothers who have untreated chlamydia are at high risk for infection; however, prophylatic antibiotic treatment is not indicated, and the efficacy of such treatment is unknown. Infants should be monitored to ensure appropriate treatment if symptoms develop. Chlamydial Infections Among Children Sexual abuse must be considered a cause of chlamydial infection in preadolescent children, although perinatally transmitted C. trachomatis infection of the nasopharynx, urogenital tract, and rectum might persist for >1 year (see Sexual Assault or Abuse of Children). Diagnostic ConsiderationsNonculture, nonamplified probe tests for chlamydia (EIA, DFA) should not be used because of the possibility of false-positive test results. With respiratory tract specimens, false-positive results can occur because of cross-reaction of test reagents with C. pneumoniae; with genital and anal specimens, false-positive results might occur because of cross-reaction with fecal flora. Recommended Regimens for Children Who Weigh
<45 kg
Recommended Regimen for Children Who Weigh
>45 kg but Who Are Aged <8 Years
Recommended Regimens for Children Aged
>8 years
Other Management ConsiderationsSee Sexual Assault or Abuse of Children. Follow-Up. Follow-up cultures are necessary to ensure that treatment has been effective. Gonococcal InfectionsGonococcal Infections in Adolescents and Adults In the United States, an estimated 600,000 new N. gonorrhoeae infections occur each year (123). Gonorrhea is the second most commonly reported bacterial STD. The majority of urethral infections caused by N. gonorrhoeae among men produce symptoms that cause them to seek curative treatment soon enough to prevent serious sequelae, but treatment might not be soon enough to prevent transmission to others. Among women, several infections do not produce recognizable symptoms until complications (e.g., PID) have occurred. Both symptomatic and asymptomatic cases of PID can result in tubal scarring that can lead to infertility or ectopic pregnancy. Because gonococcal infections among women frequently are asymptomatic, an essential component of gonorrhea control in the United States continues to be the screening of women at high risk for STDs. The U.S. Preventive Services Task Force (USPSTF) recommends that clinicians screen all sexually active women, including those who are pregnant, for gonorrhea infection if they are at increased risk. Women aged <25 years are at highest risk for gonorrhea infection. Other risk factors for gonorrhea include a previous gonorrhea infection, other sexually transmitted infections, new or multiple sex partners, inconsistent condom use, commercial sex work, and drug use. The prevalence of gonorrhea infection varies widely among communities and patient populations. The USPSTF does not recommend screening for gonorrhea in men and women who are at low risk for infection (134). Diagnostic Considerations Because of high specificity (>99%) and sensitivity (>95%), a Gram stain of a male urethral specimen that demonstrates polymorphonuclear leukocytes with intracellular Gram-negative diplococci can be considered diagnostic for infection with N. gonorrhoeae in symptomatic men. However, because of lower sensitivity, a negative Gram stain should not be considered sufficient for ruling out infection in asymptomatic men. In addition, Gram stain of endocervical specimens, pharyngeal, or rectal specimens also are not sufficient to detect infection and, therefore, are not recommended. Specific testing for N. gonorrhoeae is recommended because of the increased utility and availability of highly sensitive and specific testing methods and because a specific diagnosis might enhance partner notification. Specific diagnosis of infection with N. gonorrhoeae may be performed by testing endocervical, vaginal, male urethral, or urine specimens. Culture, nucleic acid hybridization tests, and NAAT are available for the detection of genitourinary infection with N. gonorrhoeae (127). Culture and nucleic acid hybridization tests require female endocervical or male urethral swab specimens. NAAT offer the widest range of testing specimen types because they are FDA-cleared for use with endocervical swabs, vaginal swabs, male urethral swabs, and female and male urine. However, product inserts for each NAAT vendor must be carefully examined to assess current indications because FDA-cleared specimen types might vary. In general, culture is the most widely available option for the diagnosis of infection with N. gonorrhoeae in nongenital sites (e.g., rectum and pharynx). Nonculture tests are not FDA-cleared for use in the rectum and pharynx. Some NAATs have the potential to cross-react with nongonococcal Neisseria and related organisms that are commonly found in the throat. Some noncommercial laboratories have initiated NAAT of rectal and pharyngeal swab specimens after establishing the performance of the test to meet CLIA requirements. Because nonculture tests cannot provide antimicrobial susceptibility results, in cases of persistent gonococcal infection after treatment, clinicians should perform both culture and antimicrobial susceptibility testing. All patients tested for gonorrhea should be tested for other STDs, including chlamydia, syphilis, and HIV. Dual Therapy for Gonococcal and Chlamydial InfectionsPatients infected with N. gonorrhoeae frequently are coinfected with C. trachomatis; this finding has led to the recommendation that patients treated for gonococcal infection also be treated routinely with a regimen that is effective against uncomplicated genital C. trachomatis infection (135). Because the majority of gonococci in the United States are susceptible to doxycycline and azithromycin, routine cotreatment might also hinder the development of antimicrobial-resistant N. gonorrhoeae. Because of the high sensitivity of NAATs for chlamydial infection, patients with a negative chlamydial NAAT result at the time of treatment for gonorrhea do not need to be treated for chlamydia as well. However, if chlamydial test results are not available or if a non-NAAT was negative for chlamydia, patients should be treated for both gonorrhea and chlamydia. Quinolone-Resistant N. gonorrhoeae (QRNG) QRNG continues to spread, making the treatment of gonorrhea with quinolones such as ciprofloxacin inadvisable in many areas and populations (136). Resistance to ciprofloxacin usually indicates resistance to other quinolones as well. QRNG is common in parts of Europe, the Middle East, Asia, and the Pacific. In the United States, QRNG is becoming increasingly common. Previously, CDC had advised that quinolones not be used in California and Hawaii because of the high prevalence of QRNG in these areas (137). The prevalence of QRNG has increased in other areas of the United States, which has resulted in changes in recommended treatment regimens by other states and local areas. QRNG prevalence will continue to increase, and quinolones will eventually not be advisable for the treatment of gonorrhea. The CDC website (http://www.cdc.gov/std/gisp) or state health departments can provide the most current information. In 2004, of 6,322 isolates collected by CDC's Gonococcal Isolate Surveillance Project (GISP), 6.8% were resistant to ciprofloxacin (minimum inhibitory concentrations [MICs] >1.0 µg/mL). Excluding isolates from California and Hawaii, 3.6% of isolates were QRNG. QRNG was more common among MSM than among heterosexual men (23.9% versus 2.9%). In 2004, QRNG among heterosexual men outside of California and Hawaii was 1.4% (138). Quinolones should not be used for the treatment of gonorrhea among MSM (139) or in areas with increased QRNG prevalence in the United States (e.g., California and Hawaii) or for infections acquired while traveling abroad. Because oral alternatives to quinolones are limited, quinolones may continue to be used for heterosexual men and women in areas and populations not known to have elevated levels of resistance. Clinicians should obtain information on the sexual behavior and recent travel history (including histories from sex partners) of persons to be treated for gonorrhea to ensure appropriate antibiotic therapy. Resistance of N. gonorrhoeae to fluoroquinolones and other antimicrobials is expected to continue to spread; therefore, state and local surveillance for antimicrobial resistance is crucial for guiding local therapy recommendations. GISP, which samples approximately 3% of all U.S. men who have gonococcal infections, is a mainstay of surveillance. However, surveillance by clinicians also is critical. Clinicians who have diagnosed N. gonorrhoeae infection in a person who was previously treated with a recommended regimen and who probably has not been reexposed should perform culture and susceptibility testing of relevant clinical specimens and report the case to the local health department. Uncomplicated Gonococcal Infections of the Cervix, Urethra, and Rectum Recommended Regimens*
* Quinolones should not be used for infections in MSM or in those with a history of recent foreign travel or partners' travel, infections acquired in California or Hawaii, or infections acquired in other areas with increased QRNG prevalence. Recommended Regimens for MSM or Heterosexuals with a History of Recent Travel*
* Quinolones should not be used for infections in MSM or in those with a history of recent foreign travel or partners' travel, infections acquired in California or Hawaii, or infections acquired in other areas with increased QRNG prevalence. To maximize compliance with recommended therapies, medications for gonococcal infections should be dispensed on site. Ceftriaxone in a single injection of 125 mg provides sustained, high bactericidal levels in the blood. Extensive clinical experience indicates that ceftriaxone is safe and effective for the treatment of uncomplicated gonorrhea at all anatomic sites, curing 98.9% of uncomplicated urogenital and anorectal infections in published clinical trials (140). Cefixime has an antimicrobial spectrum similar to that of ceftriaxone, but the 400 mg oral dose does not provide as high, nor as sustained, a bactericidal level as that provided by the 125 mg dose of ceftriaxone. In published clinical trials, the 400 mg dose cured 97.4% of uncomplicated urogenital and anorectal gonococcal infections (140). The advantage of cefixime is that it can be administered orally. Updates on the availability of cefixime are available from CDC or state health departments. Ciprofloxacin is no longer universally effective against N. gonorrhoeae in the United States (138). However, ciprofloxacin is safe, inexpensive, and can be administered orally. In published clinical trials of uncomplicated urogenital and anorectal infections in the absence of QRNG, a dose of 500 mg of ciprofloxacin provides sustained bactericidal levels with cure rates of 99.8% (140). If QRNG is suspected, ceftriaxone IM or cefixime PO (by mouth) should be used. If neither of these regimens are feasible options, then one of the alternative nonquinolone regimens in this report should be considered. Similar to ciprofloxacin, ofloxacin is no longer universally effective against N. gonorrhoeae in the United States. The 400 mg oral dose of ofloxacin has been effective for treatment of uncomplicated urogenital and anorectal infections; in clinical trials, 98.6% of infections were cured (140). Levofloxacin, the active l-isomer of ofloxacin, can be used in place of ofloxacin as a single dose of 250 mg. Alternative Regimens
Several other antimicrobials are active against N. gonorrhoeae, but none have substantial advantages over the recommended regimens. Spectinomycin is expensive and must be injected; however, it has been effective in published clinical trials, curing 98.2% of uncomplicated urogenital and anorectal gonococcal infections (140). Spectinomycin is useful for the treatment of patients who cannot tolerate cephalosporins and quinolones. Single-dose cephalosporin regimens (other than ceftriaxone 125 mg IM and cefixime 400 mg orally) that are safe and highly effective against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime (500 mg, administered IM), cefoxitin (2 g, administered IM with probenecid 1 g orally), and cefotaxime (500 mg, administered IM). None of the injectable cephalosporins offer any advantage over ceftriaxone. Single-dose quinolone regimens include gatifloxacin 400 mg orally, norfloxacin 800 mg orally, and lomefloxacin 400 mg orally. These regimens appear to be safe and effective for the treatment of uncomplicated gonorrhea, but data regarding their use are limited. None of the regimens appear to offer any advantage over ciprofloxacin, ofloxacin, or levofloxacin, and they are not effective against QRNG. Some evidence suggests that cefpodoxime and cefuroxime axetil 1 g orally might be additional oral alternatives in the treatment of uncomplicated urogenital gonorrhea; additional information on alternative oral regimens are available at http://www.cdc.gov/std. Cefpodoxime proxetil 200 mg PO is less active against N. gonorrhoeae than cefixime and also does not quite meet the minimum efficacy criteria (demonstrated efficacy with lower 95% confidence interval [CI] of >95% in summed clinical trials) with cure rates, 96.5% (CI = 94.8%--98.9%) for urogenital and rectal infection; efficacy in treating pharyngeal infection is unsatisfactory, 78.9% (CI = 54.5%--94%). Clinical studies are being conducted to assess whether cefpodoxime 400 mg PO is an acceptable oral alternative. Treatment with cefuroxime axetil 1 g PO does not quite meet the minimum efficacy criteria for urogenital and rectal infection (95.9%; CI = 94.5%--97.3%) and, its efficacy in treating pharyngeal infection is unacceptable (56.9%; CI = 42.2%--70.7%). Azithromycin 2 g orally is effective against uncomplicated gonococcal infection but is expensive and causes gastrointestinal distress and is not recommended for treatment of gonorrhea. Although azithromycin 1 g theoretically meets alternative regimen criteria, it is not recommended because of concerns regarding the possible rapid emergence of antimicrobial resistance. N. gonorrhoeae in the United States is not adequately susceptible to penicillins, tetracyclines, and macrolides (e.g., erythromycin) for these antimicrobials to be recommended. Uncomplicated Gonococcal Infections of the Pharynx Gonococcal infections of the pharynx are more difficult to eradicate than infections at urogenital and anorectal sites. Few antimicrobial regimens can reliably cure >90% of gonococcal pharyngeal infections. Although chlamydial coinfection of the pharynx is unusual, coinfection at genital sites sometimes occurs. Therefore, treatment for both gonorrhea and chlamydia is recommended. Recommended Regimens*
* Quinolones should not be used for infections in MSM or in those with a history of recent foreign travel or partners' travel, infections acquired in California or Hawaii, or infections acquired in other areas with increased QRNG prevalence. Recommended Regimens for MSM or Heterosexuals with a History of Recent Travel
Follow-Up Patients who have uncomplicated gonorrhea and who are treated with any of the recommended or alternative regimens do not need a test of cure. Patients who have symptoms that persist after treatment should be evaluated by culture for N. gonorrhoeae, and any gonococci isolated should be tested for antimicrobial susceptibility. Persistent urethritis, cervicitis, or proctitis also might be caused by C. trachomatis or other organisms. A high prevalence of N. gonorrhoeae infection is observed in patients who have had gonorrhea in the preceding several months (141,142). The majority of infections identified after treatment with one of the recommended regimens result from reinfection rather than treatment failure, indicating a need for improved patient education and referral of sex partners. Clinicians should consider advising all patients with gonorrhea to be retested 3 months after treatment. If patients do not seek medical care for retesting in 3 months, providers are encouraged to test these patients whenever they next seek medical care within the following 12 months, regardless of whether the patient believes that their sex partners were treated. Retesting is distinct from test of cure to detect therapeutic failure, which is not recommended. Management of Sex Partners Effective clinical management of patients with treatable STDs requires treatment of the patients' recent sex partners to prevent reinfection and curtail further transmission. Patients should be instructed to refer their sex partners for evaluation and treatment. Sex partners of patients with N. gonorrhoeae infection whose last sexual contact with the patient was within 60 days before onset of symptoms or diagnosis of infection in the patient should be evaluated and treated for N. gonorrhoeae and C. trachomatis infections. If a patient's last sexual intercourse was >60 days before onset of symptoms or diagnosis, the patient's most recent sex partner should be treated. Patients should be instructed to avoid sexual intercourse until therapy is completed and until they and their sex partners no longer have symptoms. For patients with gonorrhea whose partners' treatment cannot be ensured or is unlikely, delivery of antibiotic therapy (i.e., either a prescription or medication) by heterosexual male or female patients to their partners is an option (see Partner Management). Use of this approach (25,27) should always be accompanied by efforts to educate partners about symptoms and to encourage partners to seek clinical evaluation. Male patients must inform female partners of their infection and be given accompanying materials about the importance of seeking medical evaluation for PID (especially if symptomatic). Possible undertreatment of PID in female partners and possible missed opportunities to diagnose other STDs are of concern and have not been evaluated in comparisons with patient-delivered therapy and partner referral. Patient-delivered therapy for patients with gonorrhea should routinely include treatment for chlamydia. This approach should not be considered a routine partner management strategy in MSM because of the high risk of coexisting undiagnosed STDs or HIV infection. Special Considerations Allergy, Intolerance, and Adverse ReactionsPersons who cannot tolerate cephalosporins or quinolones should be treated with spectinomycin. Because spectinomycin is unreliable (52% effective) against pharyngeal infections, patients who have suspected or known pharyngeal infection should have a pharyngeal culture 3--5 days after treatment to verify eradication of infection. PregnancyPregnant women should not be treated with quinolones or tetracyclines. Those infected with N. gonorrhoeae should be treated with a recommended or alternate cephalosporin. Women who cannot tolerate a cephalosporin should be administered a single 2-g dose of spectinomycin IM. Either azithromycin or amoxicillin is recommended for treatment of presumptive or diagnosed C. trachomatis infection during pregnancy (see Chlamydial Infections). Administration of Quinolones to AdolescentsFluoroquinolones have not been recommended for persons aged <18 years because studies have indicated that they can damage articular cartilage in some young animals. However, no joint damage attributable to quinolone therapy has been observed in children treated with prolonged ciprofloxacin regimens (143). Therefore, children who weigh >45 kg can be treated with any regimen recommended for adults (See Gonococcal Infections). HIV InfectionPatients who have gonococcal infection and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. Gonococcal Conjunctivitis In the only published study of the treatment of gonococcal conjunctivitis among U.S. adults, all 12 study participants responded to a single 1-g IM injection of ceftriaxone (144). The following recommendation reflects the opinions of consultants knowledgeable in the field of STDs. Recommended Regimen
Consider lavage of the infected eye with saline solution once. Management of Sex PartnersPatients should be instructed to refer their sex partners for evaluation and treatment (see Gonococcal Infections, Management of Sex Partners). Disseminated Gonococcal Infection (DGI) DGI results from gonococcal bacteremia. DGI frequently results in petechial or pustular acral skin lesions, asymmetrical arthralgia, tenosynovitis, or septic arthritis. The infection is complicated occasionally by perihepatitis and rarely by endocarditis or meningitis. Some strains of N. gonorrhoeae that cause DGI may cause minimal genital inflammation. No studies on the treatment of DGI among adults have been published since publication of the last CDC STD treatment guidelines publication. DGI treatment recommendations reflect the opinions of consultants. No treatment failures have been reported with the recommended regimens. TreatmentHospitalization is recommended for initial therapy, especially for patients who might not comply with treatment, for those in whom diagnosis is uncertain, and for those who have purulent synovial effusions or other complications. Patients should be examined for clinical evidence of endocarditis and meningitis. Patients treated for DGI should be treated presumptively for concurrent C. trachomatis infection, unless appropriate testing excludes this infection. Recommended Regimen
Alternative Regimens
* Quinolones should not be used for infections in MSM or in those with a history of recent foreign travel or partners' travel, infections acquired in California or Hawaii, or infections acquired in other areas with increased QRNG prevalence. All of the preceding regimens should be continued for 24--48 hours after improvement begins, at which time therapy may be switched to one of the following regimens to complete at least 1 week of antimicrobial therapy. Cefixime 400 mg orally twice daily
* Quinolones should not be used for infections in MSM or in those with a history of recent foreign travel or partners' travel, infections acquired in California or Hawaii, or infections acquired in other areas with increased QRNG prevalence. Management of Sex PartnersGonococcal infection frequently is asymptomatic in sex partners of patients who have DGI. As with uncomplicated gonococcal infections, patients should be instructed to refer their sex partners for evaluation and treatment (see Gonococcal Infection, Management of Sex Partners). Gonococcal Meningitis and Endocarditis Recommended Regimen
Therapy for meningitis should be continued for 10--14 days; therapy for endocarditis should be continued for at least 4 weeks. Treatment of complicated DGI should be undertaken in consultation with a specialist. Management of Sex PartnersPatients should be instructed to refer their sex partners for evaluation and treatment (see Gonococcal Infection, Management of Sex Partners). Gonococcal Infections Among Infants Gonococcal infection among infants usually results from exposure to infected cervical exudate at birth. It is usually an acute illness that manifests 2--5 days after birth. The prevalence of infection among infants depends on the prevalence of infection among pregnant women, whether pregnant women are screened for gonorrhea, and whether newborns receive ophthalmia prophylaxis. The most severe manifestations of N. gonorrhoeae infection in newborns are ophthalmia neonatorum and sepsis, which can include arthritis and meningitis. Less severe manifestations include rhinitis, vaginitis, urethritis, and reinfection at sites of fetal monitoring. Ophthalmia Neonatorum Caused by N. gonorrhoeae In the United States, although N. gonorrhoeae causes ophthalmia neonatorum less frequently than C. trachomatis and nonsexually transmitted agents, identifying and treating this infection is especially important because ophthalmia neonatorum can result in perforation of the globe of the eye and blindness. Diagnostic ConsiderationsInfants at increased risk for gonococcal ophthalmia are those who do not receive ophthalmia prophylaxis and those whose mothers have had no prenatal care or whose mothers have a history of STDs or substance abuse. Gonococcal ophthalmia is strongly suspected when intracellular gram-negative diplococci are identified in conjunctival exudate, justifying presumptive treatment for gonorrhea after appropriate cultures for N. gonorrhoeae are obtained. Appropriate chlamydial testing should be done simultaneously. Presumptive treatment for N. gonorrhoeae might be indicated for newborns who are at increased risk for gonococcal ophthalmia and who have conjunctivitis but do not have gonococci in a Gram-stained smear of conjunctival exudate. In all cases of neonatal conjunctivitis, conjunctival exudates should be cultured for N. gonorrhoeae and tested for antibiotic susceptibility before a definitive diagnosis is made. A definitive diagnosis is vital because of the public health and social consequences of a diagnosis of gonorrhea. Nongonococcal causes of neonatal ophthalmia include Moraxella catarrhalis and other Neisseria species that are indistinguishable from N. gonorrhoeae on Gram-stained smear but can be differentiated in the microbiology laboratory. Recommended Regimen
Topical antibiotic therapy alone is inadequate and is unnecessary if systemic treatment is administered. Other Management Considerations Simultaneous infection with C. trachomatis should be considered when a patient does not improve after treatment. Both mother and infant should be tested for chlamydial infection at the same time that gonorrhea testing is conducted (see Ophthalmia Neonatorum Caused by C. trachomatis). Ceftriaxone should be administered cautiously to hyperbilirubinemic infants, especially those born prematurely. Follow-UpInfants who have gonococcal ophthalmia should be hospitalized and evaluated for signs of disseminated infection (e.g., sepsis, arthritis, and meningitis). One dose of ceftriaxone is adequate therapy for gonococcal conjunctivitis. Management of Mothers and Their Sex PartnersThe mothers of infants who have gonococcal infection and the mothers' sex partners should be evaluated and treated according to the recommendations for treating gonococcal infections in adults (see Gonococcal Infections in Adolescents and Adults). DGI and Gonococcal Scalp Abscesses in Newborns Sepsis, arthritis, and meningitis (or any combination of these conditions) are rare complications of neonatal gonococcal infection. Localized gonococcal infection of the scalp can result from fetal monitoring through scalp electrodes. Detection of gonococcal infection in neonates who have sepsis, arthritis, meningitis, or scalp abscesses requires cultures of blood, CSF, and joint aspirate on chocolate agar. Specimens obtained from the conjunctiva, vagina, oropharynx, and rectum that are cultured on gonococcal selective medium are useful for identifying the primary site(s) of infection, especially if inflammation is present. Positive Gram-stained smears of exudate, CSF, or joint aspirate provide a presumptive basis for initiating treatment for N. gonorrhoeae. Diagnoses based on Gram-stained smears or presumptive identification of cultures should be confirmed with definitive tests on culture isolates. Recommended Regimens
Prophylactic Treatment for Infants Whose Mothers Have Gonococcal Infection Infants born to mothers who have untreated gonorrhea are at high risk for infection. Recommended Regimen in the Absence of Signs of Gonococcal Infection
Other Management Considerations Both mother and infant should be tested for chlamydial infection. Follow-UpFollow-up examination is not required. Management of Mothers and Their Sex Partners The mothers of infants who have gonococcal infection and the mothers' sex partners should be evaluated and treated according to the recommendations for treatment of gonococcal infections in adults (see Gonococcal Infections). Gonococcal Infections Among Children Sexual abuse is the most frequent cause of gonococcal infection in pre-adolescent children (see Sexual Assault or Abuse of Children). Vaginitis is the most common manifestation of gonococcal infection in preadolescent girls. PID after vaginal infection is probably less common in children than among adults. Among sexually abused children, anorectal and pharyngeal infections with N. gonorrhoeae are common and frequently asymptomatic. Diagnostic ConsiderationsBecause of the legal implications of a diagnosis of N. gonorrhoeae infection in a child, only standard culture procedures for the isolation of N. gonorrhoeae should be used for children. Nonculture gonococcal tests for gonococci (e.g., Gram-stained smear, nucleic acid hybridization tests, EIA, and NAAT) should not be used without standard culture; none of these tests have been approved by FDA for use with specimens obtained from the oropharynx, rectum, or genital tract of children. Specimens from the vagina, urethra, pharynx, or rectum should be streaked onto selective media for isolation of N. gonorrhoeae, and all presumptive isolates of N. gonorrhoeae should be identified definitively by at least two tests that involve different principles (e.g., biochemical, enzyme substrate, or serologic). Isolates should be preserved to enable additional or repeated testing. Recommended Regimens for Children Who Weigh >45 kg
Fluoroquinolones have not been recommended for persons aged <18 years because they have damaged articular cartilage in young animals. However, no such joint damage clearly attributable to quinolone therapy has been observed in children, even in those receiving multiple-dose regimens. Recommended Regimens for Children Who Weigh
<45 kg and Who Have
Uncomplicated Gonococcal Vulvovaginitis, Cervicitis, Urethritis, Pharyngitis, or Proctitis
Alternative Regimen
Recommended Regimen for Children Who Weigh
<45 kg and Who Have Bacteremia or Arthritis
Recommended Regimen for Children Who Weigh >45
kg and Who Have Bacteremia or Arthritis Follow-UpFollow-up cultures are unnecessary if ceftriaxone is used. If spectinomycin is used to treat pharyngitis, a follow-up culture is necessary to ensure that treatment was effective. Other Management ConsiderationsOnly parenteral cephalosporins are recommended for use in children. Ceftriaxone is approved for all gonococcal infections in children; cefotaxime is approved for gonococcal ophthalmia only. Oral cephalosporins used for treatment of gonococcal infections in children have not been adequately evaluated. All children who have gonococcal infections should be evaluated for coinfection with syphilis and C. trachomatis. (For a discussion of concerns regarding sexual assault, refer to Sexual Assault or Abuse of Children). Ophthalmia Neonatorum ProphylaxisTo prevent gonococcal ophthalmia neonatorum, a prophylactic agent should be instilled into the eyes of all newborn infants; this procedure is required by law in the majority of states. All of the recommended prophylactic regimens in this section prevent gonococcal ophthalmia. However, the efficacy of these preparations in preventing chlamydial ophthalmia is less clear, and they do not eliminate nasopharyngeal colonization by C. trachomatis. The diagnosis and treatment of gonococcal and chlamydial infections in pregnant women is the best method for preventing neonatal gonococcal and chlamydial disease. Not all women, however, receive prenatal care. Ocular prophylaxis is warranted because it can prevent sight-threatening gonococcal ophthalmia and because it is safe, easy to administer, and inexpensive. ProphylaxisRecommended Regimens
One of these recommended preparations should be instilled into both eyes of every neonate as soon as possible after delivery. If prophylaxis is delayed (i.e., not administered in the delivery room), a monitoring system should be established to ensure that all infants receive prophylaxis. All infants should be administered ocular prophylaxis, regardless of whether they are delivered vaginally or by cesarean section. Single-use tubes or ampules are preferable to multiple-use tubes. Bacitracin is not effective. Use of povidone iodine has not been studied adequately. Diseases Characterized by Vaginal DischargeManagement of Patients Who Have Vaginal InfectionsVaginitis is usually characterized by a vaginal discharge and/or vulvar itching and irritation, and a vaginal odor might be present. The three diseases most frequently associated with vaginal discharge are BV (replacement of the normal vaginal flora by an overgrowth of anaerobic microorganisms, mycoplasmas, and Gardnerella vaginalis), trichomoniasis (T. vaginalis), and candidiasis (usually caused by Candida albicans). Cervicitis can sometimes cause a vaginal discharge. Although vulvovaginal candidiasis (VVC) usually is not transmitted sexually, it is included in this section because it is frequently diagnosed in women being evaluated for STDs. Various diagnostic methods are available to identify the etiology of an abnormal vaginal discharge. Laboratory testing fails to identify the cause of vaginitis in a minority of women. The cause of vaginal symptoms usually can be determined by pH and microscopic examination of fresh samples of the discharge. The pH of the vaginal secretions can be determined by narrow-range pH paper; an elevated pH (i.e., >4.5) is common with BV or trichomoniasis but might not be highly specific. Discharge can be further examined by diluting one sample in one to two drops of 0.9% normal saline solution on one slide and a second sample in 10% potassium hydroxide (KOH) solution. An amine odor detected immediately after applying KOH suggests BV. Cover slips are placed on the slides, and they are examined under a microscope at low- and high-dry power. Motile T. vaginalis or clue cells (epithelial cells with borders obscured by small bacteria), which are characteristic of BV, usually are identified easily in the saline specimen. WBCs without evidence of trichomonads or yeast are usually suggestive of cervicitis (see Cervicitis). The yeast or pseudohyphae of Candida species are more easily identified in the KOH specimen. However, the absence of trichomonads or pseudohyphae does not rule out these infections because several studies have demonstrated the presence of these pathogens by culture or PCR after a negative microscopic examination. The presence of objective signs of vulvar inflammation in the absence of vaginal pathogens, along with a minimal amount of discharge, suggests the possibility of mechanical, chemical, allergic, or other noninfectious irritation of the vulva. Culture for T. vaginalis is more sensitive than microscopic examination. In settings where microscopy is not available, alternative point-of-care tests may be used to diagnose vaginitis. Bacterial VaginosisBV is a polymicrobial clinical syndrome resulting from replacement of the normal H2O2--producing Lactobacillus sp. in the vagina with high concentrations of anaerobic bacteria (e.g., Prevotella sp. and Mobiluncus sp.), G. vaginalis, and Mycoplasma hominis. BV is the most prevalent cause of vaginal discharge or malodor; however, more than 50% of women with BV are asymptomatic. The cause of the microbial alteration is not fully understood. BV is associated with having multiple sex partners, a new sex partner, douching, and lack of vaginal lactobacilli; whether BV results from acquisition of a sexually transmitted pathogen is unclear. Women who have never been sexually active are rarely affected. Treatment of male sex partners has not been beneficial in preventing the recurrence of BV. Diagnostic Considerations BV can be diagnosed by the use of clinical criteria or Gram stain. Clinical criteria require three of the following symptoms or signs:
When a Gram stain is used, determining the relative concentration of lactobacilli (long Gram-positive rods), Gram-negative and Gram-variable rods and cocci (i.e., G. vaginalis, Prevotella, Porphyromonas, and peptostreptococci), and curved Gram-negative rods (Mobiluncus) characteristic of BV is considered the gold standard laboratory method for diagnosing BV. Culture of G. vaginalis is not recommended as a diagnostic tool because it is not specific. However, a DNA probe-based test for high concentrations of G. vaginalis (AffirmTM VP III, Becton Dickinson, Sparks, Maryland) might have clinical utility. Cervical Pap tests have no clinical utility for the diagnosis of BV because of low sensitivity. Other commercially available tests that might be useful for the diagnosis of BV include a card test for the detection of elevated pH and trimethylamine (QuickVue Advance Quidel, San Diego, California) and prolineaminopeptidase (Pip Activity TestCardTM, Quidel, San Diego, California). Treatment The established benefits of therapy for BV in nonpregnant women are to 1) relieve vaginal symptoms and signs of infection and 2) reduce the risk for infectious complications after abortion or hysterectomy. Other potential benefits might include a reduction in risk for other infections (e.g., HIV and other STDs). All women who have symptomatic disease require treatment. BV during pregnancy is associated with adverse pregnancy outcomes, including premature rupture of the membranes, preterm labor, preterm birth, intraamniotic infection, and postpartum endometritis. The established benefit of therapy for BV in pregnant women is to relieve vaginal symptoms and signs of infection. Additional potential benefits of therapy include 1) reducing the risk for infectious complications associated with BV during pregnancy and 2) reducing the risk for other infections (e.g., other STDs or HIV). The results of several investigations indicate that treatment of pregnant women with BV who are at high risk for preterm delivery (i.e., those who previously delivered a premature infant) might reduce the risk for prematurity (145--147). Therefore, clinicians should consider evaluation and treatment of high-risk pregnant women with asymptomatic BV. The bacterial flora that characterizes BV have been recovered from the endometria and salpinges of women who have PID. BV has been associated with endometritis, PID, and vaginal cuff cellulitis after invasive procedures, including endometrial biopsy, hysterectomy, hysterosalpingography, placement of an IUD, cesarean section, and uterine curettage. The results of two randomized controlled trials have indicated that treatment of BV with metronidazole substantially reduced postabortion PID (148,149). Three trials that evaluated the use of anaerobic antimicrobial coverage (i.e., metronidazole) for routine operative prophylaxis before abortion and seven trials that evaluated this additional coverage for women undergoing hysterectomy demonstrated a substantial reduction in postoperative infectious complications (148--156). Because of the increased risk for postoperative infectious complications associated with BV, some specialists suggest that before performing surgical abortion or hysterectomy, providers should screen for and treat women with BV in addition to providing routine prophylaxis. However, more information is needed before recommending treatment of asymptomatic BV before other invasive procedures. Recommended Regimens
Patients should be advised to avoid consuming alcohol during treatment with metronidazole and for 24 hours thereafter. Clindamycin cream is oil-based and might weaken latex condoms and diaphragms for 5 days after use. Refer to clindamycin product labeling for additional information. Topical clindamycin preparations should not be used in the second half of pregnancy. The recommended metronidazole regimens are equally efficacious. One randomized trial evaluated the clinical equivalency of intravaginal metronidazole gel 0.75% once daily versus twice daily and demonstrated similar cure rates 1 month after therapy (157). Alternative Regimens
Metronidazole 2 g single-dose therapy has the lowest efficacy for BV and is no longer a recommended or alternative regimen. FDA has cleared metronidazole 750 mg extended release tablets once daily for 7 days and a single dose of clindamycin intravaginal cream. Limited data have been published that compares the clinical or microbiologic equivalencies of these regimens with other regimens. Cure rates do not differ between intravaginal clindamycin cream and ovules (158). Several studies have evaluated the clinical and microbiologic efficacy of using lactobacillus intravaginal suppositories to restore normal flora and treat BV. However, no currently available lactobacillus suppository was determined to be better than placebo 1 month after therapy for either clinical or microbiologic cure. No data support the use of douching for treatment or relief of symptoms. Follow-Up Follow-up visits are unnecessary if symptoms resolve. Because recurrence of BV is not unusual, women should be advised to return for additional therapy if symptoms recur. A treatment regimen different from the original regimen may be used to treat recurrent disease. However, women with multiple recurrences should be managed in consultation with a specialist. One randomized trial for persistent BV indicated that metronidazole gel 0.75% twice per week for 6 months after completion of a recommended regimen was effective in maintaining a clinical cure for 6 months (159). Management of Sex Partners The results of clinical trials indicate that a woman's response to therapy and the likelihood of relapse or recurrence are not affected by treatment of her sex partner(s). Therefore, routine treatment of sex partners is not recommended. Special Considerations Allergy or Intolerance to the Recommended TherapyIntravaginal clindamycin cream is preferred in case of allergy or intolerance to metronidazole. Intravaginal metronidazole gel can be considered for patients who do not tolerate systemic metronidazole, but patients allergic to oral metronidazole should not be administered intravaginal metronidazole. PregnancyAll pregnant women who have symptomatic disease require treatment. BV has been associated with adverse pregnancy outcomes (e.g., premature rupture of the membranes, chorioamnionitis, preterm labor, preterm birth, intraamniotic infection, postpartum endometritis, and postcesarean wound infection). Some specialists prefer using systemic therapy to treat possible subclinical upper genital tract infections. Treatment of BV in asymptomatic pregnant women at high risk for preterm delivery (i.e., those who have previously delivered a premature infant) with a recommended oral regimen has reduced preterm delivery in three of four randomized controlled trials (145,146,160,161); some specialists recommend screening and oral treatment of these women. However, the optimal treatment regimens have not been established. Screening (if conducted) and treatment should be performed during the first prenatal visit. Two trials that evaluated the efficacy of metronidazole during pregnancy used the 250-mg regimen (145,146). However, some specialists suggest using a regimen of 500 mg twice daily in pregnant women. One small trial demonstrated that treatment with oral metronidazole 500 mg twice daily was equally effective as metronidazole gel, with cure rates of 70% (162). These regimens were not effective in reducing preterm birth in any group of women. Multiple studies and meta-analyses have not demonstrated an association between metronidazole use during pregnancy and teratogenic or mutagenic effects in newborns (164--166). Recommended Regimens for Pregnant Women
Whether treatment of asymptomatic pregnant women with BV who are at low risk for preterm delivery reduces adverse outcomes of pregnancy is unclear. One trial in which oral clindamycin was used demonstrated a reduction in spontaneous preterm birth (147). Several trials have evaluated the use of intravaginal clindamycin during pregnancy to reduce preterm birth and treat asymptomatic BV. One trial in which women were treated before 20 weeks' gestation demonstrated a reduction in preterm birth (166). In three other trials, intravaginal clindamycin cream was administered at 16--32 weeks' gestation, and an increase in adverse events (e.g., low birthweight and neonatal infections) was observed in newborns (167--169). Therefore, intravaginal clindamycin cream should only be used during the first half of pregnancy. Follow-Up of Pregnant WomenTreatment of BV in asymptomatic pregnant women who are at high risk for preterm delivery might prevent adverse pregnancy outcomes. Therefore, a follow-up evaluation 1 month after completion of treatment should be considered to evaluate whether therapy was effective. HIV InfectionPatients who have BV and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. BV appears to be more persistent in HIV-positive women. TrichomoniasisTrichomoniasis is caused by the protozoan T. vaginalis. Some men who are infected with T. vaginalis might not have symptoms; others have NGU. Many infected women have symptoms characterized by a diffuse, malodorous, yellow-green vaginal discharge with vulvar irritation. However, some women have minimal or no symptoms. Diagnosis of vaginal trichomoniasis is usually performed by microscopy of vaginal secretions, but this method has a sensitivity of only approximately 60%--70% and requires immediate evaluation of wet preparation slide for optimal results. Other FDA-cleared tests for trichomoniasis in women include OSOM Trichomonas Rapid Test (Genzyme Diagnostics, Cambridge, Massachusetts), an immunochromatographic capillary flow dipstick technology, and the Affirm™ VP III (Becton Dickenson, San Jose, California), a nucleic acid probe test that evaluates for T. vaginalis, G. vaginalis, and C. albicans. These tests are both performed on vaginal secretions and have a sensitivity >83% and a specificity >97%. Both tests are point-of-care diagnostics. The results of the OSOM Trichomonas Rapid Test are available in approximately 10 minutes, and results of the Affirm™ VP III are available within 45 minutes. Although these tests tend to be more sensitive than vaginal wet preparation, false positives might occur especially in low prevalence populations. Culture is the most sensitive and specific commercially available method of diagnosis. In women in whom trichomoniasis is suspected but not confirmed by microscopy, vaginal secretions should be cultured for T. vaginalis. In men, wet preparation is insensitive, and culture testing of urethral swab, urine, and semen is required for optimal sensitivity. No FDA-cleared PCR test for T. vaginalis is available in the United States, but such testing might be available from commercial laboratories that have developed their own PCR tests. Recommended Regimens
Alternative Regimen
Patients should be advised to avoid consuming alcohol during treatment with metronidazole or tinidazole. Abstinence from alcohol use should continue for 24 hours after completion of metronidazole or 72 hours after completion of tinidazole. The nitroimidazoles comprise the only class of drugs useful for the oral or parenteral therapy of trichomoniasis. Of these drugs, metronidazole and tinidazole are available in the United States and are cleared by the FDA for the treatment of trichomoniasis. In randomized clinical trials, the recommended metronidazole regimens have resulted in cure rates of approximately 90%--95%, and the recommended tinidazole regimen has resulted in cure rates of approximately 86%--100%. The appropriate treatment of sex partners might increase these reported rates. Randomized controlled trials comparing single 2 g doses of metronidazole and tinidazole suggest that tinidazole is equivalent to, or superior to, metronidazole in achieving parasitologic cure and resolution of symptoms (170). Treatment of patients and sex partners results in relief of symptoms, microbiologic cure, and reduction of transmission. Metronidazole gel is considerably less efficacious for the treatment of trichomoniasis (<50%) than oral preparations of metronidazole. Topically applied antimicrobials (e.g., metronidazole gel) are unlikely to achieve therapeutic levels in the urethra or perivaginal glands; therefore, use of the gel is not recommended. Several other topically applied antimicrobials occasionally have been used for treatment of trichomoniasis; however, these preparations probably do not have greater efficacy than metronidazole gel. Follow-Up Follow-up is unnecessary for men and women who become asymptomatic after treatment or who are initially asymptomatic. Some strains of T. vaginalis can have diminished susceptibility to metronidazole; however, infections caused by the majority of these organisms respond to tinidazole or higher doses of metronidazole. Low-level metronidazole resistance has been identified in 2%--5% of cases of vaginal trichomoniasis. High-level resistance is rare. Tinidazole has a longer serum half-life and reaches higher levels in genitourinary tissues than metronidazole. In addition, many T. vaginalis isolates have lower minimum inhibitory concentrations (MICs) to tinidazole than metronidazole. If treatment failure occurs with metronidazole 2 g single dose and reinfection is excluded, the patient can be treated with metronidazole 500 mg orally twice daily for 7 days or tinidazole 2 g single dose. For patients failing either of these regimens, clinicians should consider treatment with tinidazole or metronidazole at 2 g orally for 5 days. If these therapies are not effective, further management should be discussed with a specialist. The consultation should ideally include determination of the susceptibility of T. vaginalis to metronidazole and tinidazole. Consultation and T. vaginalis susceptibility testing is available from CDC (telephone: 770-488-4115; website: http://www.cdc.gov/std). Management of Sex Partners Sex partners of patients with T. vaginalis should be treated. Patients should be instructed to avoid sex until they and their sex partners are cured (i.e., when therapy has been completed and patient and partner(s) are asymptomatic). Special Considerations Allergy, Intolerance, and Adverse ReactionsMetronidazole and tinidazole are both nitroimidazoles. Patients with an immediate-type allergy to a nitroimidazole can be managed by metronidazole desensitization in consultation with a specialist (171,172). Topical therapy with drugs other than nitroimidazoles can be attempted, but cure rates are low (<50%). PregnancyVaginal trichomoniasis has been associated with adverse pregnancy outcomes, particularly premature rupture of membranes, preterm delivery, and low birthweight. However, data do not suggest that metronidazole treatment results in a reduction in perinatal morbidity. Although some trials suggest the possibility of increased prematurity or low birthweight after metronidazole treatment, limitations of the studies prevent definitive conclusions regarding risks of treatment (173,174). Treatment of T. vaginalis might relieve symptoms of vaginal discharge in pregnant women and might prevent respiratory or genital infection of the newborn and further sexual transmission. Clinicians should counsel patients regarding the potential risks and benefits of treatment. Some specialists would defer therapy in asymptomatic pregnant women until after 37 weeks' gestation. In addition, these pregnant women should be provided careful counseling regarding condom use and the continued risk of sexual transmission. Women may be treated with 2 g of metronidazole in a single dose. Metronidazole is pregnancy category B (animal studies have revealed no evidence of harm to the fetus, but no adequate, well-controlled studies among pregnant women have been conducted). Multiple studies and meta-analyses have not demonstrated a consistent association between metronidazole use during pregnancy and teratogenic or mutagenic effects in infants (163--165). Tinidazole is pregnancy category C (animal studies have demonstrated an adverse event, and no adequate, well-controlled studies in pregnant women have been conducted), and its safety in pregnant women has not been well-evaluated. In lactating women who are administered metronidazole, withholding breastfeeding during treatment and for 12--24 hours after the last dose will reduce the exposure of metronidazole to the infant. While using tinidazole, interruption of breastfeeding is recommended during treatment and for 3 days after the last dose. HIV InfectionPatients who have trichomoniasis and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. The incidence, persistence, and recurrence of trichomoniasis in HIV-infected women are not correlated with immune status. Vulvovaginal CandidiasisVVC usually is caused by C. albicans but occasionally is caused by other Candida sp. or yeasts. Typical symptoms of VVC include pruritus, vaginal soreness, dyspareunia, external dysuria, and abnormal vaginal discharge. None of these symptoms is specific for VVC. An estimated 75% of women will have at least one episode of VVC, and 40%--45% will have two or more episodes. On the basis of clinical presentation, microbiology, host factors, and response to therapy, VVC can be classified as either uncomplicated or complicated (Box 2). Approximately 10%--20% of women will have complicated VVC, suggesting diagnostic and therapeutic considerations. Uncomplicated VVC Diagnostic Considerations in Uncomplicated VVCA diagnosis of Candida vaginitis is suggested clinically by the presence of external dysuria and vulvar pruritus, pain, swelling, and redness. Signs include vulvar edema, fissures, excoriations, or thick curdy vaginal discharge. The diagnosis can be made in a woman who has signs and symptoms of vaginitis when either 1) a wet preparation (saline, 10% KOH) or Gram stain of vaginal discharge demonstrates yeasts or pseudohyphae or 2) a culture or other test yields a positive result for a yeast species. Candida vaginitis is associated with a normal vaginal pH (<4.5). Use of 10% KOH in wet preparations improves the visualization of yeast and mycelia by disrupting cellular material that might obscure the yeast or pseudohyphae. Examination of a wet mount with KOH preparation should be performed for all women with symptoms or signs of VVC, and women with a positive result should receive treatment. For those with negative wet mounts, vaginal cultures for Candida should be considered for those with any sign or multiple symptoms. If Candida cultures cannot be done, empiric treatment can be considered for symptomatic women with any sign of VVC on examination when the wet mount is negative. Identifying Candida by culture in the absence of symptoms or signs is not an indication for treatment because approximately 10%--20% of women harbor Candida sp. and other yeasts in the vagina. VVC can occur concomitantly with STDs. The majority of healthy women with uncomplicated VVC have no identifiable precipitating factors. TreatmentShort-course topical formulations (i.e., single dose and regimens of 1--3 days) effectively treat uncomplicated VVC. The topically applied azole drugs are more effective than nystatin. Treatment with azoles results in relief of symptoms and negative cultures in 80%--90% of patients who complete therapy. Recommended Regimens Intravaginal Agents:
Oral Agent:
* Over-the-counter preparations. The creams and suppositories in this regimen are oil-based and might weaken latex condoms and diaphragms. Refer to condom product labeling for further information. Intravaginal preparations of butaconazole, clotrimazole, miconazole, and tioconazole are available over-the-counter (OTC). Women whose condition has previously been diagnosed with VVC are not necessarily more likely to be able to diagnose themselves; therefore, any woman whose symptoms persist after using an OTC preparation, or who has a recurrence of symptoms within 2 months, should be evaluated with office-based testing. Unnecessary or inappropriate use of OTC preparations is common and can lead to a delay in the treatment of other vulvovaginitis etiologies, which can result in adverse clinical outcomes. Follow-UpPatients should be instructed to return for follow-up visits only if symptoms persist or recur within 2 months of onset of initial symptoms. Management of Sex PartnersVVC is not usually acquired through sexual intercourse; treatment of sex partners is not recommended but may be considered in women who have recurrent infection. A minority of male sex partners might have balanitis, which is characterized by erythematous areas on the glans of the penis in conjunction with pruritus or irritation. These men benefit from treatment with topical antifungal agents to relieve symptoms. Special ConsiderationsAllergy, Intolerance, and Adverse ReactionsTopical agents usually cause no systemic side effects, although local burning or irritation might occur. Oral agents occasionally cause nausea, abdominal pain, and headache. Therapy with the oral azoles has been associated rarely with abnormal elevations of liver enzymes. Clinically important interactions can occur when these oral agents are administered with other drugs, including astemizole, calcium channel antagonists, cisapride, coumadin, cyclosporin A, oral hypoglycemic agents, phenytoin, protease inhibitors, tacrolimus, terfenadine, theophylline, trimetrexate, and rifampin. Complicated VVC Recurrent Vulvovaginal Candidiasis (RVVC)RVVC, usually defined as four or more episodes of symptomatic VVC in 1 year, affects a small percentage of women (<5%). The pathogenesis of RVVC is poorly understood, and the majority of women with RVVC have no apparent predisposing or underlying conditions. Vaginal cultures should be obtained from patients with RVVC to confirm the clinical diagnosis and to identify unusual species, including nonalbicans species, particularly Candida glabrata (C. glabrata does not form pseudohyphae or hyphae and is not easily recognized on microscopy). C. glabrata and other nonalbicans Candidia species are observed in 10%--20% of patients with RVVC. Conventional antimycotic therapies are not as effective against these species as against C. albicans. TreatmentEach individual episode of RVVC caused by C. albicans responds well to short duration oral or topical azole therapy. However, to maintain clinical and mycologic control, some specialists recommend a longer duration of initial therapy (e.g., 7--14 days of topical therapy or a 100 mg, 150 mg, or 200 mg oral dose of fluconazole every third day for a total of 3 doses (day 1, 4, and 7) to attempt mycologic remission before initiating a maintenance antifungal regimen. Maintenance RegimensOral fluconazole (i.e., 100-mg, 150-mg, or 200-mg dose) weekly for 6 months is the first line of treatment. If this regimen is not feasible, some specialists recommend topical clotrimazole 200 mg twice a week, clotrimazole (500-mg dose vaginal suppositories once weekly), or other topical treatments used intermittently. Suppressive maintenance antifungal therapies are effective in reducing RVVC. However, 30%--50% of women will have recurrent disease after maintenance therapy is discontinued. Routine treatment of sex partners is controversial. C. albicans azole resistance is rare in vaginal isolates, and susceptibility testing is usually not warranted for individual treatment guidance. Severe VVC Severe vulvovaginitis (i.e., extensive vulvar erythema, edema, excoriation, and fissure formation) is associated with lower clinical response rates in patients treated with short courses of topical or oral therapy. Either 7--14 days of topical azole or 150 mg of fluconazole in two sequential doses (second dose 72 hours after initial dose) is recommended. Nonalbicans VVC The optimal treatment of nonalbicans VVC remains unknown. Options include longer duration of therapy (7--14 days) with a nonfluconazole azole drug (oral or topical) as first-line therapy. If recurrence occurs, 600 mg of boric acid in a gelatin capsule is recommended, administered vaginally once daily for 2 weeks. This regimen has clinical and mycologic eradication rates of approximately 70% (175). If symptoms recur, referral to a specialist is advised. Compromised Host Women with underlying debilitating medical conditions (e.g., those with uncontrolled diabetes or those receiving corticosteroid treatment) do not respond as well to short-term therapies. Efforts to correct modifiable conditions should be made, and more prolonged (i.e., 7--14 days) conventional antimycotic treatment is necessary. Pregnancy VVC frequently occurs during pregnancy. Only topical azole therapies, applied for 7 days, are recommended for use among pregnant women. HIV Infection The incidence of VVC in HIV-infected women is unknown. Vaginal Candida colonization rates among HIV-infected women are higher than among those for seronegative women with similar demographic characteristics and high-risk behaviors, and the colonization rates correlate with increasing severity of immunosuppression. Symptomatic VVC is more frequent in seropositive women and similarly correlates with severity of immunodeficiency. In addition, among HIV-infected women, systemic azole exposure is associated with the isolation of nonalbicans Candida species from the vagina. Based on available data, therapy for VVC in HIV-infected women should not differ from that for seronegative women. Although long-term prophylactic therapy with fluconazole at a dose of 200 mg weekly has been effective in reducing C. albicans colonization and symptomatic VVC (176), this regimen is not recommended for routine primary prophylaxis in HIV-infected women in the absence of recurrent VVC (50). Given the frequency at which RVVC occurs in the immmunocompetent healthy population, the occurrence of RVVC should not be considered an indication for HIV testing. Pelvic Inflammatory DiseasePID comprises a spectrum of inflammatory disorders of the upper female genital tract, including any combination of endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis. Sexually transmitted organisms, especially N. gonorrhoeae and C. trachomatis, are implicated in many cases; however, microorganisms that comprise the vaginal flora (e.g., anaerobes, G. vaginalis, Haemophilus influenzae, enteric Gram-negative rods, and Streptococcus agalactiae) also have been associated with PID. In addition, cytomegalovirus (CMV), M. hominis, U. urealyticum, and M. genitalium might be associated with some cases of PID. All women who are diagnosed with acute PID should be tested for N. gonorrhoeae and C. trachomatis and should be screened for HIV infection. Diagnostic ConsiderationsAcute PID is difficult to diagnose because of the wide variation in the symptoms and signs. Many women with PID have subtle or mild symptoms. Delay in diagnosis and treatment probably contributes to inflammatory sequelae in the upper reproductive tract. Laparoscopy can be used to obtain a more accurate diagnosis of salpingitis and a more complete bacteriologic diagnosis. However, this diagnostic tool frequently is not readily available, and its use is not easy to justify when symptoms are mild or vague. Moreover, laparoscopy will not detect endometritis and might not detect subtle inflammation of the fallopian tubes. Consequently, a diagnosis of PID usually is based on clinical findings. The clinical diagnosis of acute PID is imprecise (177,178). Data indicate that a clinical diagnosis of symptomatic PID has a positive predictive value (PPV) for salpingitis of 65%--90% compared with laparoscopy. The PPV of a clinical diagnosis of acute PID depends on the epidemiologic characteristics of the population, with higher PPVs among sexually active young women (particularly adolescents), among patients attending STD clinics, or in other settings where the rates of gonorrhea or chlamydia are high. In all settings, however, no single historical, physical, or laboratory finding is both sensitive and specific for the diagnosis of acute PID (i.e., can be used both to detect all cases of PID and to exclude all women without PID). Combinations of diagnostic findings that improve either sensitivity (i.e., detect more women who have PID) or specificity (i.e., exclude more women who do not have PID) do so only at the expense of the other. For example, requiring two or more findings excludes more women who do not have PID but also reduces the number of women with PID who are identified. Many episodes of PID go unrecognized. Although some cases are asymptomatic, others are not diagnosed because the patient or the health-care provider fails to recognize the implications of mild or nonspecific symptoms or signs (e.g., abnormal bleeding, dyspareunia, and vaginal discharge). Because of the difficulty of diagnosis and the potential for damage to the reproductive health of women, even by apparently mild or subclinical PID, health-care providers should maintain a low threshold for the diagnosis of PID. The optimal treatment regimen and long-term outcome of early treatment of women with asymptomatic or subclinical PID are unknown. The following recommendations for diagnosing PID are intended to help health-care providers recognize when PID should be suspected and when they need to obtain additional information to increase diagnostic certainty. Diagnosis and management of other common causes of lower abdominal pain (e.g., ectopic pregnancy, acute appendicitis, and functional pain) are unlikely to be impaired by initiating empiric antimicrobial therapy for PID. Empiric treatment of PID should be initiated in sexually active young women and other women at risk for STDs if they are experiencing pelvic or lower abdominal pain, if no cause for the illness other than PID can be identified, and if one or more of the following minimum criteria are present on pelvic examination:
The requirement that all three minimum criteria be present before the initiation of empiric treatment could result in insufficient sensitivity for the diagnosis of PID. The presence of signs of lower genital tract inflammation, in addition to one of the three minimum criteria, increases the specificity of diagnosis. In deciding upon the initiation of empiric treatment, clinicians should also consider the risk profile of the patient for STDs. More elaborate diagnostic evaluation frequently is needed because incorrect diagnosis and management might cause unnecessary morbidity. These additional criteria may be used to enhance the specificity of the minimum criteria. The following additional criteria can be used to enhance the specificity of the minimum criteria and support a diagnosis of PID:
The majority of women with PID have either mucopurulent cervical discharge or evidence of WBC on a microscopic evaluation of a saline preparation of vaginal fluid. If the cervical discharge appears normal and no WBCs are observed on the wet prep of vaginal fluid, the diagnosis of PID is unlikely, and alternative causes of pain should be investigated. A wet prep of vaginal fluid offers the ability to detect the presence of concomitant infections (e.g., bacterial vaginosis and trichomoniasis). The most specific criteria for diagnosing PID include the following:
A diagnostic evaluation that includes some of these more extensive studies might be warranted in some cases. Endometrial biopsy is warranted in women undergoing laparoscopy who do not have visual evidence of salpingitis, as some women with PID have endometritis alone. TreatmentPID treatment regimens must provide empiric, broad spectrum coverage of likely pathogens. Several antimicrobial regimens have been effective in achieving clinical and microbiologic cure in randomized clinical trials with short-term follow-up. However, only a limited number of investigations have assessed and compared these regimens with regard to elimination of infection in the endometrium and fallopian tubes or determined the incidence of long-term complications (e.g., tubal infertility and ectopic pregnancy) after antimicrobial regimens (179,180). All treatment regimens should be effective against N. gonorrhoeae and C. trachomatis because negative endocervical screening for these organisms does not rule out upper reproductive tract infection. The need to eradicate anaerobes from women who have PID has not been determined definitively. Anaerobic bacteria have been isolated from the upper reproductive tract of women who have PID, and data from in vitro studies have revealed that some anaerobes (e.g., Bacteroides fragilis) can cause tubal and epithelial destruction. In addition, BV also is present in many women who have PID (181).Until treatment regimens that do not adequately cover these microbes have been demonstrated to prevent long-term sequelae (e.g., infertility and ectopic pregnancy) as successfully as the regimens that are effective against these microbes, the use of regimens with anaerobic activity should be considered. Treatment should be initiated as soon as the presumptive diagnosis has been made because prevention of long-term sequelae is dependent on immediate administration of appropriate antibiotics. When selecting a treatment regimen, health-care providers should consider availability, cost, patient acceptance, and antimicrobial susceptibility. Some specialists have recommended that all patients with PID be hospitalized so that bed rest and supervised treatment with parenteral antibiotics can be initiated. However, in women with PID of mild or moderate clinical severity, outpatient therapy can provide short- and long-term clinical outcomes similar to inpatient therapy. Limited data support the use of outpatient therapy in women with more severe clinical presentations. The decision of whether hospitalization is necessary should be based on the discretion of the health-care provider. The following criteria for hospitalization are suggested:
Many practitioners have preferred to hospitalize adolescent women whose condition is diagnosed as acute PID. No evidence is available suggesting that adolescents benefit from hospitalization for treatment of PID. Younger women with mild-to-moderate acute PID have similar outcomes with either outpatient therapy or inpatient therapy. Further, clinical response to outpatient treatment is similar among younger and older women. The decision to hospitalize adolescents with acute PID should be based on the same criteria used for older women. Whether women in their later reproductive years benefit from hospitalization for treatment of PID also is unclear, although women aged >35 years who are hospitalized with PID are more likely than younger women to have a complicated clinical course. Parenteral Treatment For women with PID of mild or moderate severity, parenteral and oral therapy appears to have similar clinical efficacy. Many randomized trials have demonstrated the efficacy of both parenteral and oral regimens (180,182,183). In the majority of clinical trials, parenteral treatment for at least 48 hours has been used after the patient has demonstrated substantial clinical improvement. Clinical experience should guide decisions regarding transition to oral therapy, which usually can be initiated within 24 hours of clinical improvement. The majority of clinicians recommend at least 24 hours of direct inpatient observation for patients who have tubo-ovarian abscesses. Recommended Parenteral Regimen A
Because of the pain associated with infusion, doxycycline should be administered orally when possible, even when the patient is hospitalized. Oral and IV administration of doxycycline provide similar bioavailability. Parenteral therapy may be discontinued 24 hours after a patient improves clinically, and oral therapy with doxycycline (100 mg twice a day) should continue to complete 14 days of therapy. When tubo-ovarian abscess is present, many health-care providers use clindamycin or metronidazole with doxycycline for continued therapy, rather than doxycycline alone, because it provides more effective anaerobic coverage. Clinical data are limited regarding the use of other second- or third-generation cephalosporins (e.g., ceftizoxime, cefotaxime, and ceftriaxone), which also might be effective therapy for PID and may replace cefotetan or cefoxitin. However, these cephalosporins are less active than cefotetan or cefoxitin against anaerobic bacteria. Recommmended Parenteral Regimen B
Although use of a single daily dose of gentamicin has not been evaluated for the treatment of PID, it is efficacious in analogous situations. Parenteral therapy can be discontinued 24 hours after a patient improves clinically; continuing oral therapy should consist of doxycycline 100 mg orally twice a day or clindamycin 450 mg orally four times a day to complete a total of 14 days of therapy. When tubo-ovarian abscess is present, many health-care providers use clindamycin for continued therapy, rather than doxycycline, because clindamycin provides more effective anaerobic coverage. Alternative Parenteral RegimensLimited data support the use of other parenteral regimens, but the following three regimens have been investigated in at least one clinical trial, and they have broad spectrum coverage.
Levofloxacin 500 mg IV once daily*
* Quinolones should not be used in persons with a history of recent foreign travel or partners' travel, infections acquired in California or Hawaii, or infections acquired in other areas with increased QRNG prevalence. IV ofloxacin has been investigated as a single agent; however, because of concerns regarding its spectum, metronidazole may be included in the regimen. Levofloxacin is as effective as ofloxacin and may be substituted; its single daily dosing makes it advantageous from a compliance perspective. One trial demonstrated high short-term clinical cure rates with azithromycin, either alone for 1 week (at least one IV dose followed by oral therapy) or with a 12-day course of metronidazole (184). Ampicillin/sulbactam plus doxycycline is effective coverage against C. trachomatis, N. gonorrhoeae, and anaerobes and for patients who have tubo-ovarian abscess. Oral Treatment Oral therapy can be considered for women with mild-to-moderately severe acute PID, as the clinical outcomes among women treated with oral therapy are similar to those treated with parenteral therapy. The following regimens provide coverage against the frequent etiologic agents of PID. Patients who do not respond to oral therapy within 72 hours should be reevaluated to confirm the diagnosis and should be administered parenteral therapy on either an outpatient or inpatient basis. Recommended Regimen A
* Quinolones should not be used in persons with a history of recent foreign travel or partners' travel, infections acquired in California or Hawaii, or infections acquired in other areas with increased QRNG prevalence. Oral ofloxacin has been investigated as a single agent in two clinical trials, and it is effective against both N. gonorrhoeae and C. trachomatis (185,186). Despite the results of these trials, lack of anaerobic coverage with ofloxacin is a concern; the addition of metronidazole to the treatment regimen provides this coverage. Levofloxacin is as effective as ofloxacin and may be substituted. Azithromycin has been demonstrated in one randomized trial to be an effective regimen for acute PID (184). The addition of metronidazole should be considered, as anaerobic organisms are suspected in the etiology of the majority of PID cases. Metronidazole will also treat BV, which frequently is associated with PID. Regimen B The optimal choice of a cephalosporin for Regimen B is unclear; although cefoxitin has better anaerobic coverage, ceftriaxone has better coverage against N. gonorrhoeae. Clinical trials have demonstrated that a single dose of cefoxitin is effective in obtaining short-term clinical response in women who have PID. However, the theoretical limitations in cefoxitin's coverage of anaerobes might require the addition of metronidazole to the treatment regimen (182). Metronidazole also will effectively treat BV, which is frequently associated with PID. No data have been published regarding the use of oral cephalosporins for the treatment of PID. Limited data suggest that the combination of oral metronidazole and doxycycline after primary parenteral therapy is safe and effective (187). Alternative Oral RegimensAlthough information regarding other outpatient regimens is limited, one other regimen has undergone at least one clinical trial and has broad spectrum coverage. Amoxicillin/clavulanic acid and doxycycline was effective in obtaining short-term clinical response in a single clinical trial; however, gastrointestinal symptoms might limit compliance with this regimen. Follow-Up Patients should demonstrate substantial clinical improvement (e.g., defervescence; reduction in direct or rebound abdominal tenderness; and reduction in uterine, adnexal, and cervical motion tenderness) within 3 days after initiation of therapy. Patients who do not improve within this period usually require hospitalization, additional diagnostic tests, and surgical intervention. If no clinical improvement has occurred within 72 hours after outpatient oral or parenteral therapy (using the criteria for clinical improvement described previously), an examination should be performed. Subsequent hospitalization, parenteral therapy, and diagnostic evaluation, including the consideration of diagnostic laparoscopy for alternative diagnoses, are recommended in women without clinical improvement. Some specialists also recommend rescreening for C. trachomatis and N. gonorrhoeae 4--6 weeks after therapy is completed in women with documented infection with these pathogens. All women diagnosed with acute PID should be offered HIV testing. Management of Sex Partners Male sex partners of women with PID should be examined and treated if they had sexual contact with the patient during the 60 days preceding the patient's onset of symptoms. Evaluation and treatment are imperative because of the risk for reinfection of the patient and the strong likelihood of urethral gonococcal or chlamydial infection in the sex partner. Male partners of women who have PID caused by C. trachomatis and/or N. gonorrhoeae frequently are asymptomatic. Sex partners should be treated empirically with regimens effective against both of these infections, regardless of the etiology of PID or pathogens isolated from the infected woman. Even in clinical settings in which only women are treated, arrangements should be made to provide care for male sex partners of women who have PID. When providing care for male sex partners is not feasible, health-care providers should ensure that sex partners are referred for appropriate treatment. Prevention Prevention of chlamydial infection by screening and treating high-risk women reduces the incidence of PID (125). Theoretically, the majority of cases of PID can be prevented by screening all women or those determined to be at high risk (based on age or other factors) by using DNA amplification on cervical specimens (in women receiving pelvic examinations) and on urine specimens (in women not undergoing examinations). Although BV is associated with PID, whether the incidence of PID can be reduced by identifying and treating women with BV is unclear (181). Special Considerations Pregnancy. Because of the high risk for maternal morbidity and preterm delivery, pregnant women who have suspected PID should be hospitalized and treated with parenteral antibiotics. HIV Infection. Differences in the clinical manifestations of PID between HIV-infected women and HIV-negative women have not been well-delineated. In previous observational studies, HIV-infected women with PID were more likely to require surgical intervention. More comprehensive observational and controlled studies (published since the 2002 STD Treatment Guidelines) have demonstrated that HIV-infected women with PID had similar symptoms when compared with uninfected controls (125,188--190). They were more likely to have a tubo-ovarian abscess but responded equally well to standard parenteral and oral antibiotic regimens when compared with HIV-negative women. The microbiologic findings for HIV- positive and HIV-negative women were similar, except HIV-infected women had higher rates of concomitant M. hominis, candida, streptococcal, and HPV infections and HPV-related cytologic abnormalities. Whether the management of immunodeficient HIV-infected women with PID requires more aggressive interventions (e.g., hospitalization or parenteral antimicrobial regimens) has not been determined. IUD. Intrauterine contraceptive devices are becoming a popular contraceptive choice for women. Both levonorgestrel- and copper-containing devices are marketed in the United States. The risk of PID associated with IUD use is primarily confined to the first 3 weeks after insertion and is uncommon thereafter (191). Given the popularity of IUDs, practitioners might encounter PID in IUD users. No evidence suggests that IUDs should be removed in women diagnosed with acute PID. However, caution should be exercised if the IUD remains in place, and close clinical follow-up is mandatory. The rate of treatment failure and recurrent PID in women continuing to use an IUD is unknown. No data exist on antibiotic selection and treatment outcomes according to type of IUD (e.g., copper or levonorgestrel). EpididymitisAcute epididymitis is a clinical syndrome consisting of pain, swelling, and inflammation of the epididymis of <6 weeks. Chronic epididymitis is characterized by a 3-month or longer history of symptoms of discomfort and/or pain in the scrotum, testicle, or epididymis that is localized on clinical examination. Chronic epididymitis has been subcategorized into inflammatory chronic epididymitis, obstructive chronic epididymitis, and chronic epididymalgia (192). Among sexually active men aged <35 years, acute epididymitis is most frequently caused by C. trachomatis or N. gonorrhoeae. Acute epididymitis caused by sexually transmitted enteric organisms (e.g., Escherichia coli) also occurs among men who are the insertive partner during anal intercourse. Sexually transmitted acute epididymitis usually is accompanied by urethritis, which frequently is asymptomatic and is usually never accompanied by bacteriuria. In men aged >35 years, sexually transmitted epididymitis is uncommon. However, bacteriuria secondary to obstructive urinary disease is relatively common. In this group, nonsexually transmitted epididymitis is associated with urinary-tract instrumentation or surgery, systemic disease, or immunosuppression. Although the majority of patients can be treated on an outpatient basis, hospitalization should be considered when severe pain suggests other diagnoses (e.g., torsion, testicular infarction, or abscess) or when patients are febrile or might be noncompliant with an antimicrobial regimen. Diagnostic ConsiderationsMen who have acute epididymitis typically have unilateral testicular pain and tenderness; hydrocele and palpable swelling of the epididymis usually are present. Although the inflammation and swelling usually begin in the tail of the epididymis, they can spread to involve the rest of the epididymis and testicle. The spermatic cord is usually tender and swollen.Testicular torsion, a surgical emergency, should be considered in all cases, but it occurs more frequently among adolescents and in men without evidence of inflammation or infection. Emergency testing for torsion might be indicated when the onset of pain is sudden, pain is severe, or the test results available during the initial examination do not support a diagnosis of urethritis or urinary-tract infection. If the diagnosis is questionable, a specialist should be consulted immediately because testicular viability might be compromised. Radionuclide scanning of the scrotum is the most accurate radiologic method of diagnosis, although it is not routinely available. Color duplex doppler ultrasonography has a sensitivity of 70% and a specificity of 88% in diagnosing acute epididymitis. The evaluation of men for epididymitis should include one of the following:
Culture, nucleic acid hybridization tests, and nucleic acid amplification tests are available for the detection of both N. gonorrhoeae and C. trachomatis. Culture and nucleic acid hybridization tests require urethral swab specimens, whereas amplification tests can be performed on urine specimens. Because of their higher sensitivity, amplification tests are preferred for the detection of C. trachomatis. Depending on the risk, patients whose conditions have been diagnosed as a new STD should receive testing for other STDs. Treatment Empiric therapy is indicated before laboratory test results are available. The goals of treatment of acute epididymitis caused by C. trachomatis or N. gonorrhoeae are 1) microbiologic cure of infection, 2) improvement of signs and symptoms, 3) prevention of transmission to others, and 4) a decrease in potential complications (e.g., infertility or chronic pain). As an adjunct to therapy, bed rest, scrotal elevation, and analgesics are recommended until fever and local inflammation have subsided. Recommended Regimens For acute epididymitis most likely caused by gonococcal or chlamydial infection:
For acute epididymitis most likely caused by enteric organisms or for patients allergic to cephalosporins
and/or tetracyclines: Follow-Up Failure to improve within 3 days of the initiation of treatment requires reevaluation of both the diagnosis and therapy. Swelling and tenderness that persist after completion of antimicrobial therapy should be evaluated comprehensively. The differential diagnosis includes tumor, abscess, infarction, testicular cancer, TB, and fungal epididymitis. Management of Sex Partners Patients who have acute epididymitis, confirmed or suspected to be caused by N. gonorrhoeae or C. trachomatis, should be instructed to refer sex partners for evaluation and treatment if their contact with the index patient was within the 60 days preceding onset of the patient's symptoms. Patients should be instructed to avoid sexual intercourse until they and their sex partners are cured (i.e., until therapy is completed and patient and partners no longer have symptoms). Special Considerations HIV InfectionPatients who have uncomplicated acute epididymitis and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. Fungi and mycobacteria, however, are more likely to cause acute epididymitis in immunosuppressed patients than in immunocompetent patients. HPV InfectionMore than 100 types of HPV exist; more than 30 types can infect the genital area. The majority of HPV infections are asymptomatic, unrecognized, or subclinical. Genital HPV infection is common and usually self-limited. Genital HPV infection occurs more frequently than visible genital warts among both men and women and cervical cell changes among women. Genital HPV infection can cause genital warts, usually associated with HPV types 6 or 11. Other HPV types that infect the anogenital region (e.g., high-risk HPV types 16, 18, 31, 33, and 35) are strongly associated with cervical neoplasia. Persistent infection with high-risk types of HPV is the most important risk factor for cervical neoplasia. HPV TestsA definitive diagnosis of HPV infection is based on detection of viral nucleic acid (i.e., DNA or RNA) or capsid protein. Tests that detect several types of HPV DNA in cells scraped from the cervix are available and might be useful in the triage of women with atypical squamous cells of undetermined significance (ASC-US) or in screening women aged >30 years in conjunction with the Pap test (see Cervical Cancer Screening for Women Who Attend STD Clinics or Have a History of of STDs). Women determined to have HPV infection on such testing should be counseled that HPV infection is common, infection is frequently transmitted between partners, and that infection usually goes away on its own. If any Pap test or biopsy abnormalities have been observed, further evaluation is recommended. Screening women or men with the HPV test, outside of the above recommendations for use of the test with cervical cancer screening, is not recommended. TreatmentIn the absence of genital warts or cervical SIL, treatment is not recommended for subclinical genital HPV infection, whether it is diagnosed by colposcopy, biopsy, acetic acid application, or through the detection of HPV by laboratory tests. Genital HPV infection frequently goes away on its own, and no therapy has been identified that can eradicate infection. In the presence of coexistent SIL, management should be based on histopathologic findings. Genital WartsHPV types 6 or 11 are commonly found before, or at the time of, detection of genital warts; however, the use of HPV testing for genital wart diagnosis is not recommended. Genital warts are usually flat, papular, or pedunculated growths on the genital mucosa. Diagnosis of genital warts is made by visual inspection and may be confirmed by biopsy, although biopsy is needed only under certain circumstances (e.g., if the diagnosis is uncertain; the lesions do not respond to standard therapy; the disease worsens during therapy; the patient is immunocompromised; or warts are pigmented, indurated, fixed, bleeding, or ulcerated). No data support the use of HPV nucleic acid tests in the routine diagnosis or management of visible genital warts. The application of 3%--5% acetic acid usually turns HPV-infected genital mucosal tissue to a whitish color. However, acetic acid application is not a specific test for HPV infection, and the specificity and sensitivity of this procedure for screening have not been defined. Therefore, the routine use of this procedure for screening to detect HPV infection is not recommended. However, some clinicians, who are experienced in the management of genital warts, have determined that this test is useful for identifying flat genital warts. In addition to the external genitalia (i.e., penis, vulva, scrotum, perineum, and perianal skin), genital warts can occur on the uterine cervix and in the vagina, urethra, anus, and mouth. Intra-anal warts are observed predominantly in patients who have had receptive anal intercourse; these warts are distinct from perianal warts, which can occur in men and women who do not have a history of anal sex. In addition to the genital area, HPV types 6 and 11 have been associated with conjunctival, nasal, oral, and laryngeal warts. Genital warts are usually asymptomatic, but depending on the size and anatomic location, genital warts can be painful, friable, or pruritic. HPV types 16, 18, 31, 33, and 35 are found occasionally in visible genital warts and have been associated with external genital (i.e., vulvar, penile, and anal) squamous intraepithelial neoplasia (i.e., squamous cell carcinoma in situ, bowenoid papulosis, Erythroplasia of Queyrat, or Bowen's disease of the genitalia). These HPV types also have been associated with vaginal, anal, and CIN and anogenital and some head and neck squamous cell carcinomas. Patients who have visible genital warts are frequently infected simultaneously with multiple HPV types. TreatmentThe primary goal of treating visible genital warts is the removal of the warts. In the majority of patients, treatment can induce wart-free periods. If left untreated, visible genital warts might resolve on their own, remain unchanged, or increase in size or number. Treatment possibly reduces, but does not eliminate, HPV infection. Existing data indicate that currently available therapies for genital warts might reduce, but probably do not eradicate, HPV infectivity. Whether the reduction in HPV viral DNA, resulting from treatment, impacts future transmission remains unclear. No evidence indicates that the presence of genital warts or their treatment is associated with the development of cervical cancer. RegimensTreatment of genital warts should be guided by the preference of the patient, the available resources, and the experience of the health-care provider. No definitive evidence suggests that any of the available treatments are superior to any other and no single treatment is ideal for all patients or all warts. The use of locally developed and monitored treatment algorithms has been associated with improved clinical outcomes and should be encouraged. Because of uncertainty regarding the effect of treatment on future transmission of HPV and the possibility of spontaneous resolution, an acceptable alternative for some persons is to forego treatment and wait for spontaneous resolution. The majority of patients have <10 genital warts, with a total wart area of 0.5--1.0 cm2. These warts respond to various treatment modalities. Factors that might influence selection of treatment include wart size, wart number, anatomic site of wart, wart morphology, patient preference, cost of treatment, convenience, adverse effects, and provider experience. Factors that might affect response to therapy include the presence of immunosuppression and compliance with therapy. The majority of patients require a course of therapy rather than a single treatment. In general, warts located on moist surfaces or in intertriginous areas respond better to topical treatment than do warts on drier surfaces. The treatment modality should be changed if a patient has not improved substantially. The majority of genital warts respond within 3 months of therapy. The response to treatment and its side effects should be evaluated throughout the course of therapy. Complications occur rarely if treatments for warts are employed properly. Patients should be warned that persistent hypopigmentation or hyperpigmentation occurs commonly with ablative modalities. Depressed or hypertrophic scars are uncommon but can occur, especially if the patient has had insufficient time to heal between treatments. Rarely, treatment can result in disabling chronic pain syndromes (e.g., vulvodynia or analdynia, and hyperesthesia of the treatment site) or, in the case of rectal warts, painful defecation or fistulas. A limited number of case reports of severe systemic effects from podophyllin resin and interferon have been documented. Treatment regimens are classified into patient-applied and provider-applied modalities. Patient-applied modalities are preferred by some patients because they can be administered in the privacy of the patient's home. To use patient-applied modalities effectively, compliance with the treatment regimen is important along with the ability to identify and reach all genital warts. Recommended Regimens for External Genital Warts Patient-Applied:
Provider-Administered: Alternative Regimens Podofilox 0.5% solution or gel, an antimitotic drug that destroys warts, is relatively inexpensive, easy to use, safe, and self-applied by patients. The majority of patients experience mild-to-moderate pain or local irritation after treatment. Imiquimod is a topically active immune enhancer that stimulates production of interferon and other cytokines. Local inflammatory reactions are common with the use of imiquimod; these reactions include redness and irritation and are usually mild to moderate. Traditionally, follow-up visits are not required for patients using self-administered therapy. However, follow-up might be useful several weeks into therapy to determine the appropriateness of medication use and the response to treatment. Cryotherapy destroys warts by thermal-induced cytolysis. Health-care providers must be trained on the proper use of this therapy because over- and undertreatment might result in complications or low efficacy. Pain after application of the liquid nitrogen, followed by necrosis and sometimes blistering, is common. Local anesthesia (topical or injected) might facilitate therapy if warts are present in many areas or if the area of warts is large. Podophyllin resin, which contains several compounds, including antimitotic podophyllin lignans, is another treatment option. The resin is most frequently compounded at 10%--25% in a tincture of benzoin. However, podophyllin resin preparations differ in the concentration of active components and contaminants. The shelf life and stability of podophyllin preparations are unknown. A thin layer of podophyllin resin must be applied to the warts and allowed to air dry before the treated area comes into contact with clothing; overapplication or failure to air dry can result in local irritation caused by spread of the compound to adjacent areas. Both TCA and BCA are caustic agents that destroy warts by chemical coagulation of proteins. Although these preparations are widely used, they have not been investigated thoroughly. TCA solutions have a low viscosity comparable with that of water and can spread rapidly if applied excessively; therefore, they can damage adjacent tissues. Both TCA and BCA should be applied sparingly and allowed to dry before the patient sits or stands. If pain is intense, the acid can be neutralized with soap or sodium bicarbonate. Surgical therapy has the advantage of usually eliminating warts at a single visit. However, such therapy requires substantial clinical training, additional equipment, and a longer office visit. After local anesthesia is applied, the visible genital warts can be physically destroyed by electrocautery, in which case no additional hemostasis is required. Care must be taken to control the depth of electrocautery to prevent scarring. Alternatively, the warts can be removed either by tangential excision with a pair of fine scissors or a scalpel or by curettage. Because the majority of warts are exophytic, this procedure can be accomplished with a resulting wound that only extends into the upper dermis. Hemostasis can be achieved with an electrocautery unit or a chemical styptic (e.g., an aluminum chloride solution). Suturing is neither required nor indicated in the majority of cases if surgical removal is performed properly. Surgical therapy is most beneficial for patients who have a large number or area of genital warts. Carbon dioxide laser and surgery might be useful in the management of extensive warts or intraurethral warts, particularly for those patients who have not responded to other treatments. Interferons, both natural or recombinant, have been used for the treatment of genital warts. They have been administered systemically (i.e., subcutaneously at a distant site or IM) and intralesionally (i.e., injected into the warts). Systemic interferon is not effective. The efficacy and recurrence rates of intralesional interferon are comparable to other treatment modalities. Administration of intralesional interferon is associated with stinging, burning, and pain at the injection site. Interferon is probably effective because of its antiviral and/or immunostimulating effects. Interferon therapy is not recommended as a primary modality because of inconvenient routes of administration, frequent office visits, and the association between its use and a high frequency of systemic adverse effects. Because of the shortcomings associated with all available treatments, some clinics employ combination therapy (i.e., the simultaneous use of two or more modalities on the same wart at the same time). No data support the use of more than one therapy at a time to improve efficacy of treatment, and some specialists believe that combining modalities might increase complications.
Recommended Regimens for Cervical Warts
Recommended Regimens for Vaginal Warts
Recommended Regimens for Urethral Meatus Warts
Although data evaluating the use of podofilox and imiquimod for the treatment of distal meatal warts are limited, some specialists recommend their use in some patients. Recommended Regimens for Anal Warts
Warts on the rectal mucosa should be managed in consultation with a specialist. Many persons with warts on the anal mucosa also have warts on the rectal mucosa, so persons with anal warts can benefit from an inspection of the rectal mucosa by digital examination or anoscopy. CounselingGenital HPV InfectionEducation and counseling are vital aspects of managing patients with genital warts. Patients can be educated through patient education materials, including pamphlets, hotlines, and websites (http://www.ashastd.org or http://www.cdc.gov/std/hpv). Attempts should be made to convey the following key messages:
Genital Warts
Follow-UpAfter visible genital warts have cleared, a follow-up evaluation might be helpful. Patients should be cautioned to watch for recurrences, which occur most frequently during the first 3 months. External genital warts can be difficult to identify, so it might be useful for patients to have a follow-up evaluation 3 months after treatment. Earlier follow-up visits also might be useful for some patients to document the absence of warts, to monitor for or treat complications of therapy, and to provide an additional opportunity for patient education and counseling. Women should be counseled to undergo regular Pap screening as recommended for women without genital warts. Management of Sex PartnersExamination of sex partners is not necessary for the management of genital warts because no data indicate that reinfection plays a role in recurrences. In addition, providing treatment for genital warts solely for the purpose of preventing future transmission cannot be recommended because the value of treatment in reducing infectivity is unknown. However, sex partners of patients who have genital warts might benefit from counseling and examination to assess the presence of genital warts and other STDs. The counseling of sex partners provides an opportunity for these partners to 1) learn that HPV infection is common and probably shared between partners and 2) receive STD evaluation and screening and Pap screening if they are female. Female sex partners of patients who have genital warts should be reminded that cytologic screening for cervical cancer is recommended for all sexually active women. Special ConsiderationsPregnancy Imiquimod, podophyllin, and podofilox should not be used during pregnancy. However, because genital warts can proliferate and become friable during pregnancy, many specialists advocate their removal during pregnancy. HPV types 6 and 11 can cause respiratory papillomatosis in infants and children. The route of transmission (i.e., transplacental, perinatal, or postnatal) is not completely understood. Whether cesarean section prevents respiratory papillomatosis in infants and children is unclear; therefore, cesarean delivery should not be performed solely to prevent transmission of HPV infection to the newborn. Cesarean delivery might be indicated for women with genital warts if the pelvic outlet is obstructed or if vaginal delivery would result in excessive bleeding. Pregnant women with genital warts should be counseled concerning the low risk for warts on the larynx (recurrent respiratory papillomatosis) in their infants or children (193). No controlled studies have suggested that cesarean section prevents this condition. HIV Infection No data suggest that treatment modalities for external genital warts should be different in the setting of HIV-infection. However, persons who are immunosuppressed because of HIV or other reasons might have larger or more numerous warts, might not respond as well as immunocompetent persons to therapy for genital warts, and might have more frequent recurrences after treatment (194,195). Squamous cell carcinomas arising in or resembling genital warts might occur more frequently among immunosuppressed persons, therefore, requiring biopsy for confirmation of diagnosis. Because of the increased incidence of anal cancer in HIV-infected homosexual men, screening for anal SIL by cytology in this population is recommended by some specialists. However, evidence is limited concerning the natural history of anal intraepithelial neoplasias, the reliability of screening methods, the safety and response to treatments, and the programmatic considerations that would support this screening approach. Until additional data are generated on screening for anal SIL, this screening approach cannot be recommended. Squamous Cell Carcinoma in Situ Patients in whom squamous cell carcinoma in situ of the genitalia is diagnosed should be referred to a specialist for treatment. Ablative modalities usually are effective, but careful follow-up is essential. The risk for these lesions leading to invasive squamous cell carcinoma of the external genitalia in immunocompetent patients is unknown but is probably low. Female partners of male patients who have squamous cell carcinoma in situ are at high risk for cervical abnormalities. Cervical Cancer Screening for Women Who Attend STD Clinics or Have a History of STDsWomen with a history of STDs might be at increased risk for cervical cancer, and women attending STD clinics might have other risk factors that place them at even greater risk. Prevalence studies indicate that precursor lesions for cervical cancer occur approximately five times more frequently among women attending STD clinics than among women attending family planning clinics (196). Cervical cancer screening using the Pap test is an effective, low-cost screening test for preventing invasive cervical cancer. Recommendations for cervical cancer screening intervals vary in the United States, but the American Cancer Society and American College of Obstetricians and Gynecologists guidelines recommend annual screening for women aged 21--30 years and then every 2--3 years for women aged >30 years if three consecutive annual Pap tests are negative (197,198). RecommendationsDuring the appointment in which a pelvic examination for STD screening is performed, the health-care provider should inquire about the result of the patient's most recent Pap test and discuss the following information with the patient:
If a woman has not had a Pap test during the previous 12 months, a Pap test may be obtained as part of the routine pelvic examination. Health-care providers should be aware that many women frequently equate having a pelvic examination with having a Pap test; they believe that a Pap test was taken when they actually received only a pelvic examination. They might, therefore, over report having had a recent Pap test. Therefore, in STD clinics, having a protocol for conducting cervical cancer screening should be highly encouraged and obtaining a Pap test strongly considered during the routine clinical evaluation of women who do not have clinical-record documentation of a normal Pap test within the preceding 12 months. A woman might benefit from receiving printed information concerning Pap tests and a report containing a statement that a Pap test was obtained during her clinic visit. If possible, a copy of the Pap test result should be provided to the patient for her records when it becomes available. Follow-UpSTD clinics offering cervical cancer screening are encouraged to use cytopathology laboratories that report results by using the Bethesda System of classification (199).††† If the results of the Pap test are abnormal, follow-up care should be provided according to the ASCCP Consensus Guidelines for Management of Abnormal Cervical Cytology (198), or information regarding follow-up care is available at http//www.asccp.org. If resources in STD clinics do not allow follow-up of abnormal results, protocols for referral of women needing follow-up and case management should be in place. Pap tests indicating low- or high-grade SIL should always include referral to a clinician who can perform a colposcopic examination of the lower genital tract and, if indicated, colposcopically directed biopsy. For patients with an equivocal Pap test report indicating ASC-US, three options are available for follow-up management: 1) immediate colposcopy, 2) repeat Pap tests at 6-month intervals for 3 intervals, or 3) an HPV DNA test. Women with ASC-US may be considered for immediate colposcopy if concerns for patient adherence with recommended follow-up or for other clinical indications are a factor. The presence of high grade histological changes after ASC-US Pap test reports usually is <10%. If repeat Pap tests are used to follow ASC-US results, a test should be performed every 6 months until 3 negative results are noted before the women returns to cervical cancer screening at a normal interval for age. If subsequent Pap tests demonstrate progression to SIL, follow-up should be conducted according to ASCCP Consensus Guidelines (i.e., frequent colposcopy and directed cervical biopsy). If specific infections other than HPV are identified, the patient might need to have a repeat Pap test after appropriate treatment for those infections. In the majority of instances, even in the presence of some severe infections, Pap tests will be reported as satisfactory for evaluation, so they may be read and final reports produced without the necessity to treat and repeat the Pap test. When repeating the Pap test is necessary because of an unsatisfactory for interpretation report, the repeat test must be interpreted by the laboratory as satisfactory and also be negative before returning the woman to Pap tests at regularly scheduled intervals. A third strategy for managing patients with ASC-US Pap test results involves testing for HPV DNA. Whereas conducting HPV testing in some STD clinics might not be possible or appropriate because of inadequate resources, such testing might be appropriate in other public health clinic settings. Only one FDA-cleared test exists, the Digene Hybrid Capture II. The HPV DNA test may be performed by 1) co-collecting a specimen; 2) using a supplied swab at the time of the Pap test, if conventional cytology is used; 3) reflex testing, if liquid-based cytology is used and enough residual material is available in the cytology test vial; or 4) scheduling a separate follow-up appointment when the Pap test report results are known. If the high-risk HPV DNA test is positive, women are referred immediately for colposcopy, and if indicated, directed cervical biopsy. Because many public health clinics, including the majority of STD clinics, cannot provide clinical follow-up of abnormal Pap tests, women with Pap tests demonstrating low or high grade SIL or ASC-US usually need a referral to other local health-care providers or clinics for colposcopy and biopsy. Clinics and health-care providers who offer Pap test screening services but cannot provide appropriate colposcopic follow-up of abnormal Pap tests should arrange referral to health-care facilities in which 1) a patient will be promptly evaluated and treated and 2) the results of the evaluation will be reported to the referring clinic or health-care provider. Clinics and health-care providers should develop protocols that identify women who miss follow-up appointments so that these women can be located and scheduled for needed studies and management, and they should reevaluate such protocols routinely. Pap test results, type and location of follow-up appointments, and results of follow-up appointments should be clearly documented in the clinic record. The establishment of colposcopy and biopsy services in local health departments, especially in circumstances in which referrals are difficult and follow-up is unlikely, should be considered if resources are available. Other Management ConsiderationsOther considerations in performing Pap tests include the following:
Special Considerations PregnancyPregnant women should have a Pap test as part of routine prenatal care. A cytobrush and an Ayers spatula might be used for obtaining Pap tests in pregnant women. HIV InfectionSeveral studies have documented an increased prevalence of SIL in HIV-infected women (200,201). The following recommendations for Pap test screening among HIV-infected women are consistent with other guidelines published by the U.S. Department of Health and Human Services (50) and are based partially on the opinions of professionals knowledgeable about the care and management of cervical cancer and HIV infection in women. After obtaining a complete history of previous cervical disease, HIV-infected women should be provided a comprehensive gynecologic examination, including a pelvic examination and Pap test, as part of their initial evaluation. A Pap test should be obtained twice in the first year after diagnosis of HIV infection and, if the results are normal, annually thereafter. If the results of the Pap test are abnormal, care should be provided according to the ASCCP Consensus Guidelines for Management of Abnormal Cervical Cytology (198). Women with cytological reports of ASC-US, low or high-grade SIL or squamous cell carcinoma, regardless of CD4+ count or antiretroviral treatment status, should undergo colposcopy and directed biopsy. Colposcopy and biopsy are not indicated in HIV-positive women with negative Pap test reports. Vaccine Preventable STDsSome STDs can be effectively prevented through preexposure vaccination. Vaccines are under development or are undergoing clinical trials for certain STDs, including HIV and HSV. However, the only vaccines currently available are for prevention of HAV, HBV, and HPV infection. Vaccination efforts focus largely on integrating the use of these available vaccines into STD prevention and treatment activities. Every person being evaluated or treated for an STD, who is not already vaccinated, should receive hepatitis B vaccination. In addition, some persons (e.g., MSM and illegal-drug users) should receive hepatitis A vaccination. Hepatitis AHepatitis A, caused by infection with HAV, has an incubation period of approximately 28 days (range: 15--50 days). HAV replicates in the liver and is shed in high concentrations in feces from 2 weeks before to 1 week after the onset of clinical illness. HAV infection produces a self-limited disease that does not result in chronic infection or chronic liver disease. However, 10%--15% of patients might experience a relapse of symptoms during the 6 months after acute illness. Acute liver failure from hepatitis A is rare (overall case-fatality rate: 0.5%). The risk for symptomatic infection is directly related to age, with >80% of adults having symptoms compatible with acute viral hepatitis and the majority of children having either asymptomatic or unrecognized infection. Antibody produced in response to HAV infection persists for life and confers protection against reinfection. HAV infection is primarily transmitted by the fecal-oral route, by either person-to-person contact, or through consumption of contaminated food or water. Although viremia occurs early in infection and can persist for several weeks after onset of symptoms, bloodborne transmission of HAV is uncommon. HAV occasionally might be detected in saliva in experimentally infected animals, but transmission by saliva has not been demonstrated. In the United States, nearly half of all reported hepatitis A cases have no specific risk factor identified. Among adults with identified risk factors, the majority of cases are among MSM, persons who use illegal drugs, and international travelers (202). Because transmission of HAV during sexual activity probably occurs because of fecal-oral contact, measures typically used to prevent the transmission of other STDs (e.g., use of condoms) do not prevent HAV transmission. In addition, efforts to promote good personal hygiene have not been successful in interrupting outbreaks of hepatitis A. Vaccination is the most effective means of preventing HAV transmission among persons at risk for infection, many of whom might seek services in STD clinics. DiagnosisThe diagnosis of hepatitis A cannot be made on clinical grounds alone and requires serologic testing. The presence of IgM antibody to HAV is diagnostic of acute HAV infection. A positive test for total anti-HAV indicates immunity to HAV infection but does not differentiate current from previous HAV infection. Although usually not sensitive enough to detect the low level of protective antibody after vaccination, anti-HAV tests might be positive after hepatitis A vaccination. TreatmentPatients with acute hepatitis A usually require only supportive care, with no restrictions in diet or activity. Hospitalization might be necessary for patients who become dehydrated because of nausea and vomiting and is critical for patients with signs or symptoms of acute liver failure. Medications that might cause liver damage or are metabolized by the liver should be used with caution among persons with hepatitis A. PreventionTwo products are available for the prevention of HAV infection: hepatitis A vaccine (Table 2) and immune globulin (Ig) for IM administration. Hepatitis A vaccines are prepared from formalin-inactivated, cell-culture--derived HAV and have been available in the United States since 1995, initially for persons aged >2 years. In 2005, the vaccines were approved by FDA for persons aged >12 months. Administered IM in a 2-dose series, these vaccines induce protective antibody levels in virtually all adults. By 1 month after the first dose, 94%--100% of adults have protective antibody levels; 100% of adults develop protective antibody after a second dose. In randomized controlled trials, the equivalent of 1 dose of hepatitis A vaccine administered before exposure has been 94%--100% effective in preventing clinical hepatitis A (3). Kinetic models of antibody decline indicate that protective levels of antibody persist for at least 20 years. A combined hepatitis A and hepatitis B vaccine have been developed and licensed for use as a 3-dose series in adults aged >18 years (see Table 3, Hepatitis B). When administered IM on a 0-, 1-, and 6-month schedule, the vaccine has equivalent immunogenicity to that of the monovalent vaccines.
Preexposure ImmunizationPersons in the following groups who are likely to be treated in STD clinic settings should be offered hepatitis A vaccine: 1) all MSM; 2) illegal drug users (both injecting and noninjecting drugs); and 3) persons with CLD, including persons with chronic HBV and HCV infection who have evidence of CLD. Prevaccination Serologic Testing for SusceptibilityApproximately one third of the U.S. population has serologic evidence of previous HAV infection, which increases directly with age and reaches 75% among persons aged >70 years. Screening for HAV infection might be cost-effective in populations where the prevalence of infection is likely to be high (e.g., persons aged >40 years and persons born in areas of high HAV endemicity). The potential cost-savings of testing should be weighed against the cost and the likelihood that testing will interfere with initiating vaccination. Vaccination of a person who is already immune is not harmful. Postvaccination Serologic TestingPostvaccination serologic testing is not indicated because the majority of persons respond to the vaccine. In addition, the commercially available serologic test is not sensitive enough to detect the low, but protective, levels of antibody produced by vaccination. Postexposure ProphylaxisPreviously unvaccinated persons exposed to HAV (e.g., through household or sexual contact or by sharing illegal drugs with a person who has hepatitis A) should be administered a single IM dose of Ig (0.02 mL/kg) as soon as possible but not >2 weeks after exposure. Persons who have had 1 dose of hepatitis A vaccine at least 1 month before exposure to HAV do not need Ig. If hepatitis A vaccine is recommended for a person receiving Ig, it can be administered simultaneously at a separate anatomic injection site. The use of hepatitis A vaccine alone is not recommended for PEP. Special ConsiderationsLimited data indicate that vaccination of persons with CLD and of HIV-infected persons results in lower seroprotection rates and antibody concentrations (50). In HIV-infected persons, antibody response might be directly related to CD4+ levels. Hepatitis BHepatitis B is caused by infection with HBV. The incubation period from the time of exposure to onset of symptoms is 6 weeks to 6 months. HBV is found in highest concentrations in blood and in lower concentrations in other body fluids (e.g., semen, vaginal secretions, and wound exudates). HBV infection can be self-limited or chronic. In adults, only approximately half of newly acquired HBV infections are symptomatic, and approximately 1% of reported cases result in acute liver failure and death. Risk for chronic infection is inversely related to age at infection: approximately 90% of infected infants and 30% of infected children aged <5 years become chronically infected, compared with 2%--6% of adults. Among persons with chronic HBV infection, the risk for premature death from cirrhosis or hepatocellular carcinoma (HCC) is 15%--25%. HBV is efficiently transmitted by percutaneous or mucous membrane exposure to infectious blood or body fluids that contain blood. The primary risk factors that have been associated with infection among adolescents and adults are unprotected sex with an infected partner, unprotected sex with more than one partner, MSM, history of other STDs, and illegal injecting-drug use. CDC's national strategy to eliminate transmission of HBV infection includes 1) prevention of perinatal infection through routine screening of all pregnant women for HBsAg and immunoprophylaxis of infants born to HBsAg-positive mothers and infants born to mothers with unknown HBsAg status, 2) routine infant vaccination, 3) vaccination of previously unvaccinated children and adolescents through age 18 years, and 4) vaccination of previously unvaccinated adults at increased risk for infection (2,4). High vaccination coverage rates, with subsequent declines in acute hepatitis B incidence, have been achieved among infants and adolescents (2,203,204). In contrast, vaccination coverage among the majority of high-risk adult groups (e.g., persons with more than one sex partner in the previous 6 months, MSM, and IDUs) have remained low, and the majority of new infections occur in these high-risk groups (4,205--207). STD clinics and other settings that provide services targeted to high-risk adults are ideal sites in which to provide hepatitis B vaccination to adults at risk for HBV infection. All unvaccinated adults seeking services in these settings should be assumed to be at risk for hepatitis B and should receive hepatitis B vaccination. DiagnosisDiagnosis of acute or chronic HBV infection requires serologic testing (Table 4). HBsAg is present in both acute and chronic infection. The presence of IgM antibody to hepatitis B core antigen (IgM anti-HBc) is diagnostic of acute or recently acquired HBV infection. Antibody to HBsAg (anti-HBs) is produced after a resolved infection and is the only HBV antibody marker present after immunization. The presence of HBsAg and total anti-HBc, with a negative test for IgM anti-HBc, indicates chronic HBV infection. The presence of anti-HBc alone might indicate a false-positive result or acute, resolved, or chronic infection. TreatmentNo specific therapy is available for persons with acute hepatitis B; treatment is supportive. Persons with chronic HBV infection should be referred for evaluation to a physician experienced in the management of CLD. Therapeutic agents approved by FDA for treatment of chronic hepatitis B can achieve sustained suppression of HBV replication and remission of liver disease in some persons. In addition, patients with chronic hepatitis B might benefit from screening to detect HCC at an early stage. PreventionTwo products have been approved for hepatitis B prevention: hepatitis B immune globulin (HBIG) and hepatitis B vaccine. HBIG provides temporary (i.e., 3--6 months) protection from HBV infection and is typically used as PEP either as an adjunct to hepatitis B vaccination in previously unvaccinated persons or alone in persons who have not responded to vaccination. HBIG is prepared from plasma known to contain high concentrations of anti-HBs. The recommended dose of HBIG is 0.06 mL/kg. Hepatitis B vaccine contains HBsAg produced in yeast by recombinant DNA technology and provides protection from HBV infection when used for both preexposure immunization and PEP. The two available monovalent hepatitis B vaccines for use in adolescents and adults are Recombivax HB® (Merck and Co., Inc., Whitehouse Station, New Jersey) and Engerix- B® (GlaxoSmithKline Biologicals, Pittsburgh, Pennsylvania). A combination vaccine (hepatitis A and hepatitis B) for use in adults, Twinrix® (GlaxoSmithKline Biologicals, Pittsburgh, Pennsylvania), also is available. The recommended HBV dose varies by product and age of recipient (Table 3). When selecting a hepatitis B vaccination schedule, the health-care provider should consider the need to achieve completion of the vaccine series. Approved adolescent and adult schedules for both monovalent hepatitis B vaccine (i.e., Engerix-B® and Recombivax HB®) include the following: 0, 1, and 6 months; 0, 1, and 4 months; and 0, 2, and 4 months. A 4-dose schedule of Engerix-B® at 0, 1, 2, and 12 months is licensed for all age groups. A 2-dose schedule of Recombivax HB® adult formulation (10 µg) is licensed for adolescents aged 11--15 years. When scheduled to receive the second dose, adolescents aged >15 years should be switched to a 3-dose series, with doses 2 and 3 consisting of the pediatric formulation (5 µg) administered on an appropriate schedule. Twinrix® may be administered to persons aged >18 years at risk for both HAV and HBV infections at 0, 1, and 6 months. Hepatitis B vaccine should be administered IM in the deltoid muscle and may be administered simultaneously with other vaccines. For adolescents and adults, the needle length should be 1--2 inches, depending on the recipient's weight (1 inch for females weighing <70 kg), 1.5 inches for males weighing <120 kg; and 2 inches for males weighing >120 kg and females >100 kg). A 22- to 25-gauge needle is recommended. If the vaccine series is interrupted after the first or second dose of vaccine, the missed dose should be administered as soon as possible. The series does not need to be restarted after a missed dose. In adolescents and healthy adults aged <40 years, approximately 30%--55% acquire a protective antibody response (anti-HBs >10 mIU/mL) after the first vaccine dose, 75% after the second, and >90% after the third. Vaccine-induced immune memory has been demonstrated to persist for at least 15--20 years. Periodic testing to determine antibody levels in immunocompetent persons is not necessary, and booster doses of vaccine are not recommended. Hepatitis B vaccination is generally well-tolerated by the majority of recipients. Pain at the injection site and low-grade fever are reported by a minority of recipients. Evidence for a causal association between receipt of hepatitis B vaccination and anaphylaxis exists, which is estimated to occur in 1 of 1.1 million doses of vaccine administered among children and adolescents; no deaths have been reported after anaphylaxis. Vaccine is contraindicated in persons with a history of anaphylaxis after a previous dose of hepatitis B vaccine and in persons with a known anaphylactic reaction to any vaccine component. No evidence for a causal association has been demonstrated for other adverse events reported after administration of hepatitis B vaccine. Preexposure VaccinationHepatitis B vaccination is recommended for all unvaccinated adolescents, all unvaccinated adults at risk for HBV infection, and all adults seeking protection from HBV infection. For adults, acknowledgement of a specific risk factor is not a requirement for vaccination. Hepatitis B vaccine should be routinely offered to all unvaccinated persons attending STD clinics and to all unvaccinated persons seeking treatment for STDs in other settings. Other settings where all unvaccinated adults should be assumed to be at risk for hepatitis B and should receive hepatitis B vaccination include correctional facilities, facilities providing drug abuse treatment and prevention services, health-care settings serving MSM, and HIV testing and treatment facilities. All persons who receive clinical services in these settings should be offered hepatitis B vaccine, unless they have a reliable vaccination history (i.e., a written, dated record of each dose of a complete series). In all settings, vaccination should be initiated even though completion of the vaccine series might not be ensured. Prevaccination Antibody ScreeningPrevaccination serologic testing for susceptibility may be considered to reduce the cost of vaccinating adult populations that have an expected high prevalence of HBV infection (i.e., >20%--30%) (e.g., IDUs and MSM [especially in older age groups]). In addition, prevaccination testing for susceptibility is recommended for unvaccinated household, sexual, and needle-sharing contacts of HBsAg-positive persons. Anti-HBc is the test of choice for prevaccination testing; persons who are anti-HBc--positive should be tested for HBsAg. If persons are determined to be HBsAg negative, no further action is required. If persons are determined to be HBsAg positive, the person should be referred for medical follow-up, including counseling and evaluation for antiviral treatment (see Management of HBsAg-Positive Persons). In addition, all household members, sex partners, and needle-sharing partners of HBsAg-positive persons should be vaccinated. Serologic testing should not be a barrier to vaccination of susceptible persons, especially in populations that are difficult to access. In the majority of situations, the first vaccine dose should be administered immediately after collection of the blood sample for serologic testing. Vaccination of persons who are immune to HBV infection because of current or previous infection or vaccination does not increase the risk for adverse events. Postvaccination Testing for Serologic ResponseSerologic testing for immunity is not necessary after routine vaccination of adolescents or adults. Testing after vaccination is recommended for persons whose subsequent clinical management depends on knowledge of their immune status (e.g., health-care workers or public safety workers at high risk for continued percutaneous or mucosal exposure to blood or body fluids). In addition, testing is recommended for 1) HIV-infected persons and other immunocompromised persons to determine the need for revaccination and the type of follow-up testing; and 2) sex and needle-sharing partners of HBsAg-positive persons to determine the need for revaccination and for other methods to protect themselves from HBV infection. If indicated, testing should be performed 1--2 months after administration of the last dose of the vaccine series by using a method that allows determination of a protective level of anti-HBs (>10 mIU/mL). Persons determined to have anti-HBs levels of <10 mIU/mL after the primary vaccine series should be revaccinated with a 3-dose series, followed by anti-HBs testing 1--2 months after the third dose. Persons who do not respond to revaccination should be tested for HBsAg. If HBsAg positive, the person should receive appropriate management (see Management of HBsAg-Positive Persons); if HBsAg negative, the person should be considered susceptible to HBV infection and counseled concerning precautions to prevent HBV infection and the need for HBIG PEP for any known exposure (see PEP). Postexposure ProphylaxisBoth passive-active PEP with HBIG and hepatitis B vaccination and active PEP with hepatitis B vaccination alone have been demonstrated to be highly effective in preventing transmission after exposure to HBV (2). HBIG alone also has been demonstrated to be effective in preventing HBV transmission, but with the availability of hepatitis B vaccine, HBIG typically is used as an adjunct to vaccination. Exposure to HBsAg-Positive Source. Unvaccinated persons or persons known not to have responded to a complete hepatitis B vaccine series should receive both HBIG and hepatitis vaccine as soon as possible (preferably <24 hours) after a discrete, identifiable exposure to blood or body fluids that contain blood from an HBsAg-positive source (Table 5). Hepatitis B vaccine should be administered simultaneously with HBIG in a separate injection site, and the vaccine series should be completed by using the age-appropriate vaccine dose and schedule (Table 3). Exposed persons who are in the process of being vaccinated but who have not completed the vaccine series should receive the appropriate dose of HBIG (i.e., 0.06 mL/kg) and should complete the vaccine series. Exposed persons who are known to have responded to vaccination are considered protected and need no further vaccine doses. Persons who have written documentation of a complete hepatitis B vaccine series and who did not receive postvaccination testing should receive a single vaccine booster dose. Alternatively, these persons can be managed according to guidelines for management of persons with occupational exposure to blood or body fluids that contain blood (207). Exposure to Source with Unknown HBsAg Status. Unvaccinated persons who have a discrete, identifiable exposure to blood or body fluids containing blood from a source with unknown HBsAg status should receive the hepatitis B vaccine series, with the first dose initiated as soon as possible after exposure (preferably within 24 hours) and the series completed by using the age-appropriate dose and schedule. Exposed persons who are not fully vaccinated should complete the vaccine series. Exposed persons with written documentation of a complete hepatitis B vaccine series require no further treatment. Special ConsiderationsPregnancy. All pregnant women receiving STD services should be tested for HBsAg, regardless of whether they have been previously tested or vaccinated. All HBsAg-positive pregnant women should be reported to state and local perinatal hepatitis B prevention programs. HBsAg-negative pregnant women seeking STD treatment who have not been previously vaccinated should receive hepatitis B vaccination. Additional information regarding management of HBsAg-positive pregnant women and their infants is available at http://www.cdc.gov/mmwr/PDF/rr/rr5416.pdf. HIV Infection. HIV infection can impair the response to hepatitis B vaccination. HIV-infected persons should be tested for anti-HBs 1--2 months after the third vaccine dose (see Postvaccination Testing for Serologic Response). Modified dosing regimens, including a doubling of the standard antigen dose and administration of additional doses, might increase the response rate. Management of HBsAg-Positive PersonsThis section provides recommendations for management of all HBsAg-positive persons. Additional recommendations for management of HBsAg-positive persons who are coinfected with HIV are available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5315a1.htm.
When seeking medical or dental care, HBsAg-positive persons should be advised to inform those responsible for their care of their HBsAg status so that they can be appropriately evaluated and managed. Information regarding HBsAg-positive women who are pregnant is available in this report (see Special Populations, Pregnant Women). Other counseling messages also should be considered.
Hepatitis CHepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States; approximately 2.7 million persons are chronically infected (204). Although HCV is not efficiently transmitted sexually, persons at risk for infection through injection-drug use might seek care in STD treatment facilities, HIV counseling and testing facilities, correctional facilities, drug treatment facilities, and other public health settings where STD and HIV prevention and control services are available. Persons newly infected with HCV typically are either asymptomatic or have a mild clinical illness. HCV RNA can be detected in blood within 1--3 weeks after exposure. The average time from exposure to antibody to HCV (anti-HCV) seroconversion is 8--9 weeks, and anti-HCV can be detected in >97% of persons by 6 months after exposure. Chronic HCV infection develops in 60%--85% of HCV-infected persons; 60%--70% of chronically infected persons have evidence of active liver disease. The majority of infected persons might not be aware of their infection because they are not clinically ill. However, infected persons serve as a source of transmission to others and are at risk for CLD or other HCV-related chronic diseases for decades after infection. HCV is most efficiently transmitted through large or repeated percutaneous exposure to infected blood (e.g., through transfusion of blood from unscreened donors or through use of injecting drugs), although less efficient, occupational, perinatal, and sexual exposures also can result in transmission of HCV. The role of sexual activity in the transmission of HCV has been controversial. Case-control studies have reported an association between acquiring HCV infection and exposure to a sex contact with HCV infection or exposure to multiple sex partners. Surveillance data also indicate that 15%--20% of persons reported with acute HCV infection have a history of sexual exposure in the absence of other risk factors (204,208). Case reports of acute HCV infection among HIV-positive MSM who deny injecting-drug use have indicated that this occurrence is frequently associated with other STDs (e.g., syphilis) (209,210). In contrast, a low prevalence (average: 1.5%) of HCV infection has been demonstrated in studies of long-term spouses of patients with chronic HCV infection who had no other risk factors for infection, and multiple published studies have demonstrated the prevalence of HCV infection among MSM who have not reported a history of injecting-drug use to be no higher than that of heterosexuals (211--213). Because sexual transmission of bloodborne viruses is more efficient among homosexual men compared with heterosexual men and women, the reason that HCV infection rates are not substantially higher among MSM compared with heterosexuals is unclear. Overall, these findings indicate that sexual transmission of HCV is possible but inefficient. Additional data are needed to determine whether sexual transmission of HCV might be increased in the context of HIV infection or other STDs. Diagnosis and TreatmentAnti-HCV testing is recommended for routine screening of asymptomatic persons based on their risk for infection or based on a recognized exposure (see Hepatitis C, Prevention). For such persons, testing for HCV infection should include the use of an FDA-cleared test for antibody to HCV (i.e., immunoassay, EIA, or enhanced chemiluminescence assay and, if recommended, a supplemental antibody test) (214). Persons counseled and tested for HCV infection and determined to be anti-HCV positive should be evaluated (by referral or consultation, if appropriate) for presence of active infection, presence or development of CLD, and for possible treatment. Reverse transcriptase polymerase chain reaction to detect HCV RNA may be used to confirm the diagnosis of current HCV infection, and an elevated alanine aminotransferase (ALT) level is biochemical evidence of CLD. Combination therapy with pegylated interferon and ribavirin is the treatment of choice for patients with chronic hepatitis C. Because of advances in the field of antiviral therapy for acute and chronic hepatitis C, clinicians should consult with specialists knowledgeable about management of hepatitis C infection. PreventionNo vaccine for hepatitis C is available, and prophylaxis with immune globulin is not effective in preventing HCV infection after exposure. Reducing the burden of HCV infection and disease in the United States requires implementation of both primary and secondary prevention activities (208). Primary prevention reduces or eliminates HCV transmission; secondary prevention activities reduce liver and other chronic diseases in HCV-infected persons by identifying them and providing appropriate medical management and antiviral therapy, if appropriate. Persons seeking care in STD clinics or other primary-care settings should be screened to identify those who should be offered HCV counseling and testing. In STD clinics and other settings that serve large numbers of persons at high risk for bloodborne infections (e.g., correctional settings), the major risk factor for which to screen for HCV infection is injection of illegal drugs. In addition, for clinical management issues, all persons with HIV infection should also be offered HCV counseling and testing. Other risk factors for which routine HCV testing is recommended include persons
Persons who test positive for anti-HCV (see Diagnosis and Treatment) should be provided information regarding 1) how to protect their liver from further harm, 2) how to prevent transmission to others, and 3) the need for medical evaluation for CLD and possible treatment.
Persons who test negative for anti-HCV who had an exposure previously should be reassured that they are not infected. Regardless of test results, persons who use or inject illegal drugs should be counseled to
Postexposure Follow-UpNo PEP has been demonstrated to be effective against HCV. Testing to determine whether HCV infection has developed is recommended for health-care workers after percutaneous or permucosal exposures to HCV-positive blood and for children born to HCV-positive women. Special Considerations PregnancyRoutine testing for HCV infection is not recommended for all pregnant women. Pregnant women with a known risk factor for HCV infection should be offered counseling and testing. Patients should be advised that approximately five of every 100 infants born to HCV-infected woman become infected. This infection occurs predominantly during or near delivery, and no treatment or delivery method is known to decrease this risk. The risk is increased by the presence of maternal HCV viremia at delivery and also is greater (2--3 times) if the woman is coinfected with HIV. Breastfeeding does not appear to transmit HCV, although HCV-positive mothers should consider abstaining from breastfeeding if their nipples are cracked or bleeding. Infants born to HCV-positive mothers should be tested for HCV infection and, if positive, evaluated for the presence of CLD. HIV InfectionBecause of the high prevalence of HIV/HCV coinfection and because of critical clinical management issues for coinfected persons, all HIV-infected persons should be tested for HCV. Because a small percentage of coinfected persons fail to acquire HCV antibodies, HCV RNA should be tested in HIV-positive persons with unexplained liver disease who are anti-HCV negative. The course of liver disease is more rapid in HIV/HCV coinfected persons, and the risk for cirrhosis is nearly twice that in persons with HCV infection alone. Treatment of HCV in coinfected persons might improve tolerance to highly active antiretroviral therapy (HAART) for HIV infection because of the increased risk for hepatotoxicity from HAART with HCV infection. However, anti-HCV treatment in coinfected persons is still investigational, and based on ongoing clinical trials, more data are needed to determine the best regimens. Proctitis, Proctocolitis, and EnteritisSexually transmitted gastrointestinal syndromes include proctitis, proctocolitis, and enteritis. Evaluation for these syndromes should include appropriate diagnostic procedures (e.g., anoscopy or sigmoidoscopy, stool examination, and culture). Proctitis is inflammation of the rectum (i.e., the distal 10--12 cm) that might be associated with anorectal pain, tenesmus, or rectal discharge. N. gonorrhoeae, C. trachomatis (including LGV serovars), T. pallidum, and HSV are the most common sexually transmitted pathogens involved. In patients coinfected with HIV, herpes proctitis might be especially severe. Proctitis occurs predominantly among persons who participate in receptive anal intercourse. Proctocolitis is associated with symptoms of proctitis and diarrhea or abdominal cramps and inflammation of the colonic mucosa, extending to 12 cm above the anus. Fecal leukocytes might be detected on stool examination, depending on the pathogen. Pathogenic organisms include Campylobacter sp., Shigella sp., Entamoeba histolytica, and, rarely, LGV serovars of C. trachomatis. CMV or other opportunistic agents might be involved in immunosuppressed HIV-infected patients. Proctocolitis can be acquired by the oral route or by oral-anal contact, depending on the pathogen. Enteritis usually results in diarrhea and abdominal cramping without signs of proctitis or proctocolitis; it occurs among persons whose sexual practices include oral-anal contact. In otherwise healthy persons, Giardia lamblia is most frequently implicated. When outbreaks of gastrointestinal illness occur among social or sexual networks of MSM, clinicians should consider sexual transmission as a mode of spread and provide counseling accordingly. Among HIV-infected patients, gastrointestinal illness can be caused by other infections that usually are not sexually transmitted, including CMV, Mycobacterium avium-intracellulare, Salmonella sp., Campylobacter sp., Shigella sp., Cryptosporidium, Microsporidium, and Isospora. Multiple stool examinations might be necessary to detect Giardia, and special stool preparations are required to diagnose cryptosporidiosis and microsporidiosis. In addition, enteritis might be directly caused by HIV infection. When laboratory diagnostic capabilities are available, treatment decisions should be based on the specific diagnosis. Diagnostic and treatment recommendations for all enteric infections are beyond the scope of these guidelines. TreatmentAcute proctitis of recent onset among persons who have recently practiced receptive anal intercourse is usually sexually acquired (215,216). Such patients should be examined by anoscopy and should be evaluated for infection with HSV, N. gonorrhoeae, C. trachomatis, and T. pallidum. If an anorectal exudate is detected on examination or if polymorphonuclear leukocytes are detected on a Gram-stained smear of anorectal secretions, the following therapy may be prescribed while awaiting additional laboratory tests. Recommended Regimen Patients with suspected or documented herpes proctitis should be managed in the same manner as those with genital herpes (see Genital HSV Infections). If painful perianal ulcers are present or mucosal ulcers are detected on anoscopy, presumptive therapy should include a regimen for treating genital herpes. In addition, LGV proctitis and proctocolitis also should be considered. Appropriate diagnostic testing for LGV should be conducted in accordance with state or federal guidelines, and doxycycline therapy should be administered 100 mg orally twice daily for 3 weeks. Follow-UpFollow-up should be based on specific etiology and severity of clinical symptoms. Reinfection might be difficult to distinguish from treatment failure. Management of Sex PartnersPartners of patients with sexually transmitted enteric infections should be evaluated for any diseases diagnosed in the index patient. Ectoparasitic InfectionsPediculosis PubisPatients who have pediculosis pubis (i.e., pubic lice) usually seek medical attention because of pruritus or because they notice lice or nits on their pubic hair. Pediculosis pubis is usually transmitted by sexual contact. Recommended Regimens Alternative Regimens Reported resistance to pediculcides has been increasing and is widespread. Malathion may be used when treatment failure is believed to have occurred because of resistance (217). The odor and long duration of application for malathion make it a less attractive alternative than the recommended pediculcides. Ivermectin has been successfully used to treat lice but has only been evaluated in small studies. Lindane is not recommended as first-line therapy because of toxicity. It should only be used as an alternative because of inability to tolerate other therapies or if other therapies have failed. Lindane toxicity, as indicated by seizure and aplastic anemia, has not been reported when treatment was limited to the recommended 4-minute period. Permethrin has less potential for toxicity than lindane. Other Management Considerations The recommended regimens should not be applied to the eyes. Pediculosis of the eyelashes should be treated by applying occlusive ophthalmic ointment to the eyelid margins twice a day for 10 days. Bedding and clothing should be decontaminated (i.e., machine-washed, machine-dried using the heat cycle, or dry cleaned) or removed from body contact for at least 72 hours. Fumigation of living areas is not necessary. Patients with pediculosis pubis should be evaluated for other STDs. Follow-Up Patients should be evaluated after 1 week if symptoms persist. Re-treatment might be necessary if lice are found or if eggs are observed at the hair-skin junction. Patients who do not respond to one of the recommended regimens should be re-treated with an alternative regimen. Management of Sex Partners Sex partners within the previous month should be treated. Patients should avoid sexual contact with their sex partner(s) until patients and partners have been treated and reevaluated to rule out persistent disease. Special Considerations PregnancyPregnant and lactating women should be treated with either permethrin or pyrethrins with piperonyl butoxide; lindane is contraindicated in pregnancy. HIV InfectionPatients who have pediculosis pubis and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. ScabiesThe predominant symptom of scabies is pruritus. Sensitization to Sarcoptes scabiei occurs before pruritus begins. The first time a person is infested with S. scabiei, sensitization takes up to several weeks to develop. However, pruritus might occur within 24 hours after a subsequent reinfestation. Scabies in adults frequently is sexually acquired, although scabies in children usually is not. Recommended Regimens
Alternative Regimens
Lindane is not recommended as first-line therapy because of toxicity. It should only be used as an alternative if the patient cannot tolerate other therapies or if other therapies have failed. Lindane should not be used immediately after a bath or shower, and it should not be used by persons who have extensive dermatitis, women who are pregnant or lactating, or children aged <2 years. Lindane resistance has been reported in some areas of the world, including parts of the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia after lindane use also has been reported. Permethrin is effective and safe and less expensive than ivermectin. One study demonstrated increased mortality among elderly, debilitated persons who received ivermectin, but this observation has not been confirmed in subsequent reports (218). Other Management Considerations Bedding and clothing should be decontaminated (i.e., either machine-washed, machine-dried using the hot cycle, or dry cleaned) or removed from body contact for at least 72 hours. Fumigation of living areas is unnecessary. Crusted Scabies Crusted scabies (i.e., Norwegian scabies) is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Patients who are receiving systemic or potent topical glucocorticoids, organ transplant recipients, mentally retarded or physically incapacitated persons, HIV-infected or human T-lymphotrophic virus-1-infected persons, and persons with various hematologic malignancies are at risk for developing crusted scabies. Crusted scabies is associated with greater transmissibility than scabies. No controlled therapeutic studies for crusted scabies have been conducted, and the appropriate treatment remains unclear. Substantial treatment failure might occur with a single topical scabicide or with oral ivermectin treatment. Some specialists recommend combined treatment with a topical scabicide and oral ivermectin or repeated treatments with ivermectin 200 ug/kg on days 1, 15, and 29. Lindane should be avoided because of the risks for neurotoxicity with heavy applications or denuded skin. Patient's fingernails should be closely trimmed to reduce injury from excessive scratching. Follow-Up Patients should be informed that the rash and pruritus of scabies might persist for up to 2 weeks after treatment. Symptoms or signs that persist for >2 weeks can be attributed to several factors. Treatment failure might be caused by resistance to medication or by faulty application of topical scabicides. Patients with crusted scabies might have poor penetration into thick scaly skin and harbor mites in these difficult-to-penetrate layers. Particular attention must be given to the fingernails of these patients. Reinfection from family members or fomites might occur in the absence of appropriate contact treatment and washing of bedding and clothing. Even when treatment is successful and reinfection is avoided, symptoms can persist or worsen as a result of allergic dermatitis. Finally, household mites can cause symptoms to persist as a result of crossreactivity between antigens. Some specialists recommend re-treatment after 1--2 weeks for patients who are still symptomatic; others recommend re-treatment only if live mites are observed. Patients who do not respond to the recommended treatment should be re-treated with an alternative regimen. Management of Sex Partners and Household Contacts Both sexual and close personal or household contacts within the preceding month should be examined and treated. Management of Outbreaks in Communities, Nursing Homes, and Other Institutional Settings Scabies epidemics frequently occur in nursing homes, hospitals, residential facilities, and other communities. Control of an epidemic can only be achieved by treatment of the entire population at risk. Ivermectin can be considered in this setting, especially if treatment with topical scabicides fails. Epidemics should be managed in consultation with a specialist. Special Considerations Infants, Young Children, and Pregnant or Lactating WomenInfants, young children, and pregnant or lactating women should not be treated with lindane. They can be treated with permethrin. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh <15 kg has not been determined. HIV InfectionPatients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV negative. HIV-infected patients and others who are immunosuppressed are at increased risk for crusted scabies. Ivermectin has been reported to be useful in small, noncontrolled studies. Such patients should be managed in consultation with a specialist. Sexual Assault and STDsAdults and AdolescentsThe recommendations in this report are limited to the identification, prophylaxis, and treatment of sexually transmitted infections and conditions commonly identified in the management of such infections. The documentation of findings, collection of nonmicrobiologic specimens for forensic purposes, and the management of potential pregnancy or physical and psychological trauma are beyond the scope of this report. Examinations of survivors of sexual assault should be conducted by an experienced clinician in a way that minimizes further trauma to the survivor. The decision to obtain genital or other specimens for STD diagnosis should be made on an individual basis. Care systems for survivors should be designed to ensure continuity (including timely review of test results), support adherence, and monitor for adverse reactions to any therapeutic or prophylactic regimens prescribed at initial examination. Laws in all 50 states strictly limit the evidentiary use of a survivor's previous sexual history, including evidence of previously acquired STDs, as part of an effort to undermine the credibility of the survivor's testimony. Evidentiary privilege against revealing any aspect of the examination or treatment is enforced in the majority of states. In unanticipated, exceptional situations, STD diagnoses may later be accessed, and the survivor and clinician may opt to defer testing for this reason. However, collection of specimens at initial examination for laboratory STD diagnosis gives the survivor and clinician the option to defer empiric prophylactic antimicrobial treatment. Among sexually active adults, the identification of sexually transmitted infection after an assault might be more important for the psychological and medical management of the patient than for legal purposes because the infection could have been acquired before the assault. Trichomoniasis, BV, gonorrhea, and chlamydial infection are the most frequently diagnosed infections among women who have been sexually assaulted. Because the prevalence of these infections is high among sexually active women, their presence after an assault does not necessarily signify acquisition during the assault. A postassault examination is, however, an opportunity to identify or prevent sexually transmitted infections, regardless of whether they were acquired during an assault. Chlamydial and gonococcal infections in women are of particular concern because of the possibility of ascending infection. In addition, HBV infection might be prevented by postexposure administration of hepatitis B vaccine. Reproductive-aged female survivors should be evaluated for pregnancy, if appropriate. Evaluation for Sexually Transmitted InfectionsInitial Examination An initial examination should include the following procedures:
Follow-Up Examinations After the initial postassault examination, follow-up examinations provide an opportunity to 1) detect new infections acquired during or after the assault; 2) complete hepatitis B immunization, if indicated; 3) complete counseling and treatment for other STDs; and 4) monitor side effects and adherence to postexposure prophylactic medication, if prescribed. Examination for STDs should be repeated within 1--2 weeks of the assault. Because infectious agents acquired through assault might not have produced sufficient concentrations of organisms to result in positive test results at the initial examination, testing should be repeated during the follow-up visit, unless prophylactic treatment was provided. If treatment was provided, testing should be conducted only if the survivor reports having symptoms. If treatment was not provided, follow-up examination should be conducted within 1 week to ensure that results of positive tests can be discussed promptly with the survivor and that treatment is provided. Serologic tests for syphilis and HIV infection should be repeated 6 weeks, 3 months, and 6 months after the assault if initial test results were negative and infection in the assailant could not be ruled out (see Sexual Assaults, Risk for Acquiring HIV Infection). Prophylaxis Many specialists recommend routine preventive therapy after a sexual assault because follow-up of survivors of sexual assault can be difficult. The following prophylactic regimen is suggested as preventive therapy:
Recommended Regimens
For patients requiring alternative treatments, refer to the sections in this report relevant to the specific agent. The efficacy of these regimens in preventing infections after sexual assault has not been evaluated. Clinicians should counsel patients regarding the possible benefits and toxicities associated with these treatment regimens; gastrointestinal side effects can occur with this combination. Providers might also consider anti-emetic medications, particularly if EC also is provided. Other Management Considerations At the initial examination and, if indicated, at follow-up examinations, patients should be counseled regarding 1) symptoms of STDs and the need for immediate examination if symptoms occur and 2) abstinence from sexual intercourse until STD prophylactic treatment is completed. Risk for Acquiring HIV Infection HIV seroconversion has occurred in persons whose only known risk factor was sexual assault or sexual abuse, but the frequency of this occurrence is probably low. In consensual sex, the risk for HIV transmission from vaginal intercourse is 0.1%--0.2% and for receptive rectal intercourse, 0.5%--3% (219). The risk for HIV transmission from oral sex is substantially lower. Specific circumstances of an assault might increase risk for HIV transmission (e.g., trauma, including bleeding) with vaginal, anal, or oral penetration; site of exposure to ejaculate; viral load in ejaculate; and the presence of an STD or genital lesions in the assailant or survivor. Children might be at higher risk for transmission because child sexual abuse is frequently associated with multiple episodes of assault and might result in mucosal trauma (see Sexual Assault or Abuse of Children). Postexposure therapy with zidovudine was associated with a reduced risk for acquiring HIV in a study of health-care workers who had percutaneous exposures to HIV-infected blood (220). On the basis of these results and the results of animal studies, PEP has been recommended for health-care workers who have occupational exposures to HIV (207). These findings have been extrapolated to other types of HIV exposure, including sexual assault (58). If HIV exposure has occurred, initiation of PEP as soon as possible after the exposure likely increases benefit. Although a definitive statement of benefit cannot be made regarding PEP after sexual assault, the possibility of HIV exposure from the assault should be assessed at the time of the postassault examination. The possible benefit of PEP in preventing HIV infection also should be discussed with the assault survivor if risk exists for HIV exposure from the assault. The likelihood of the assailant having HIV, any exposure characteristics that might increase the risk for HIV transmission, the time elapsed after the event, as well as potential benefits and risks the PEP are all factors that will impact the medical recommendation for PEP and impact the assault survivor's acceptance of that recommendation (58). Determination of assailant's HIV status at the time of the assault examination will usually be impossible. Therefore, the health-care provider should assess any available information concerning HIV-risk behaviors of the assailant(s) (e.g., a man who has sex with other men and injecting-drug or crack cocaine use), local epidemiology of HIV/AIDS, and exposure characteristics of the assault. When an assailant's HIV status is unknown, factors that should be considered in determining whether an increased risk for HIV transmission exists include 1) whether vaginal or anal penetration occurred; 2) whether ejaculation occurred on mucous membranes; 3) whether multiple assailants were involved; 4) whether mucosal lesions are present in the assailant or survivor; and 5) other characteristics of the assault, survivor, or assailant that might increase risk for HIV transmission. If PEP is offered, the following information should be discussed with the patient: 1) the unproven benefit and known toxicities of antiretrovirals; 2) the close follow-up that will be necessary; 3) the benefit of adherence to recommended dosing; and 4) the necessity of early initiation of PEP to optimize potential benefits (as soon as possible after and up to 72 hours after the assault). Providers should emphasize that PEP appears to be well-tolerated in both adults and children and that severe adverse effects are rare. Clinical management of the survivor should be implemented according to the following guidelines (58). Specialist consultation on PEP regimens is recommended if HIV exposure during the assault was possible and if PEP is being considered. The sooner PEP is initiated after the exposure, the higher the likelihood that it will prevent HIV transmission, if HIV exposure occurred; however, distress after an assault also might prevent the survivor from accurately weighing exposure risks and benefits of PEP and making an informed decision to start PEP. If use of PEP is judged to be warranted, the survivor should be offered a 3--5-day supply of PEP with a follow-up visit scheduled for additional counseling after several days. Recommendations for Postexposure Assessment of Adolescent and Adult Survivors Within 72 hours of Sexual Assault§§§
Sexual Assault or Abuse of ChildrenRecommendations in this report are limited to the identification and treatment of STDs. Management of the psychosocial aspects of the sexual assault or abuse of children is beyond the scope of these recommendations. The identification of sexually transmissible agents in children beyond the neonatal period suggests sexual abuse. The significance of the identification of a sexually transmitted agent in such children as evidence of possible child sexual abuse varies by pathogen. Postnatally acquired gonorrhea; syphilis; and nontransfusion, nonperinatally acquired HIV are usually diagnostic of sexual abuse. Sexual abuse should be suspected when genital herpes is diagnosed. The investigation of sexual abuse among children who possibly have an infection that might have been sexually transmitted should be conducted in compliance with recommendations by clinicians who have experience and training in all elements of the evaluation of child abuse, neglect, and assault. The social importance of infection that might have been acquired sexually and the recommended action regarding reporting of suspected child sexual abuse varies by the specific organism (Table 6). In all cases in which a sexually transmitted infection has been diagnosed in a child, efforts should be made to detect evidence of sexual abuse, including conducting diagnostic testing for other commonly occurring sexually transmitted infections (221). The general rule that sexually transmissible infections beyond the neonatal period are evidence of sexual abuse has exceptions. For example, rectal or genital infection with C. trachomatis among young children might be the result of perinatally acquired infection and has, in some cases, persisted for as long as 2--3 years. Genital warts have been diagnosed in children who have been sexually abused, but also in children who have no other evidence of sexual abuse. BV has been diagnosed in children who have been abused, but its presence alone does not prove sexual abuse. The majority of HBV infections in children result from household exposure to persons who have chronic HBV infection. The possibility of sexual abuse should be strongly considered if no conclusive explanation for nonsexual transmission of a sexually transmitted infection can be identified. When the only evidence of sexual abuse is the isolation of an organism or the detection of antibodies to a sexually transmissible agent, findings should be confirmed and the implications considered carefully. Evaluation for Sexually Transmitted Infections Examinations of children for sexual assault or abuse should be conducted in a manner designed to minimize pain and trauma to the child. Collection of vaginal specimens in prepubertal children can be very uncomfortable and should be performed by an experienced clinician to avoid psychological and physical trauma to the child. The decision to obtain genital or other specimens from a child to conduct an STD evaluation must be made on an individual basis. The following situations involve a high risk for STDs and constitute a strong indication for testing:
If a child has symptoms, signs, or evidence of an infection that might be sexually transmitted, the child should be tested for other common STDs before the initiation of any treatment that could interfere with the diagnosis of those other STDs. Because of the legal and psychosocial consequences of a false-positive diagnosis, only tests with high specificities should be used. The potential benefit to the child of a reliable diagnosis of an STD justifies deferring presumptive treatment until specimens for highly specific tests are obtained by providers with experience in the evaluation of sexually abused and assaulted children. The scheduling of an examination should depend on the history of assault or abuse. If the initial exposure was recent, the infectious agents acquired through the exposure might not have produced sufficient concentrations of organisms to result in positive test results. A follow-up visit approximately 2 weeks after the most recent sexual exposure may include a repeat physical examination and collection of additional specimens. To allow sufficient time for antibodies to develop, another follow-up visit approximately 12 weeks after the most recent sexual exposure might be necessary to collect sera. A single examination might be sufficient if the child was abused for an extended period and if the last suspected episode of abuse occurred substantially before the child received medical evaluation. The following recommendations for scheduling examinations serve as a general guide. The exact timing and nature of follow-up examinations should be determined on an individual basis and should be performed to minimize the possibility for psychological trauma and social stigma. Compliance with follow-up appointments might be improved when law enforcement personnel or child protective services are involved. Initial and 2-Week Follow-Up Examinations During the initial examination and 2-week follow-up examination (if indicated), the following should be performed:
HIV infection has been reported in children whose only known risk factor was sexual abuse. Serologic testing for HIV infection should be considered for abused children. The decision to test for HIV infection should be made on a case-by-case basis, depending on the likelihood of infection among assailant(s). Data are insufficient concerning the efficacy and safety of PEP among both children and adults. However, antiretroviral treatment is well-tolerated by infants and children with and without HIV infection. In addition, children who receive such treatment have a minimal risk for serious adverse reactions because of the short period recommended for prophylaxis (58,62). In considering whether to offer antiretroviral PEP, health-care providers should consider whether the child can be treated soon after the sexual exposure (i.e., within 72 hours), the likelihood that the assailant is at risk for HIV infection, and the likelihood of high compliance with the prophylactic regimen. The potential benefit of treating a sexually abused child should be weighed against the risk for adverse reactions. If antiretroviral PEP is being considered, a professional specializing in HIV-infected children should be consulted. Recommendations for HIV-Related Postexposure Assessment of Children within 72 Hours of Sexual Assault
Follow-Up Examination After Assault In circumstances in which transmission of syphilis, HIV, or hepatitis B is a concern but baseline tests are negative, an examination approximately 6 weeks, 3 months, and 6 months after the last suspected sexual exposure is recommended to allow time for antibodies to infectious agents to develop. In addition, results of HBsAg testing must be interpreted carefully, because HBV can be transmitted nonsexually. Decisions regarding which tests should be performed must be made on an individual basis. Presumptive Treatment The risk of a child acquiring an STD as a result of sexual abuse or assault has not been well studied. Presumptive treatment for children who have been sexually assaulted or abused is not recommended because 1) the incidence of the majority of STDs in children is low after abuse/assault, 2) prepubertal girls appear to be at lower risk for ascending infection than adolescent or adult women, and 3) regular follow-up of children usually can be ensured. However, some children or their parent(s) or guardian(s) might be concerned about the possibility of infection with an STD, even if the risk is perceived to be low by the health-care provider. Such concerns might be an appropriate indication for presumptive treatment in some settings and may be considered after all specimens for diagnostic tests relevant to the investigation have been collected. Reporting U.S. states and territories have laws that require the reporting of child abuse. Although the exact requirements differ by state, if a health-care provider has reasonable cause to suspect child abuse, a report must be made. Health-care providers should contact their state or local child-protection service agency regarding child-abuse reporting requirements in their states. References
* Regardless of history of condom use during exposure. † Providers should use a culture or test that has been cleared by the FDA or locally verified in accordance with applicable statutes. § The absence of a fourfold or greater titer for an infant does not exclude congenital syphilis. ¶ CSF test results obtained during the neonatal period can be difficult to interpret; normal values differ by gestational age and are higher in preterm infants. Values as high as 25 white blood cells (WBCs)/mm3 and/or protein of 150 mg/dL might occur among normal neonates; some specialists, however, recommend that lower values (i.e., 5 WBCs/mm3 and protein of 40 mg/dL) be considered the upper limits of normal. Other causes of elevated values should be considered when an infant is being evaluated for congenital syphilis. ** A woman treated with a regimen other than those recommended in these guidelines for treatment should be considered untreated. †† If the infant's nontreponemal test is nonreactive and the likelihood of the infant being infected is low, certain specialists recommend no evaluation but treatment of the infant with a single IM dose of benzathine penicillin G 50,000 units/kg for possible incubating syphilis, after which the infant should receive close serologic follow-up. §§ Some specialists would not treat the infant but would provide close serologic follow-up in those whose mother's nontreponemal titers decreased fourfold after appropriate therapy for early syphilis or remained stable or low for late syphilis. ¶¶ An association between oral erythromycin and infantile hypertrophic pyloric stenosis has been reported in infants aged <6 weeks who were treated with this drug. Infants treated with erythromycin should be followed for signs and symptoms of idiopathic hypertrophic pyloric stenosis (IHPS). *** Data on use of other macrolides (e.g., azithromycin and clarithromycin) for the treatment of neonatal chlamydia infection are limited. The results of one study involving a limited number of patients suggest that a short course of azithromycin, 20 mg/kg/day orally, 1 dose daily for 3 days, may be effective. ††† The Bethesda System for Reporting Cervical/Vaginal Cytologic Results uses the terms "low-grade SIL" and "high-grade SIL" for abnormal results (199). Low-grade SIL encompasses cytological changes associated with HPV and mild dysplasia. High-grade SIL includes cytological changes associated with moderate dysplasia, severe dysplasia, and carcinoma in situ. Cytological results should be distinguished from histological results obtained from biopsy specimens. §§§ Assistance with postexposure prophylaxis decisions can be obtained by calling the National Clinician's Post-Exposure Prophylaxis Hotline (PEPLine), telephone: 888-448-4911.
Terms and Abbreviations Used in This Report
|
| AIDS | Acquired immunodeficiency syndrome | IgE | Immunoglobulin E |
| ALT | Alanine aminotransferase | Ig | Immune globulin |
| anti-HBc | Antibody to hepatitis B core antigen | IgG | Immunoglobulin G |
| anti-HCV | Hepatitis C antibodies | IgM | Immunoglobulin M |
| ASC-US | Atypical squamous cells of undetermined significance | IM | Intramuscularly |
| BCA | Bichloroacetic acid | IUD | Intrauterine device |
| BV | Bacterial vaginosis | IV | Intravenous or intravenously |
| CBC | Complete blood count | KOH | Potassium hydroxide |
| CI | Confidence interval | LGV | Lymphogranuloma venereum |
| CIN | Cervical intraepithelial neoplasia | MAC | Mycobacterium avium complex |
| CLIA | Clinical Laboratory Improvement Amendments | MIC | Minimum inhibitory concentration |
| CNS | Central nervous system | MSM | Men who have sex with men |
| CSF | Cerebrospinal fluid | N-9 | Nonoxynol-9 |
| DFA | Direct fluorescent antibody | NAAT | Nucleic acid amplification test |
| DGI | Disseminated gonococcal infection | NGU | Nongonococcal urethritis |
| dL | Deciliter | Pap | Papanicolaou |
| DNA | Deoxyribonucleic acid | PCR | Polymerase chain reaction |
| EC | Emergency contraception | PEP | Postexposure prophylaxis |
| EIA | Enzyme immunoassay | PID | Pelvic inflammatory disease |
| ELISA | Enzyme-linked immunosorbent assay | PO | By mouth |
| EPT | Expedited partner therapy | PPV | Positive predictive value |
| FDA | Food and Drug Administration | QRNG | Quinolone resistant Neisseria gonorrhoeae |
| FTA-ABS | Fluorescent treponemal antibody absorbed | RNA | Ribonucleic acid |
| gG | Glycoprotein G | RPR | Rapid plasma reagin |
| GNID | Gram-negative intracellular diplococci | RT-PCR | Reverse transcriptase polymerase chain reaction |
| HAART | Highly active antiretroviral therapy | RVVC | Recurrent vulvovaginal candidiasis |
| HAV | Hepatitis A virus | SIL | Squamous intraepithelial lesion |
| HBIG | Hepatitis B immune globulin | STD | Sexually transmitted disease |
| HBsAg | Hepatitis B surface antigen | TCA | Trichloroacetic acid |
| HBV | Hepatitis B virus | TE | Toxoplasmic encephalitis |
| HCC | hepatocellular carcinoma | TP-PA | Treponema pallidum particle agglutation |
| HCV | Hepatitis C virus | VDRL | Venereal Disease Research Laboratory |
| HIV | Human immunodeficiency virus | VVC | Vulvovaginal candidiasis |
| HPV | Human papillomavirus | WB | Western blot |
| HSV | Herpes simplex virus | WBC | White blood count |
| IFA | Immunofluorescence assay | WSW | Women who have sex with women |
Sexually Transmitted Diseases Treatment Guidelines, 2006
Consultants
Chairpersons: David Atkins, MD, Agency for Healthcare Research and Quality, Rockville, Maryland; Kimberly A. Workowski, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, and Emory University, Atlanta, Georgia.
Presenters: Heidi Bauer, MD, California Sexually Transmitted Disease Control Branch, Oakland, California; Emily J. Erbelding, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland; William M. Geisler, MD, Department of Medicine, University of Alabama, Birmingham, Alabama; Margaret Hammerschlag, MD, State University of New York, Downstate Medical Center, Brooklyn, New York; Peter Leone, MD, University of North Carolina School of Medicine, Chapel Hill, North Carolina; Jeanne Marrazzo, MD, University of Washington, Harborview Medical Center, Seattle, Washington; Kenneth Hugh Mayer, MD, Brown University Medical School, Providence, Rhode Island; Pablo Sanchez, MD, University of Texas Southwestern Medical Center, Dallas, Texas; Bradley Stoner, MD, PhD, Washington University, St. Louis, Missouri; Anna Wald, MD, University of Washington, Harborview Medical Center, Seattle, Washington; George Wendel, MD, University of Texas Southwestern Medical School, Dallas, Texas; Karen Wendel, MD, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma; Harold C. Wiesenfeld, MD, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania. Moderators: Willard Cates, Jr., MD, Family Health International, Durham, North Carolina; King K. Holmes, MD, PhD, University of Washington, Harborview Medical Center, Seattle, Washington; David Martin, MD, Louisiana State University Medical Center, New Orleans, Louisiana. Rapporteurs: Hunter Handsfield, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia, University of Washington, Seattle, Washington; William McCormack, MD, State University of New York Health Science Center, Brooklyn, New York; Anne Rompalo, MD, Johns Hopkins School of Medicine, Baltimore, Maryland.
Consultants: Michael Augenbraun, MD, State University of New York Health Science Center, Brooklyn, New York; Gail Bolan, MD, California Department of Health, Oakland, California; Carolyn Deal, PhD, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; Kenneth H. Fife, MD, PhD, Indiana University School of Medicine, Indianapolis, Indiana; J. Dennis Fortenberry, MD, Indiana University School of Medicine, Indianapolis, Indiana; Edward Hook, III, MD, Department of Medicine, University of Alabama, Birmingham, Alabama; Franklyn Judson, MD, University of Colorado Department of Medicine and Preventive Medicine, Denver, Colorado; Alice A. Kraman, PharmD; Emory Healthcare, Atlanta, Georgia; Roberta B. Ness, MD, University of Pittsburgh Department of Medicine, Pittsburgh, Pennsylvania; Paul Nyirjesy, MD, Drexel University College of Medicine, Philadelphia, Pennsylvania; Jeffrey Peipert, MD, Women and Infants Hospital, Providence, Rhode Island; Jane R. Schwebke, MD, Department of Medicine, University of Alabama, Birmingham, Alabama; Mary Ann Shafer, MD, University of California, San Francisco Department of Medicine, San Francisco, California; David Soper, MD, Medical University of South Carolina, Charleston, South Carolina; Lawrence Stanberry, MD, PhD, University of Texas Medical Branch, Galveston, Texas; Heather Watts, MD, National Institute of Child Health and Development, National Institutes of Health, Bethesda, Maryland; Jonathan M. Zenilman, MD, Johns Hopkins Bayview Medical Center, Baltimore, Maryland.
Liaison Participants: Joanne Armstrong, MD, Women's Health, Aetna, Sugar Land, Texas; James R. Allen, MD, American Social Health Association, Durham, North Carolina; Margaret J. Blythe, MD, American Academy of Pediatrics, Indianapolis, Indiana; Sherry R. Crump, MD, American College of Preventive Medicine, Atlanta, Georgia; Carolyn D. Deal, PhD, National Institutes of Health, Bethesda, Maryland; Jordon Dimitrakov, MD, PhD, American Urological Association, Boston, Massachusetts; Mark FitzGerald, MD, British Association for Sexual Health and HIV, Southampton, United Kingdom; Edward Harrison, National Commission on Correctional Health Care, Chicago, Illinois; Edward W. Hook, III, MD, Infectious Disease Society of America, Birmingham, Alabama; Michel Janier, MD, PhD, International Union Against Sexually Transmitted Infections Europe, Paris, France; Abe Macher, MD, HIV/AIDS Bureau, Rockville, Maryland; Francis J. Ndowa, MD, World Health Organization, Geneva, Switzerland; Jeffrey F. Peipert, MD, American College of Obstetricians and Gynecologists, Providence, Rhode Island; Kees A. Rietmeijer, MD, PhD, Denver Public Health Department, Denver, Colorado; Richard Rothman, MD, American College of Emergency Physicians, Baltimore, Maryland; David Soper, MD, Infectious Diseases Society for Obstetrics and Gynecology, Charleston, South Carolina; Litjen Tan, PhD, American Medical Association, Chicago, Illinois; Bruce Trigg, MD, National Coalition for Sexually Transmitted Disease Directors, Albuquerque, New Mexico; Julia Valderrama, MD, Pan American Health Organization, Washington, DC; Tom Wong, MD, Public Health Agency of Canada, Ottawa, Ontario, Canada; Miriam Zieman, MD, Association of Reproductive Health Professionals, Atlanta, Georgia.
CDC, Division of Sexually Transmitted Disease Prevention Treatment Guidelines 2006 Project. Coordinator: Kimberly A. Workowski, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, and Emory University, Atlanta, Georgia.
Project Managers: Donald F. Dowda, ORISE, Oakridge, Tennessee; Richard Voigt, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia.
CDC Presenters: Joanna Buffington, MD, National Center for Infectious Diseases; Eileen Dunne, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia; Matthew Hogben, PhD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia; Emily Koumans, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia; Hershel Lawson, MD, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, Georgia; Catherine McLean, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), Atlanta, Georgia; Juliette Morgan, MD, National Center for Infectious Diseases, CDC, Atlanta, Georgia; Lori Newman, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia; Madeline Sutton, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia. CDC Consultants: Sevgi O. Aral, PhD, Stuart M. Berman, MD, John Douglas, MD, Susan J. DeLisle, Kathleen Ethier, PhD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia; Kevin Fenton, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia; John Moran, MD, National Immunization Program, CDC, Atlanta, Georgia; Julia Schillinger, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia. Support Staff: Valerie Barner, Winda Graves, Garrett Mallory, Deborah McElroy, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed), CDC, Atlanta, Georgia; Eboney Walker, NAI Personnel, Washington, DC.
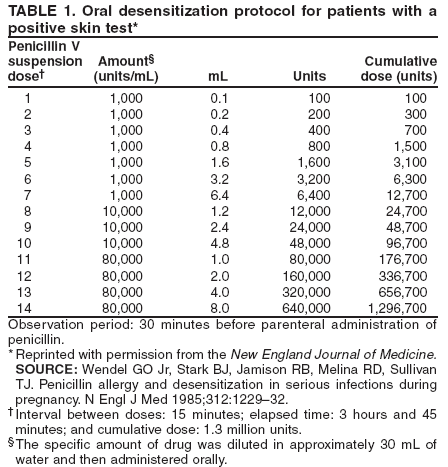
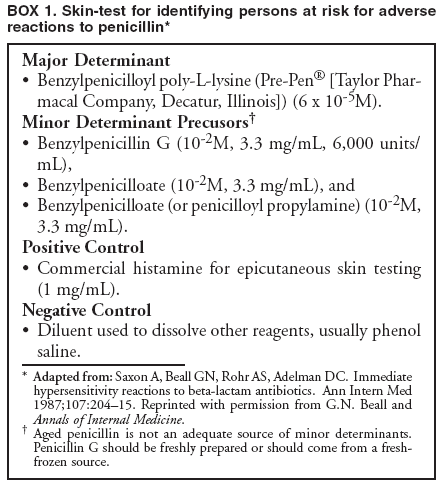
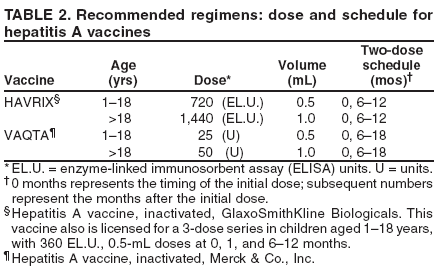
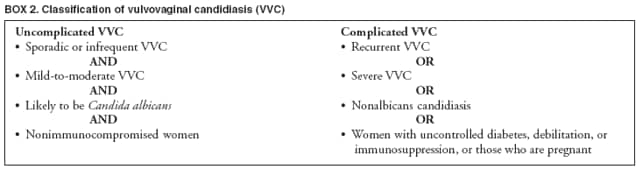
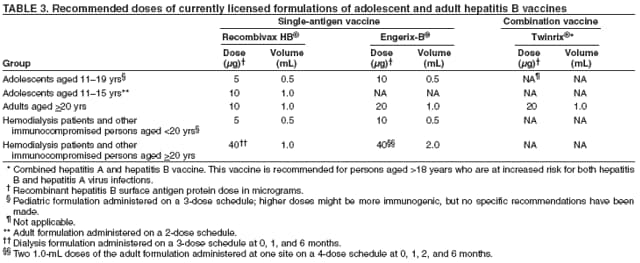
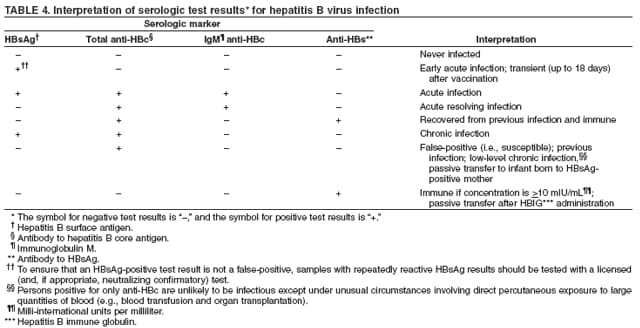
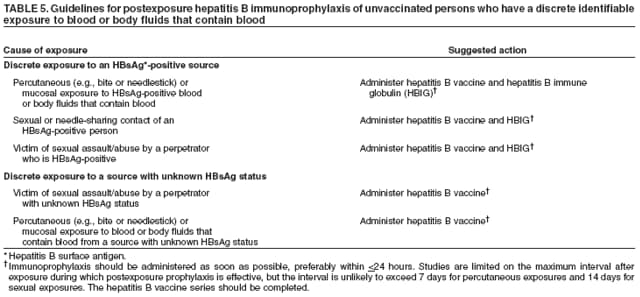
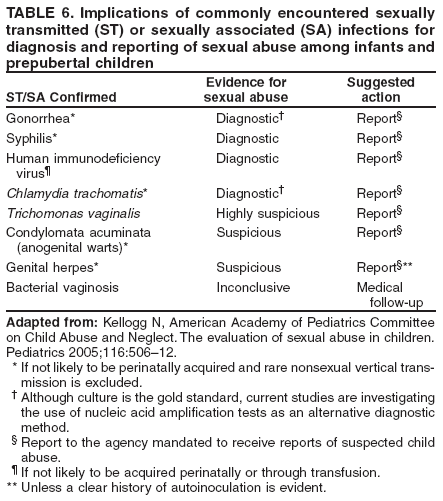
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to [email protected].Date last reviewed: 10/24/2006
|
|
|||||
|
HOME |
ABOUT MMWR |
MMWR SEARCH |
DOWNLOADS |
RSS
|
CONTACT
|
|||||
|
|
|||||
|