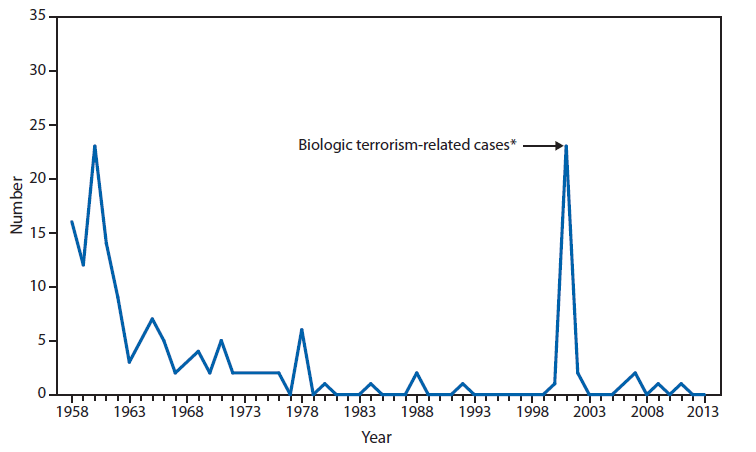
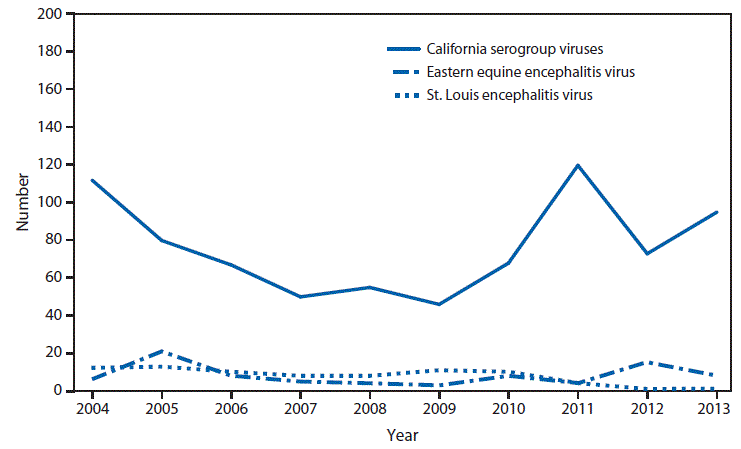
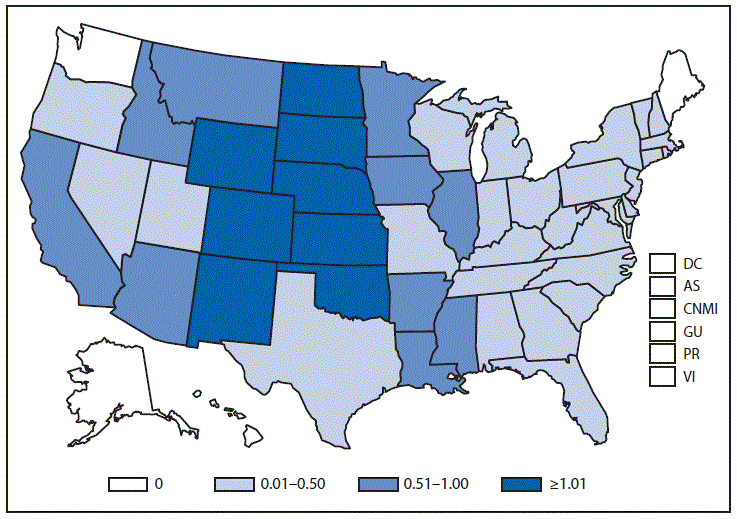
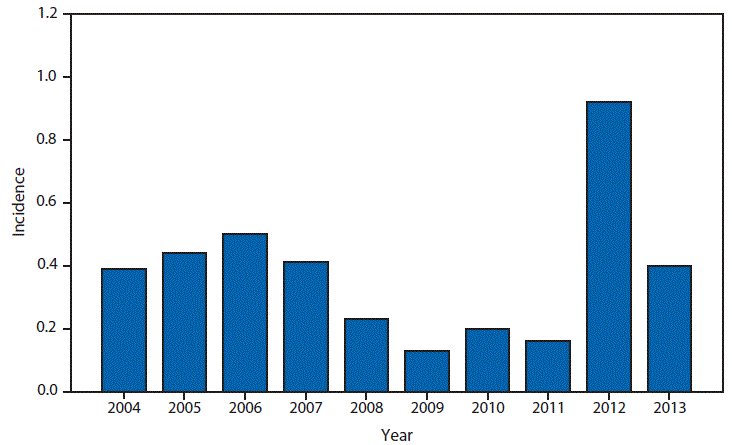
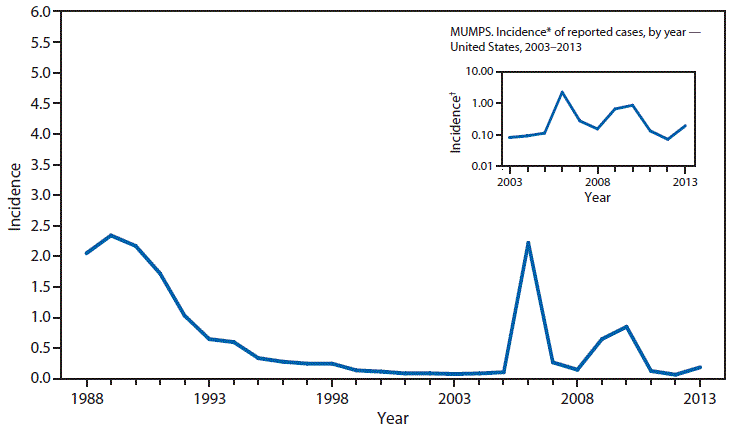
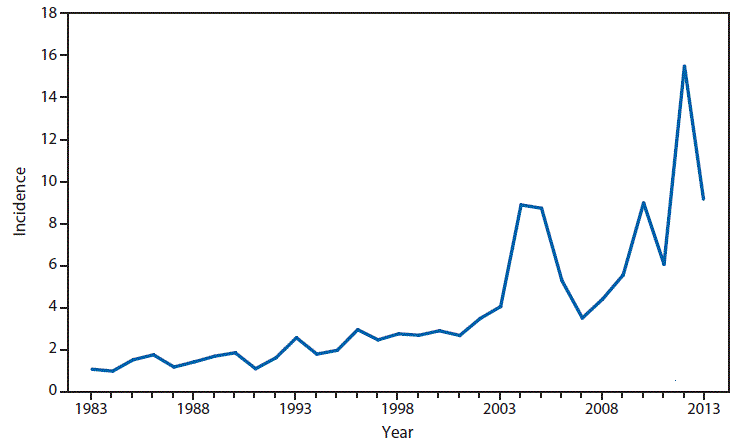
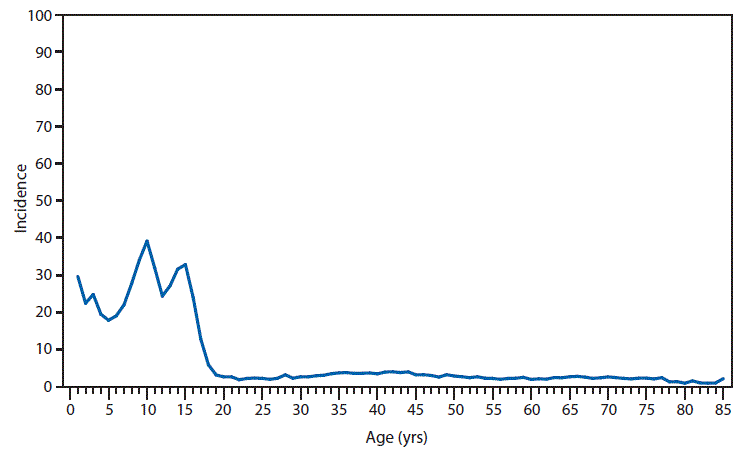
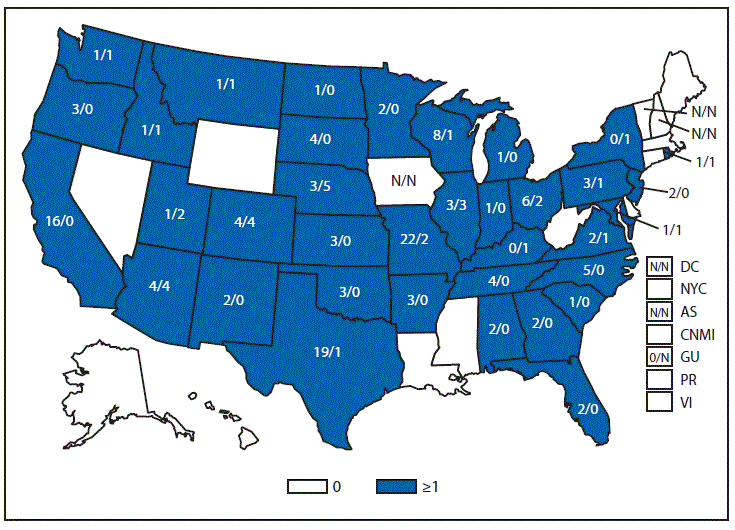
Nationally Notifiable Infectious Conditions Group
Contributors
(All group members meet the CDC and MMWR criteria for contributors)
John Abellera, MPH, Willie Anderson, Kaitlin Benedict, MPH, Tina Benoit, MPH, David Blaney, MPH, Anna Bowen, MD, Delicia Carey, PhD, Tom Chiller, MD, Amanda Conrad, MPH, Kristina Cordes, MPH, MBA, Rosalyn Dhara, MPH, Elizabeth Ervin, MPH, Julia Gargano, PhD, Elizabeth B. Gray, MPH, Marta Guerra, DVM, Rebecca L. Hall, MPH, Alesia Harvey, Michele Hlavsa, MPH, Scott Holmberg, MD, Deborah Holtzman, Jacqueline Hurd, MPH, Brendan R. Jackson, MD, Kelly A. Jackson, MPH, Ruth Jiles, PhD, Anna Satcher Johnson, MPH, Michael Judd, MPH, Grishma Kharod, MPH, Sarah Kidd, MD, Robert Kirkcaldy, MD, Monina Klevens, DDS, Barbara Knust, DVM, Ben Kupronis, MPH, Jennifer Lehman, Anastasia Litvintseva, PhD, Adriana Lopez, MHS, Kathleen Ly, MPH, Jessica R. MacNeil, MPH, Harold S. Margolis, MD, Barbara Mahon, MD, Orion McCotter, MPH, Paul Mead, MD, Prachi Mehta, PhD, Rajal Mody, MD, William Morrill, MPH, Anna Newton, MPH, Agam Rao, MD, Susan B. Redd, Pierre E. Rollin, MD, Amanda Santander, MPH, Tyler M. Sharp, PhD, Tami H. Skoff, MS, John Su, MD, Kimberly Thomas, MPH, Elizabeth Torrone, PhD, Rita Traxler, MHS, Greg S. Wallace, MD, Tony Winters, MSPH, Jonathan Yoder, MPH.
 ShareCompartir
ShareCompartir